Abstract
A reliable, nontoxic method of inducing transplantation tolerance is needed to overcome the problems of chronic organ graft rejection and immunosuppression-related toxicity. Treatment of mice with single injections of an anti-CD40 ligand antibody and CTLA4Ig, a low dose (3 Gy) of whole body irradiation, plus fully major histocompatibility complex–mismatched allogeneic bone marrow transplantation (BMT) reliably induced high levels (>40%) of stable (>8 mo) multilineage donor hematopoiesis. Chimeric mice permanently accepted donor skin grafts (>100 d), and rapidly rejected third party grafts. Progressive deletion of donor-reactive host T cells occurred among peripheral CD4+ lymphocytes, beginning as early as 1 wk after bone marrow transplantation. Early deletion of peripheral donor-reactive host CD4 cells also occurred in thymectomized, similarly treated marrow recipients, demonstrating a role for peripheral clonal deletion of donor-reactive T cells after allogeneic BMT in the presence of costimulatory blockade. Central intrathymic deletion of newly developing T cells ensued after donor stem cell engraftment had occurred. Thus, we have shown that high levels of chimerism and systemic T cell tolerance can be reliably achieved without myeloablation or T cell depletion of the host. Chronic immunosuppression and rejection are avoided with this powerful, nontoxic approach to inducing tolerance.
Keywords: tolerance, transplantation, costimulation, chimerism, bone marrow
The field of organ transplantation has enjoyed substantial progress during the last two decades, resulting in marked improvements in short-term graft survival. However, organ transplant recipients still face substantial risks of long-term morbidity and mortality. Although modern immunosuppressive regimens have led to a dramatic reduction in the incidence of acute rejection episodes, they have yet to achieve a similar effect for chronic rejection, which is still the leading cause of graft loss during long-term follow-up (1). In addition, the requirement for life-long immunosuppressive drug therapy carries a significant risk of severe side effects, including tumors, infections, and metabolic disorders. The reliable induction of donor-specific tolerance would solve both problems by obviating the need for chronic nonspecific immunosuppression and by abrogating detrimental immunological reactions against the allograft.
Costimulation mediated by interactions of CD28 and CD40 ligand (CD40L, CD154)1 on T cells, and B7 (CD80 and CD86; 2, 3) and CD40 (4–6), respectively, on APCs, is of central importance for T cell–dependent immune responses. The use of antibodies that block these two pathways has recently led to improved graft survival in several transplant models (7–10), with optimal results obtained when both pathways are blocked simultaneously (11–13). However, none of the reported treatment regimens were shown to induce permanent primary skin graft acceptance or deletional tolerance. Since deletional tolerance is the most robust form of tolerance (14, 15), leading to the absence of mature donor-reactive host cells, it may be the most desirable form of tolerance for the clinical situation. Hematopoietic cell transplantation has been known to be associated with donor-specific tolerance for over 40 yr (16). However, the routine use of hematopoietic cell transplantation to induce tolerance in the clinical setting has been prohibited thus far, largely because of the unacceptable toxicity associated with the conditioning needed to achieve engraftment of allogeneic bone marrow (BM) and because of complications associated with allogeneic bone marrow transplantation (BMT).
We now describe a method of inducing deletional T cell tolerance that uses allogeneic BMT and costimulatory blockade, leading to peripheral clonal deletion, high levels of allogeneic stem cell engraftment, and permanent tolerance without requiring host T cell depletion or myeloablative conditioning.
Materials and Methods
Animals.
Female C57BL/6 (B6: H-2b), B10.A (B10.A: H-2a), and A.SW (H-2s) mice were purchased from Frederick Cancer Research Center (Frederick, MD) or from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a specific pathogen–free microisolator environment, as previously described (17).
Conditioning and BMT.
Age-matched (6–8-wk-old) female B6 mice received 3 Gy whole body irradiation (WBI) and were injected intravenously on the same day (day 0) with unseparated BM harvested from MHC-mismatched female B10.A donors (10–12 wk old). A control group was injected intraperitoneally with depleting doses of rat IgG2b anti–mouse CD4 mAb GK1.5 and anti–mouse CD8 mAb 2.43 on days −5 and −1, as previously described (18). Murine CTLA4Ig was injected intraperitoneally as a single dose (0.5 mg) on day +2, and hamster anti– mouse CD40L mAb (MR1) was injected intraperitoneally on d0 (0.45 mg). CTLA4Ig was a gift from Bristol-Myers Squibb Pharmaceuticals, Seattle, WA; the MR1 hybridoma was provided to us by Dr. Randolph J. Noelle (Dartmouth Medical School, Lebanon, NH). In the experiments indicated, thymectomies were performed 4 wk before BMT, as described elsewhere (19, 20). The completeness of thymectomy was confirmed at the time of death 2 wk after BMT by visual inspection and two-color FACS® staining (Becton Dickinson, San Jose, CA) (CD4-FITC versus CD8-PE) of mediastinal tissue. Mice showing any evidence of remaining thymic tissue were excluded from analysis.
Flow Cytometric Analysis of Multilineage Chimerism.
Flow cytometric analysis (FCM) of multilineage chimerism was performed as previously described (21). In brief, forward angle and 90° light scatter properties were used to distinguish lymphocytes, monocytes, and granulocytes in peripheral white blood cells. Two-color FCM was used to distinguish donor and host cells of particular lineages, and the percentage of donor cells was calculated as previously described (21), by subtracting control staining from quadrants containing donor and host cells expressing a particular lineage marker, and by dividing the net percentage of donor cells by the total net percentage of donor plus host cells of that lineage. Dead cells were excluded using propidium iodide staining. Nonspecific FcγR binding was blocked by anti–mouse FcγR mAb 2.4G2 (22). FITC-conjugated mAbs included anti-CD4, anti-CD8, anti-B220 (all purchased from PharMingen, San Diego, CA), and anti-MAC1 (Caltag Labs., San Francisco, CA). Negative control mAb HOPC1-FITC, with no reactivity to mouse cells, was prepared in our laboratory. Biotinylated anti– H-2Dd mAb 34-2-12 and control mAb HOPC1 were developed with PE-streptavidin.
FCM of T Cell Receptor Vβ Families.
Peripheral blood lymphocytes were stained with FITC-conjugated anti-Vβ5.1/2, Vβ11, and Vβ8.1/2 mAbs versus PE-conjugated anti-CD4 mAb (all from PharMingen). Nonspecific PE-conjugated rat IgG2a (PharMingen) served as negative control. Two-color FCM analysis was performed on gated CD4+ cells. Splenocytes were stained with FITC-conjugated anti-Vβ5.1/2, Vβ11, and Vβ8.1/2 mAbs versus PE-conjugated anti-CD4 mAb (or anti-CD8 mAb; PharMingen) and anti–34-2-12-biotin (BIO) developed with CyChrome-streptavidin (PharMingen). Three-color FCM analysis was performed on 34-2-12–negative, CD4+ (or CD8+) cells. Thymocytes were stained with FITC-conjugated anti–TCR-β (PharMingen), anti–Vβ5.1/2, Vβ11, and Vβ8.1/2 versus BIO-conjugated KH95 (anti-Db, PharMingen) developed with PE-streptavidin. Two-color FCM analysis was performed on gated host-type class I (KH95)–high cells, and the percentage of Vβ positive cells in this gate was corrected for the percentage of TCR-high cells in the same gate, as previously described (23). For BIO.A controls, gated 34-2-12-high cells were analyzed in a similar way. Background staining (as determined by nonreactive mAb HOPC-FITC) was subtracted from the percentage of cells staining with each anti-Vβ mAb. P values were calculated using a two-tailed Student's t test.
Skin Grafting.
Full thickness tail skin from B10.A (donor-specific) and fully MHC-mismatched A.SW (third party) mice was grafted onto the lateral thoracic wall, secured with 5-0 silk sutures and bandaids, and followed by visual and tactile inspections daily for 3 wk, and then at least one inspection every week thereafter. Grafts were defined as rejected when <10% of the graft remained viable.
Results and Discussion
Stable Multilineage Hematopoietic Chimerism after Treatment with CTLA4Ig Plus MR1.
To determine whether blocking the CD28 and CD40 costimulatory pathways could allow survival of fully MHC-mismatched BM and the induction of mixed chimerism and tolerance, B6 mice were treated with 3 Gy WBI and received 1.5 × 107 unseparated BM cells from fully MHC-mismatched B10.A donors. A single dose of MR1 and of CTLA4Ig was given either alone or in combination, on days 0 and +2, respectively. Donor hematopoiesis was assessed at multiple time points after BMT by FCM of peripheral white blood cells. By staining with an mAb specific for donor class I versus various lineage markers, the net percentage of donor cells within these lineages was determined (see also Materials and Methods).
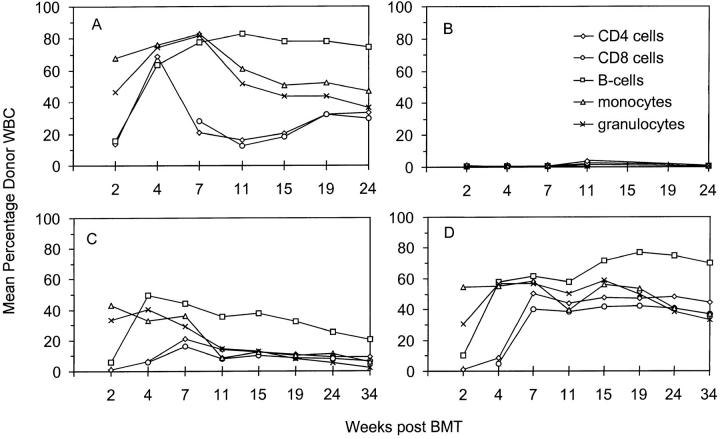
The combined administration of CTLA4Ig plus MR1 led to high levels of chimerism in all hematopoietic lineages, including T, B, and myeloid cells (Fig. 1 D). Donor reconstitution averaged >40% in all lineages by 7 wk, and remained high during the observation period of 34 wk. Especially surprising was the high level of donor representation among CD4 and CD8 cells by 7 wk after BMT, even though the hosts did not receive T cell depletion in their conditioning. Donor T cell levels in the group treated with MR1 plus CTLA4Ig were stable throughout long-term follow-up (34 wk) and were, on average, higher than those in a control group conditioned with anti-CD4– and anti-CD8–depleting mAbs (Fig. 1 A) (21, 24). 15 out of 16 mice treated with the combination of MR1 and CTLA4Ig developed high levels of chimerism (one mouse showed no detectable chimerism and was excluded from further analysis as an outlier). Treatment with MR1 alone led to high levels of donor cells among myeloid lineages and B cells at early time points after BMT, and to lower levels of chimerism among CD4 and CD8 cells. However, the induction of chimerism was less reliable in these mice than that in the group receiving both costimulatory blocking reagents, and donor chimerism was not stable in recipients of MR1 alone (Fig. 1 C). Mice receiving CTLA4Ig alone did not show chimerism in peripheral blood, as detected by FCM, at any time after BMT (Fig. 1 B). Similarly, control animals receiving WBI and BM cells alone failed to show hematopoietic chimerism (data not shown).
Figure 1.
High levels of multilineage donor chimerism in peripheral blood for 34 wk after BMT. Results from one of two similar experiments are shown as group averages. All animals received 3 Gy WBI and 1.5 × 107 allogeneic BM cells on day 0. Only MR1 plus CTLA4Ig together (D) allowed the reliable induction of stable chimerism (n = 5), with high levels of donor cells in all lineages throughout the follow-up. Administration of MR1 alone (C) led to significant levels of chimerism, but chimerism declined over time (n = 5). When CTLA4Ig was given alone (B), no chimerism was detectable by FCM (n = 4). A control group (n = 5), receiving depleting doses of anti-CD4 and anti-CD8 mAbs on day −5 and day −1 (A), showed substantial levels of donor chimerism, with long-term levels of T cell chimerism being significantly lower than B cell, granulocyte, and monocyte chimerism.
These results demonstrate that the simultaneous blockade of the CD28 and CD40 pathways allows the induction of high levels of stable multilineage chimerism, and thus allogeneic pluripotent stem cell engraftment. In previous protocols using immunocompetent hosts, this has only been achieved with regimens that involve exhaustive depletion of host T cells with mAbs or myeloablation by lethal irradiation or cytotoxic drugs (18, 21, 25–28). The toxicity associated with myeloablation is generally considered to preclude its use in organ transplant recipients. The completeness of T cell depletion necessary in experimental protocols to achieve mixed chimerism (21, 26) would also be of great concern in the clinical setting, given that the capacity of the adult human thymus to regenerate T cells might be limited after such treatment (29).
Donor-specific Skin Graft Tolerance in Chimeras Prepared with CTLA4Ig Plus MR1.
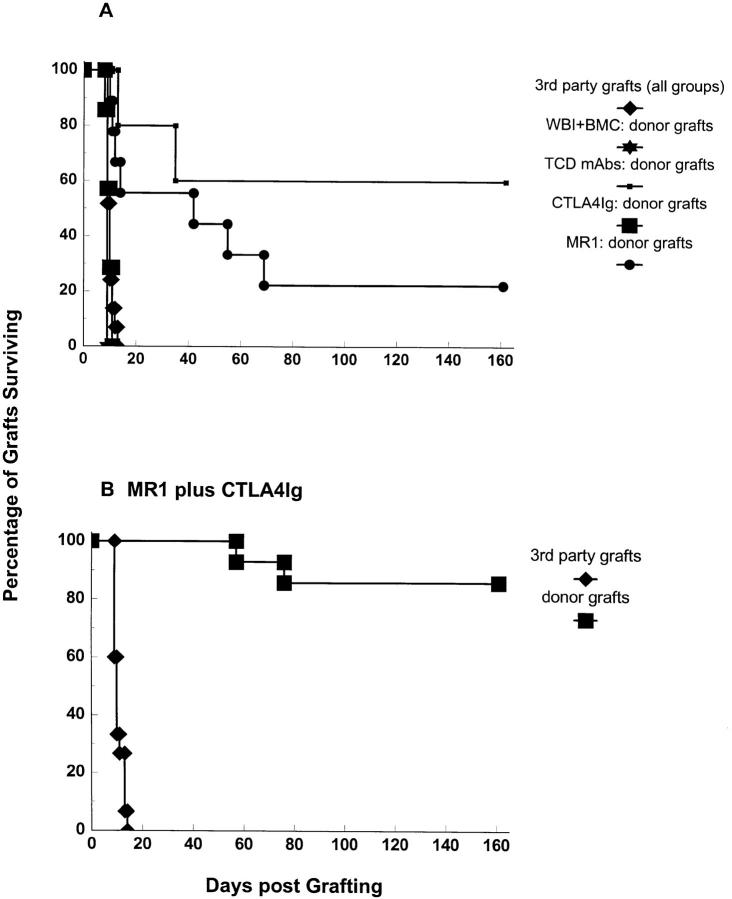
Primary skin grafting is considered the most stringent test of transplantation tolerance. We therefore grafted donor (B10.A) and third party (A.SW) full-thickness tail skin onto recipients at various time points after BMT. Mice that received both CTLA4Ig and MR1, plus 3 Gy WBI and BMT, permanently accepted donor skin grafts placed 3, 6, or 10 wk after BMT (Fig. 2 B), with the exception of two animals that rejected their grafts 57 and 76 d after graft placement, respectively. Third party grafts were readily rejected (median survival time [MST] = 10 d), demonstrating the donor specificity of the tolerance induced. This skin graft survival compares favorably even to the control animals that were conditioned with T cell– depleting antibodies, in which only 60% of donor skin grafts survived more than 100 d (Fig. 2 A). Mice treated with MR1 alone in addition to 3 Gy WBI and BMT demonstrated prolongation of donor skin graft survival (MST = 42 d; Fig. 2 A). However, only two out of nine grafts were accepted for 100 d. In contrast, mice receiving BMT after 3 Gy WBI and CTLA4Ig alone did not show prolonged survival of donor skin grafts (MST = 10 d), consistent with the absence of chimerism.
Figure 2.
Permanent survival of donor-specific skin grafts in chimeras prepared with 3 Gy WBI and allogeneic (B10.A) BM cells and treatment with MR1 plus CTLA4Ig. Combined results from two experiments are shown. Recipients were grafted with donor-specific (B10.A) and third party (A.SW) skin grafts at 3, 6, or 10 wk after BMT. Mice receiving the full treatment of BMT and MR1 plus CTLA4Ig accepted donor skin grafts (B) permanently (12 out of 14), with the exception of 2 animals that rejected their grafts at days 57 and 76, respectively. 9 grafts have been accepted in perfect condition for >110 d, and 5 grafts for >140 d. Third-party skin grafts (B) were rejected in the expected time frame (MST = 10 d). MR1 alone (A) led to prolongation of donor-specific skin graft survival (MST = 42 d), but only 2 out of 9 grafts survived >100 d. CTLA4Ig alone (A) failed to improve skin graft survival (n = 7, MST = 10 d). Control mice treated with 3 Gy WBI plus BMC (n = 4) and mice receiving 3 Gy WBI and MR1 plus CTLA4Ig alone (without BMT, data not shown) rejected donor skin within 2 wk. In a control group prepared with T cell–depleting mAbs on day −5 and day −1 plus BMT (plus 3 Gy WBI) (n = 5), donor skin grafts were permanently accepted in 60% of mice. Third party grafts were rejected within 2 wk in all groups.
These results demonstrate the presence of donor-specific tolerance across a full MHC barrier in chimeras prepared with MR1 plus CTLA4Ig. The ability of mixed chimeras prepared with MR1 and CTLA4Ig to rapidly reject third party skin grafts is evidence for their immunocompetence. Although previous studies reported the ability of costimulatory blockade alone to prolong skin graft survival (8, 9, 11), permanent skin graft acceptance and tolerance was not reliably achieved (9, 30).
Deletion of Donor-reactive T Cells in Chimeras Prepared with CTLA4Ig Plus MR1.
To examine whether deletion of donor-reactive T cells occurs when chimerism is induced with MR1 and CTLA4Ig, peripheral blood lymphocytes were analyzed for the presence of certain Vβ subunits on their TCRs. The donor strain B10.A expresses I-E, which is required to present superantigens derived from Mammary tumor virus 8 and 9 endogenous retroviruses encoded in the B6/B10 background genome. Developing thymocytes whose TCRs contain Vβ11 or Vβ5.1/2, which bind to these superantigens, are deleted in I-E-positive B10.A mice (31–33), but not in B6 mice, because they do not express I-E (32, 34).
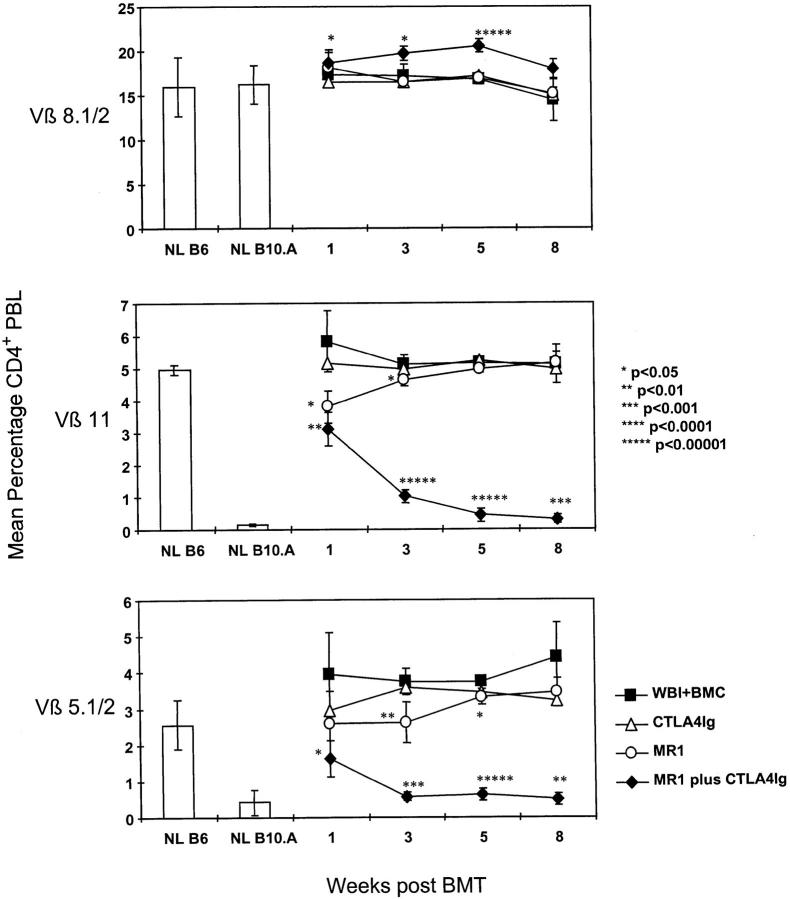
Partial deletion of Vβ5+ and Vβ11+ peripheral CD4 T cells was observed as early as 1 wk after BMT in mice receiving 3 Gy WBI followed by MR1 plus CTLA4Ig (Fig. 3). The deletion became progressively more complete over the ensuing weeks, and reached similar levels to those in chimeras prepared with T cell depletion (data not shown) (23). Deletion of these Vβ5+ and Vβ11+ cells was sustained throughout the follow-up period (>6 mo in the first experiment for which chimerism data are shown in Fig. 1). Percentages of Vβ8-bearing CD4 cells, which do not recognize superantigens on the donor or host, were not reduced at any time point, ruling out a nonspecific deletional process. Mice treated with BMT (plus 3 Gy WBI) and MR1 alone showed early partial deletion of Vβ5 and Vβ11, which was only transient in the experiment shown (Fig. 3). However, in the experiment shown in Fig. 1, deletion was still observed at later time points for the group receiving BMT (plus 3 Gy WBI) and MR1 alone, which correlated with the higher initial levels of chimerism observed for this group in this experiment. Control animals receiving 3 Gy WBI plus BMT alone or BMT (plus 3 Gy WBI) with CTLA4Ig failed to show any Vβ5 or Vβ11 deletion. As expected, deletion of Vβ5 and Vβ11 did not occur in control animals receiving WBI and MR1 plus CTLA4Ig without BMT (data not shown). Downregulation of the level of TCR expression instead of deletion seems an unlikely explanation for the reduction in Vβ5+ and Vβ11+ CD4 T cells in chimeras, since the intensity of the Vβ5 and Vβ11 staining on the cells remaining in the blood at 1 and 3 wk after BMT was similar to that in nontransplanted controls (data not shown). Thus, no evidence for TCR downmodulation was observed.
Figure 3.
Specific deletion of donor-reactive peripheral T cells in recipients of BMT and MR1 plus CTLA4Ig. Results from one of two similar experiments are shown. FCM was performed at indicated time points, with the percentage of Vβ-positive cells being determined among gated CD4+ PBLs. The mean percentage of CD4+ lymphocytes expressing Vβ5.1/2 or Vβ11 was significantly lower in mice receiving BM cells (BMC) (plus 3 Gy WBI) with MR1 plus CTLA4Ig (n = 10) than in recipients of BMC (plus 3 Gy WBI) alone (n = 4), as early as 1 wk after BMT (P <0.01 for Vβ11, P <0.05 for Vβ5). The specific deletion gradually became more complete at 3, 5, and 8 wk after BMT and was sustained for the length of follow-up. The percentage of Vβ8.1/2+ CD4 cells remained similar in all groups, demonstrating specificity of the Vβ5.1/2 or Vβ11 deletion in mixed chimeras. Mice receiving BMC (plus 3 Gy WBI) alone or in addition to CTLA4Ig (n = 4) did not show any deletion, nor did control mice treated with MR1 plus CTLA4Ig alone (without BMC; data not shown). MR1 alone led only to a slight and transient deletion in this experiment (n = 5). Error bars indicate standard deviation. P values are shown for comparison with the control group receiving 3 Gy WBI plus BMC. NL B6 denotes naive C57BL/6 control; NL B10.A denotes naive B10.A control.
These results indicate that in BMT recipients treated with MR1 plus CTLA4Ig and 3 Gy WBI, donor-reactive host T cells start to disappear from the periphery very soon after BMT. Neither CTLA4Ig nor MR1 (35, 36) is known to be directly cytotoxic to the T cells to which they bind. 3 Gy WBI causes only transient and mild leukopenia (37) and only partial T cell depletion (Sykes, M., unpublished data). This time course suggests that the deletion observed at 1 wk is not entirely due to intrathymic mechanisms, since a sufficient number of thymocytes would be unlikely to emigrate from the thymus during this period to “dilute” the preexisting peripheral repertoire to an extent that could explain the observed decrease in Vβ5+ and Vβ11+ CD4 lymphocytes in PBLs.
Extrathymic Clonal Deletion Occurs in the Early Period after BMT and Costimulatory Blockade.
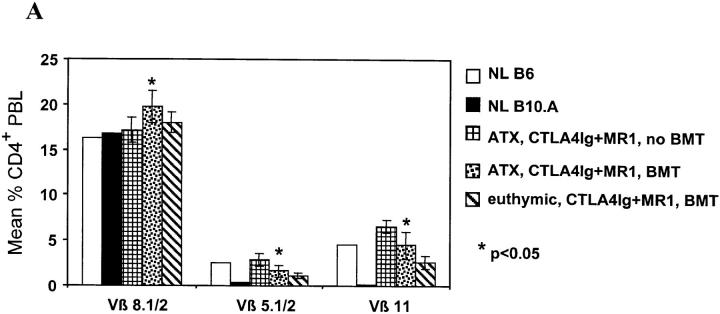
To directly determine whether peripheral deletion is responsible for the early decline in donor-reactive CD4 T cells in chimeras, thymectomized (ATX) B6 mice received B10.A BM cells after conditioning with 3 Gy WBI and MR1 plus CTLA4Ig. As shown in Fig. 4 A, ATX mice demonstrated partial deletion of Vβ5+ and Vβ11+ peripheral blood CD4 cells 1 wk after BMT, and the degree of deletion was comparable to that in euthymic recipients. Vβ8+ CD4 cells were not diminished, indicating the specificity of the deletion for superantigens presented by the donor. Similarly treated ATX mice not receiving BMT showed no reduction in the percentage of Vβ5+ or Vβ11+ CD4 cells compared to untreated B6 mice (Fig. 4 A), demonstrating that this peripheral deletion occurred specifically in response to donor marrow in BMT recipients.
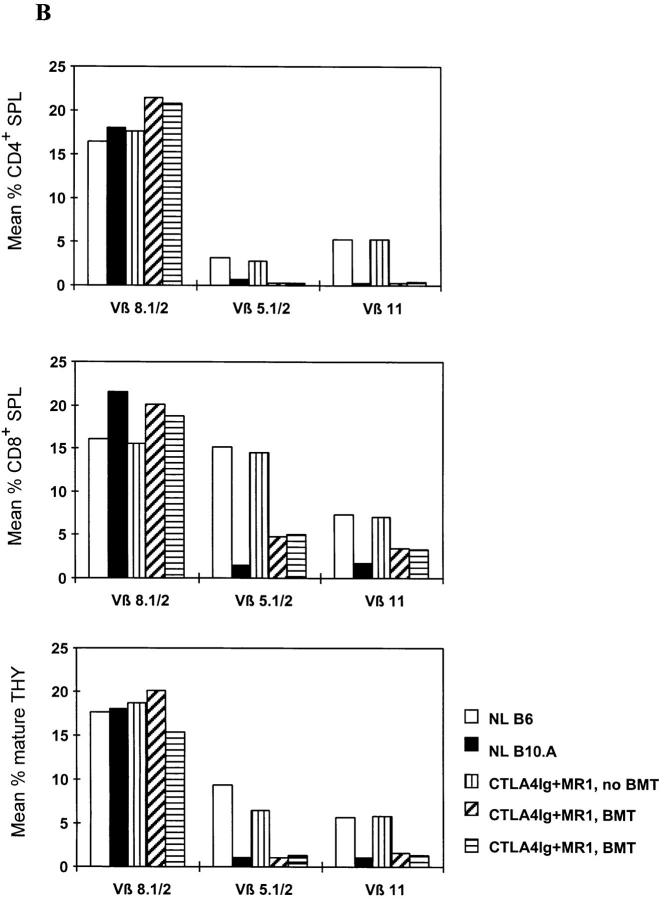
Figure 4.
Extrathymic clonal deletion after BMT and costimulatory blockade with CTLA4Ig and MR1. (A) ATX recipients (n = 6) showed specific, partial deletion of Vβ5.1/2+ and Vβ11+ CD4 cells in PBLs, 1 wk after BMT plus CTLA4Ig and MR1 and 3 Gy WBI. A similar degree of deletion was observed in euthymic controls (n = 5) prepared with the same conditioning. CD4 cells in ATX controls receiving CTLA4Ig plus MR1 and WBI without BMT (n = 4) did not show deletion of these Vβ. The percentage of Vβ+ cells was determined by FCM analysis of gated CD4+ PBLs. P values are shown for comparison between ATX BMT recipients receiving CTLA4Ig plus MR1 and ATX non-BMT controls. (B) In two euthymic chimeras killed 20 wk after BMT (under cover of 3 Gy WBI, plus treatment with CTLA4Ig plus MR1), Vβ5.1/2+ and Vβ11+CD4+ splenocytes (SPL) were deleted to the same extent as in naive B10.A controls (top). In contrast, the percentage of Vβ5.1/2+ and Vβ11+CD8+ splenocytes was reduced compared to naive B6 mice, but was substantially higher than in naive B10.A mice (middle). Mature Vβ5.1/2+ and Vβ11+ thymocytes (THY) showed deletion comparable to B10.A at the same time (bottom). A control mouse receiving WBI and CTLA4Ig plus MR1 (but no BMT) showed no deletion in either splenocytes or thymocytes. The percentage of Vβ+ cells was determined by FCM analysis in gated CD4+ (or CD8+) 34-2-12–negative splenocytes, and in gated KH-95high (i.e., Db high)-thymocytes (or Dd high in the case of BIO.A controls; see Materials and Methods). NL B6 denotes naive C57BL/6 control; NL B10.A denotes naive B10.A control.
Further evidence that peripheral deletion plays a role in the early period after BMT in this model was obtained from chimeras killed 20 wk after BMT and CTLA4Ig plus MR1. Among splenocytes of these mice, the percentage of Vβ5+ and Vβ11+ cells among CD4+ T cells was reduced to similar levels as in normal, control B10.A mice. However, the percentages of Vβ5+ and Vβ11+ cells were substantially higher among CD8+ splenocytes than among CD4+ splenocytes. Nevertheless, the percentages of CD8 splenocytes using these Vβ were significantly lower than among those of normal, control B6 mice (Fig. 4 B). As discussed below, this difference most likely reflects the dilution of the peripheral CD8 pool by new thymic emigrants that are tolerized by a central deletion mechanism in the chimeras.
Peripheral deletion has been shown to be one consequence of powerful T cell responses in vivo, but it has only been reported after marked expansion of antigen-recognizing cells (38, 39). Although 1 wk after BMT was the earliest time point at which donor-reactive host T cells were examined, we did not see evidence of such initial expansion. More recently, in vitro evidence has demonstrated that costimulatory signals play a prominent role in preventing apoptotic cell death after TCR engagement (40–42). However, apoptosis induced in vivo by antigen encountered in the presence of costimulatory blockade has not been described previously.
Evidence for Central Deletion of Donor-reactive T Cells in Long-Term Chimeras.
Mature recipient T cells (including both CD4 and CD8 cells) in the thymus showed marked deletion of Vβ5 and Vβ11 when animals were killed 20 wk after BMT (Fig. 4 B), demonstrating that newly developing donor-reactive thymocytes are effectively deleted during maturation in the thymus in long-term chimeras.
Together, our data suggest a model whereby extrathymic clonal deletion occurs early after BMT under cover of costimulatory blockade. Allogeneic pluripotent stem cell engraftment is thus permitted, and subsequent tolerization of newly-developing donor-reactive thymocytes occurs by deletional mechanisms in the thymus similar to that which occurs in animals initially treated with T cell–depleting mAbs (23). This model also explains the discrepancy in the extent of deletion of CD4 cells compared to CD8 cells in the peripheral tissues of long-term chimeras. Vβ5+ and Vβ11+ CD4 cells are subject to deletion both intrathymically and in the periphery when they recognize superantigen plus donor MHC class II. CD8 cells expressing these Vβ are efficiently deleted intrathymically (43) at the CD8+CD4+ stage of maturation. However, CD8+CD4− cells are not very effectively deleted extrathymically (38, 39, 44), although weak proliferative activity to superantigens presented by class II MHC in the periphery has been described (39, 44). It follows that the substantial difference in the degree of deletion between peripheral CD4 and CD8 cells is most likely due to the more extensive contribution of extrathymic deletion among the CD4 compared to the CD8 cells preexisting at the time of BMT. This conclusion is further supported by the observation that ATX recipients of BMT plus costimulatory blockade demonstrated a significant reduction of Vβ5+ and Vβ11+ CD4 splenocytes, but not of CD8 splenocytes, at 2 wk after BMT (data not shown).
Previously published reports showed that costimulatory blockade as the sole immunosuppressive treatment can allow the permanent acceptance of vascularized allografts grafted at the same time (8, 11, 12). However, the organ allografts themselves probably play a critical role in tolerance induction in these models, and deletional tolerance has not been demonstrated. In contrast, solid organ (skin) grafting was not required in the induction phase of tolerance in our model. Instead, the permanent engraftment of donor hematopoietic cells ensured the tolerization of preexisting host T cells and of T cells that developed subsequent to the disappearance of the costimulatory blocking agents from the circulation. This later tolerance occurred through intrathymic deletional mechanisms (Fig. 4), presumably as a consequence of the presence of donor-derived APCs in the thymus, as has been demonstrated in long-term mixed chimeras prepared with other regimes that involve initial depletion of the T cell repertoire with mAbs (23). In previous studies, administration of donor lymphocytes in the presence of anti-CD40L has been shown to allow survival of pancreatic islet allografts (45) and to prolong survival of MHC-mismatched skin allografts (9). However, chimerism and deletion were not evident in those studies. Thus, our studies provide the first demonstration that costimulatory blockade leads to peripheral deletion of donor-reactive T cells, then allows the engraftment of fully MHC-mismatched, allogeneic pluripotent stem cells, which induce central tolerance among T cells that subsequently develop in the thymus.
BMT with CTLA4Ig plus MR1 specifically eliminates donor-reactive T cells, while avoiding the nonspecific depletion or suppression of T cells, which is a component of all clinically available immunosuppressive strategies, and can lead to severe complications. This new treatment protocol would be suitable for both cadaveric and living-related organ transplantation, as it allows the reliable induction of deletional tolerance with a nontoxic conditioning regimen beginning on the day of transplantation. Since the peripheral T cell repertoire is not globally depleted by the conditioning and only a low, minimally myelosuppressive dose of WBI is given, the clinical potential of this approach is extraordinarily high.
Acknowledgments
We thank Dr. Hugh Auchincloss, Jr., and Dr. Henry Winn for critical review of the manuscript; Dr. David H. Sachs for his support and advice; and Diane Plemenos for secretarial assistance.
This study was supported by National Institutes of Health grant R01 HL-49915, and in part by a sponsored research agreement between Massachusetts General Hospital and BioTransplant, Inc. Dr. Wekerle was supported by fellowships from the Max Kade Foundation and the Austrian Science Fund (FWF). Dr. Sayegh is a recipient of the National Kidney Foundation Clinician Scientist Award.
Footnotes
Abbreviations used in this paper: ATX, thymectomized; BM, bone marrow; BMT, bone marrow transplantation; CD40L, CD40 ligand; FCM, flow cytometric analysis; MR1, hamster anti–mouse CD40L mAb; MST, median survival time; WBI, whole body irradiation.
References
- 1.Gjertson, D.W. 1991. Survival trends in long-term first cadaver-donor kidney transplants. In Clinical Transplants. P.I. Terasaki and J.M. Cecka, editors. UCLA Tissue Typing Laboratory, Los Angeles. 225–235. [PubMed]
- 2.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 3.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 5.Hancock WW, Sayegh MH, Peach R, Linsley PS, Turka LA. Costimulatory function of CD40L, CD80 and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen CP, Pearson TC. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr Opin Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 7.Lenshow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islets induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 8.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 9.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 11.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Rae H, Cho, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 12.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayegh, M.H., and L.A. Turka. 1998. The role of T cell costimulatory activation pathways in transplant rejection. N. Engl. J. Med. In press. [DOI] [PubMed]
- 14.Sykes M. Chimerism and central tolerance. Curr Opin Immunol. 1996;8:694–703. doi: 10.1016/s0952-7915(96)80088-4. [DOI] [PubMed] [Google Scholar]
- 15.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 16.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 17.Sykes M, Romick ML, Hoyles KA, Sachs DH. In vivo administration of interleukin 2 plus T cell– depleted syngeneic marrow prevents graft-versus-host disease mortality and permits alloengraftment. J Exp Med. 1990;171:645–658. doi: 10.1084/jem.171.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JFAP. Studies on mouse leukaemia: the role of the thymus in leukaemagenesis by cell-free leukemic infiltrates. Br J Cancer. 1960;14:93–97. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykes M, Harty MW, Karlhofer FM, Pearson DA, Szot G, Yokoyama W. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993;178:223–229. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita Y, Sachs DH, Khan A, Sykes M. Additional monoclonal antibody (mAB) injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti–T cell mABs and 3-Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 22.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a non-myeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 24.Tomita Y, Khan A, Sykes M. Mechanism by which additional monoclonal antibody (mAB) injections overcome the requirement for thymic irradiation to achieve mixed chimerism in mice receiving bone marrow transplantation after conditioning with anti–T cell mABs and 3-Gy whole body irradiation. Transplantation. 1996;61:477–485. doi: 10.1097/00007890-199602150-00028. [DOI] [PubMed] [Google Scholar]
- 25.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 26.Nomoto K, Yung-Yun K, Omoto K, Umesue M, Murakami Y, Matsuzaki G. Tolerance induction in a fully allogeneic combination using anti–T cell receptor-αβ monoclonal antibody, low dose irradiation, and donor bone marrow transfusion. Transplantation. 1995;59:395–401. [PubMed] [Google Scholar]
- 27.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–2829. [PubMed] [Google Scholar]
- 28.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 30.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-γ is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 31.Acha-Orbea H, Palmer E. Mls—a retrovirus exploits the immune system. Immunol Today. 1991;12:356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- 32.Tomonari K, Fairchild S. The genetic basis of negative selection of Tcrβ-V11β+ T cells. Immunogenetics. 1991;33:157–162. doi: 10.1007/BF01719234. [DOI] [PubMed] [Google Scholar]
- 33.Dyson PJ, Knight AM, Fairchild S, Simpson E, Tomonari K. Genes encoding ligands for deletion of Vβ11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991;349:531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- 34.Bill J, Kanagawa O, Woodland D, Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of Vβ11-bearing T cells. J Exp Med. 1989;169:1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foy TM, Shepherd DM, Durie FH, Aruffo A, Ledbetter JA, Noelle RJ. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Eertwegh AJM, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 38.Webb SR, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 39.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction in Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureusenterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 40.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 41.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 42.Radvanyi LG, Shi Y, Vaziri H, Sharma A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 43.MacDonald HR, Pedrazzini T, Schneider R, Louis JA, Zinkernagel RM, Hengartner H. Intrathymic elimination of Mlsa-reactive (Vβ6+) cells during neonatal tolerance induction to Mlsa-encoded antigens. J Exp Med. 1988;167:2005–2010. doi: 10.1084/jem.167.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao E-K, Kanagawa O, Sprent J. Capacity of unprimed CD4+ and CD8+T cells expressing Vβ11 receptors to respond to I-E alloantigens in vivo. J Exp Med. 1989;170:1947–1957. doi: 10.1084/jem.170.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]