Abstract
We examined the regulation of virus-specific CD8 T cell responses during chronic lymphocytic choriomeningitis virus (LCMV) infection of mice. Our study shows that within the same persistently infected host, different mechanisms can operate to silence antiviral T cell responses; CD8 T cells specific to one dominant viral epitope were deleted, whereas CD8 T cells responding to another dominant epitope persisted indefinitely. These virus-specific CD8 T cells expressed activation markers (CD69hi, CD44hi, CD62Llo) and proliferated in vivo but were unable to elaborate any antiviral effector functions. This unresponsive phenotype was more pronounced under conditions of CD4 T cell deficiency, highlighting the importance of CD8– CD4 T cell collaboration in controlling persistent infections. Importantly, in the presence of CD4 T cell help, adequate CD8 effector activity was maintained and the chronic viral infection eventually resolved. The persistence of activated virus-specific CD8 T cells without effector function reveals a novel mechanism for silencing antiviral immune responses and also offers new possibilities for enhancing CD8 T cell immunity in chronically infected hosts.
Keywords: cytotoxic T cells, unresponsiveness, chronic infections, CD4 T cells, major histocompatibility complex tetramers
CD8 T cells eliminate viruses by killing infected cells and by producing antiviral cytokines such as IFN-γ (1–6). These T cells are of importance in chronic viral infections and several studies have documented a role for CD8 CTLs in controlling persistent infections of humans with hepatitis B virus, Epstein-Barr virus, and cytomegalovirus (6–11). Also, there is now convincing evidence that decreased CD8 T cell responses are associated with high viral burden and poor prognosis in HIV-infected individuals (12–17). In such chronic infections, continuous presence of functional CD8 T cells is essential to keep the virus in check, and loss of CD8 T cell responses can lead to resurgence of virus. One condition that results in loss of CD8 responses is CD4 deficiency. Studies with murine models of chronic viral infections have shown that CD4 T cells are essential for maintaining CD8 CTL responses (18–20), and in humans there is a correlation between the decline of CD4 T cells and loss of HIV-specific CD8 T cell responses during the progression of AIDS (21). However, it is not known whether loss of CD8 T cell function under these conditions is due to deletion of virus-specific CD8 T cells or due to induction of anergy (unresponsiveness) in these cells (13, 19, 22–24). It is important to distinguish between these two mechanisms of silencing immune responses because this information is critical for designing therapeutic strategies for enhancing T cell responses in persistently infected individuals.
To address this issue and to investigate the role of CD4 T cells in regulating CD8 T cell responses, we have studied infection of normal (+/+) and CD4 knockout (−/−) mice with lymphocytic choriomeningitis virus (LCMV).1 We have used MHC class I tetramers complexed with defined viral epitopes to directly visualize antigen-specific CD8 T cells and have measured antiviral T cell effector functions using cytotoxicity and cytokine production assays (25–27). This approach allows us to simultaneously monitor the physical presence and functional activity of virus-specific CD8 T cells. Moreover, the use of MHC class I tetramers to identify antigen-specific T cells obviates the need to use TCR transgenic cells. Viral infections induce polyclonal antiviral T cell responses directed towards multiple epitopes; therefore, studies with monoclonal TCR transgenic cells may not mimic the regulation of polyclonal virus–specific CD8 T cells (4, 10, 22, 28). In this study we show that antiviral CD8 T cell responses are silenced during chronic infections by different mechanisms depending upon the viral epitope. CD8 T cells specific for the LCMV nucleoprotein (NP)396–404 epitope were deleted, whereas CD8 T cells specific to another dominant epitope, glycoprotein (GP)33–41, persisted indefinitely. These LCMV GP33–41-specific CD8 T cells expressed activation markers (CD69hi, CD44hi, and CD62Llo) but were unable to either kill virus infected cells or release antiviral cytokines such as IFN-γ. These “unresponsive” cells were found predominantly under conditions of CD4 T cell deficiency, documenting the importance of CD4 T cells in maintaining CD8 T cell responses during chronic viral infections.
Materials and Methods
Mice and Virus.
C57BL/6J (+/+) and C57BL/6-Cd4tm1Mak CD4-deficient (CD4−/−) mice (29) were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited facilities and experiments were performed in accordance with institutional guidelines. Mice were used at 6–8 wk of age.
For acute infections mice were infected by intraperitoneal injection of 2 × 105 PFU of LCMV Armstrong. To establish chronic LCMV, infection mice were infected by intravenous injection of 2 × 106 PFU of the LCMV variant t1b or clone 13. In all cases viral titers were determined by plaque assay, as previously described (30). In certain experiments mice were transiently depleted of CD4+ cells by intraperitoneal injection of partially purified anti-CD4 monoclonal antibody GK1.5 (19). The antibody was administered 1 d before and 3 d after virus infection and resulted in a >95% reduction in the number of splenic CD4 T cells.
Secondary CTL Assays and Limiting Dilution Analysis.
Secondary bulk CTL assays were performed as previously described (19). In brief, single cell suspensions of splenocytes (8 × 106 cells) were restimulated in vitro with 2 × 106 splenocytes from congenital LCMV carrier mice. After 5 d of culture, virus-specific CTL activity was determined using standard 6-h 51Cr-release assays. Limiting dilution analysis (LDA) was also performed as previously described (31). In brief, graded doses of spleen cells from infected mice were added to 96-well plates and cultured in the presence of recombinant IL-2 (50 U/ml) together with irradiated splenocytes from congenital LCMV carrier mice. After 8 d of culture, the contents of each well were split and virus-specific CTL activity was measured. For both secondary CTL and LDA, target cells for cytotoxicity assays were either noninfected MC57 (H-2b) cells or MC57 cells that had been infected 48 h previously with LCMV.
IFN-γ ELISPOT Assays.
IFN-γ–producing spleen cells were enumerated using IFN-γ–specific ELISPOT assays (26). 96-well filtration plates (Millipore Corp., Bedford, MA) were coated overnight with 0.4 μg/well of purified anti–IFN-γ. Coated plates were washed twice with PBS to remove unbound antibody and then blocked for 1–2 h with RPMI 1640 supplemented with 10% FCS. Graded doses of responder cells were added to the wells followed by 5 × 105 irradiated (1,100 rad) syngeneic splenic feeder cells. Recombinant IL-2 (50 U/ml final concentration) was added to all wells. Cultures were either untreated or stimulated with peptide epitopes (0.1 μg/ml final concentration) and the volume was adjusted to 200 μl/well.
Cultures were incubated for 40 h at 37°C in 6% CO2. After this period cells were removed by two washes with PBS and two additional washes using PBS/0.05% (vol/vol) Tween 20. Each well then received 0.4 μg biotinylated anti–IFN-γ antibody diluted in PBS/0.05% Tween 20/0.1% FCS. After overnight incubation at 4°C, unbound secondary antibody was removed by three washes with PBS/0.05% Tween 20. Horseradish peroxidase–conjugated streptavidin was diluted 1:100 in PBS/0.05% Tween 20/0.1% FCS and 100 μl of this solution was added to each well. After 1 h at room temperature, plates were washed three times with PBS. ELISPOTS were developed by the addition of 3-amino-9-ethyl-carbazole diluted in 0.1 M acetate buffer, pH 5, containing H2O2. Reactions were allowed to proceed for ∼10 min and were halted by extensive washing in water. Plates were dried and spots were counted using a stereomicroscope with indirect illumination.
Intracellular Staining.
To enumerate the number of IFN-γ–, IL-2–, IL-4–, and IL-10–producing cells, intracellular cytokine staining was performed as previously described (26). In brief, 106 freshly explanted splenocytes were cultured in flat-bottomed 96-well plates. Cells were left untreated, stimulated with LCMV-specific peptide epitopes (0.1 μg/ml), or treated with PMA (10 ng/ml) and ionomycin (500 ng/ml). In certain experiments the cytokine production profile of an OVA-specific CD4+ Th2 cell clone (provided by J. Kapp and A. Long, Emory University, Atlanta, GA) was similarly analyzed. The OVA 323–339 peptide was used to stimulate this cell line. In all cases cells were incubated for 6 h at 37°C in 6% CO2. Brefeldin A was added for the duration of the culture period to facilitate intracellular cytokine accumulation. After this period cell surface staining was performed, followed by intracellular cytokine staining using the Cytofix/Cytoperm kit (PharMingen, San Diego, CA) in accordance with the manufacturer's recommendations. For intracellular cytokine staining, the antibodies used were anti–IFN-γ (clone XMG1.2), anti–IL-2 (clone JES6-5H4), anti–IL-4 (clone 11B11), and anti–IL-10 (clone JES5-16E3). All antibodies were purchased from PharMingen.
Flow Cytometry and Tetramer Staining.
MHC class I (H-2Db) tetramers complexed with either LCMV–GP33–41 or LCMV– NP396–404 peptides were produced exactly as previously described (25, 26). Biotinylated Db peptide complexes were tetramerized by the addition of allophycocyanin-conjugated streptavidin.
Freshly explanted splenocytes (106) were stained in PBS/2% (wt/vol) BSA/0.2% (wt/vol) NaN3 using fluorochrome conjugated antibodies and MHC class I tetramers. The antibodies used were anti-CD8 (clone 53-6.7), anti-CD44 (clone IM7), anti-CD62L (clone MEL-14), and anti-CD69 (clone H1.2F3). All antibodies were purchased from PharMingen. After staining, cells were fixed in PBS/2% (wt/vol) paraformaldehyde, and events acquired using a FACSCalibur® flow cytometer (Becton Dickinson). Dead cells were excluded on the basis of forward and side light scatter. Data was analyzed using the computer program CELLQuest (Becton Dickinson).
BrdU Labeling and Staining.
The in vivo proliferation of LCMV-specific CD8 T cells was assessed by administering 5-bromo-2′-deoxyuridine (BrdU) (0.8 mg/ml) in drinking water for 8 d. After this period splenocytes were isolated and stained with anti-CD8-PE and Db(GP33–41) tetramers conjugated to allophycocyanin. Samples were also stained for BrdU incorporation, using anti-BrdU antibodies (Clone B44, Becton Dickinson) as previously described (26, 32). Flow cytometry and data analysis were performed as described above.
Results
CD8 T Cell Effector Functions Are Lost during Chronic Infection of CD4−/− Mice.
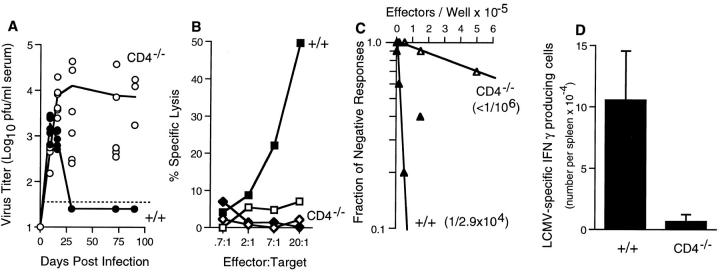
Infection of adult +/+ or CD4−/− mice with the Armstrong strain of LCMV results in an acute infection (19, 29, 33). Both +/+ and CD4−/− mice make potent LCMV-specific CD8 T cell responses and clear the virus within 1 wk. However, infection with LCMV variants that rapidly spread to many tissues in vivo leads to strikingly different outcomes in +/+ and CD4−/− mice (18, 19). An example of this is shown in Fig. 1. Adult +/+ mice infected with LCMV variant t1b (19, 34) had no detectable viremia after day 30 and virus was cleared from most of their tissues within 2 mo, except for the kidneys that contained low levels (∼103 PFU/organ) of virus for >1 yr. In contrast CD4−/− mice failed to clear the infection and these mice contained high levels of virus in all tissues tested; >106 PFU in spleen, lymph nodes, thymus, liver, lung, brain, kidney, and salivary gland (Fig. 1 A, data not shown). Virus-specific CD8 T cells, as measured by their ability to kill infected cells and to secrete IFN-γ, were present in +/+ mice but no functional CD8 T cell responses could be detected in CD4−/− mice. No LCMV-specific CD8 T cell responses were present in chronically infected CD4−/− mice either directly ex vivo or after restimulation with virus in vitro. Data from mice analyzed at day 90 after infection are shown in Fig. 1, B, C, and D.
Figure 1.
CD8 T cell effector functions are lost during chronic infection of CD4−/− mice. (A) Serum virus titers were measured in +/+ (•) and age-matched CD4−/− (○) mice at various times after infection with LCMV-t1b. The limit of detection is indicated by the dashed line. (B) CTL activity of splenocytes from +/+ (▪, □) or CD4−/− (♦, ⋄) mice at 90 d after infection with LCMV-t1b was measured after 5 d restimulation in vitro. Target cells were either uninfected (open symbols) or LCMV infected (filled symbols). (C) Limiting dilution analysis was performed using splenocytes from either +/+ (▴) or CD4−/− (▵) mice at 90 d after infection with LCMV-t1b. The frequency of LCMV-specific cells per spleen is indicated in parentheses. (D) Virus-specific IFN-γ secreting cells were enumerated using single cell ELISPOT assays. Splenocytes were isolated from either +/+ or CD4−/− mice at 90 d after inoculation with LCMV-t1b.
Epitope-dependent T Cell Deletion and Functional Unresponsiveness during Chronic Infection.
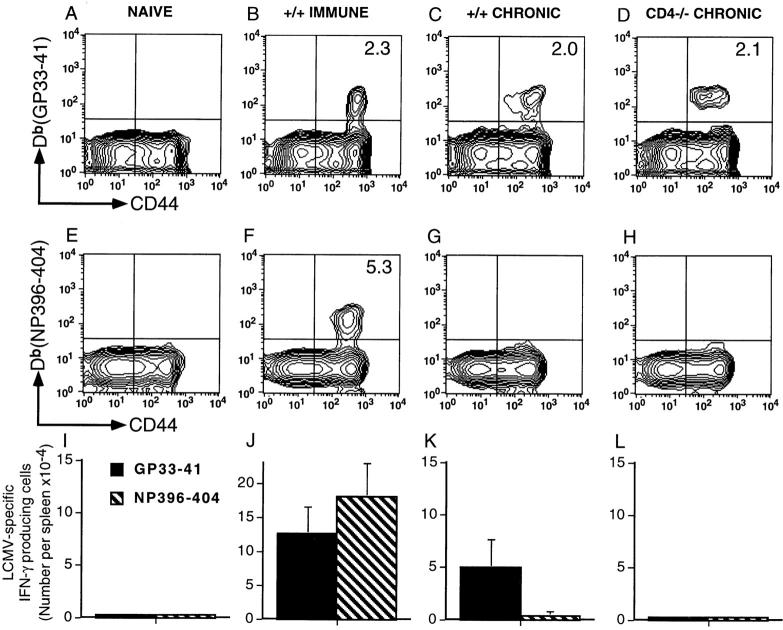
To determine if loss of CD8 T cell responses in chronically infected CD4−/− mice was due to peripheral deletion of virus-specific T cells or due to functional unresponsiveness, MHC class I tetramers complexed to immunodominant LCMV epitopes (GP33– 41 and NP396–404) were used to visualize antigen-specific CD8 T cells (Fig. 2). Uninfected (naive) and LCMV immune mice (i.e., mice that had undergone an acute LCMV Armstrong infection) were included as negative and positive controls. No virus-specific CD8 T cells were detectable (frequency <1/105) in naive mice (Fig. 2, A, E, and I), whereas immune mice contained GP33- and NP396-specific CD8 T cells at frequencies of ∼2% (1/50 CD8 T cells) and ∼5% (1/20 CD8 T cells), respectively (Fig. 2, B and F). The tetramer binding cells in LCMV immune mice were CD44hi, a marker for activated/memory T cells (35), and all of these antigen-specific cells were fully functional; ∼100% of these cells produced IFN-γ after stimulation with the appropriate peptides (Fig. 2 J). +/+ mice that had undergone a protracted (4–6 wk) LCMV-t1b infection exhibited a selective deletion of NP396-specific CD8 T cells (Fig. 2, C and G). These mice still contained GP33-specific CD8 T cells (Fig. 2 C) at a frequency (1/50) comparable to that seen in +/+ Armstrong immune mice and, as in the immune group, these GP33-specific CD8 T cells were CD44hi and most of these cells were functional based on IFN-γ production (Fig. 2 K). Thus, +/+ mice that were infected for 4–6 wk and subsequently eliminated virus from most of their tissues had lost their NP396-specific CD8 T cell responses due to peripheral deletion of these cells but still retained their GP33-specific responses. An interesting variation of this phenotype was seen in chronically infected CD4−/− mice. In these mice, similar to in +/+ mice, NP396-specific CD8 T cells were deleted (Fig. 2 H) and GP33-specific CD8 T cells were present (Fig. 2 D), but in striking contrast to +/+ mice, nearly all (>98%) of these cells were nonfunctional (Fig. 2 L). Although CD4−/− mice contained as many GP33 tetramer binding cells as +/+ mice, and these cells were also CD44hi, few if any of these virus-specific CD8 T cells were able to secrete IFN-γ or kill LCMV-infected cells. Taken together, these results show that the mechanism of peripheral tolerance during chronic viral infections can vary depending upon the epitope specificity of the T cells; in the case of NP396-specific CD8 T cells, silencing of the response was due to physical deletion of the antigen-specific cells, whereas for GP33-specific CD8 T cells, loss of function was due to unresponsiveness. Most importantly, these data show that virus-specific T cells can persist during chronic viral infection but these T cells are incapable of eliciting their normal array of antiviral effector functions (i.e., cytotoxicity and IFN-γ production).
Figure 2.
T cell deletion and functional unresponsiveness are distinct mechanisms for silencing antiviral immune responses. Naive +/+ mice, +/+ immune mice (161 d after LCMV Armstrong infection; 2 × 105 PFU, i.p.), and +/+ or CD4−/− mice infected with LCMV-t1b (2 × 106 PFU, i.v.) 60 d previously were checked for the physical presence and functional responsiveness of LCMV-specific CD8 T cells. (A–H) LCMV-specific CD8 T cells were visualized using MHC class I tetramers complexed to viral peptides. Cells were costained with anti-CD8–PE, anti-CD44–FITC, and either Db(GP33–41) or Db(NP396–404) tetramers conjugated to allophycocyanin. The histograms show gated CD8 lymphocytes, and the percentage of CD8 cells costaining with either Db(GP33–41) or Db(NP396–404) are indicated in the corresponding upper right quadrant. Where not shown values are <0.2%. (I–L) Numbers of LCMV-specific IFN-γ producing cells were enumerated using single-cell cytokine ELISPOT assays. The number of splenocytes producing IFN-γ after stimulation with either GP33–41 (black bars) or NP396–404 peptides (hatched bars) are shown (± SD).
In certain conditions (36, 37), immune “deviation” can occur, resulting in the synthesis of an alternative set of cytokines by responding T cells. To address this possibility, intracellular cytokine staining was performed. After 6 h stimulation in vitro with GP33 peptide, freshly explanted spleen cells from both LCMV-t1b–infected +/+ mice and CD4−/− mice failed to produced IL-2, IL-4, or IL-10 (data not shown). Identical intracellular staining procedures detected significant amounts of IL-4 and IL-10 after in vitro stimulation of an OVA-specific CD4 Th2 cell clone. However, IFN-γ was produced by spleen cells from LCMV-t1b–infected +/+ mice, but splenocytes isolated from similarly infected CD4−/− mice did not produce detectable levels of IFN-γ. Thus, during chronic infection, GP33-specific CD8 T cells do not exhibit an altered pattern of cytokine production. Moreover, in the absence of CD4 T cell help, these cells fail to elicit any detectable effector functions and the viral infection is not resolved.
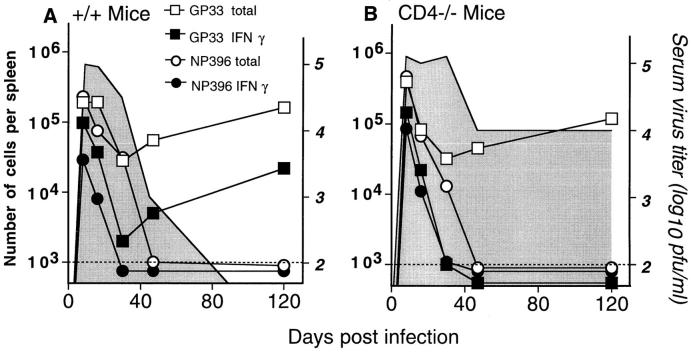
The kinetics of LCMV epitope–specific responses were examined during the course of chronic infection to determine when NP396-specific CD8 T cells were deleted and when GP33-specific cells became unresponsive (Fig. 3). In both +/+ and CD4−/− mice infected with LCMV clone 13 (30), NP396-specific CD8 T cells were deleted by day 50 after infection. After expansion and differentiation into effectors during the first week after infection, NP396-specific CD8 T cells became progressively unresponsive and were then deleted in the periphery. In striking contrast, GP33-specific CD8 T cells persisted indefinitely. During the initial phase of infection both groups of mice contained a mixture of functional and nonfunctional GP33-specific CD8 T cells. In +/+ mice, a fraction (10–20%) of these T cells remained functional and the LCMV infection was eventually resolved (Fig. 3 A). However, in CD4−/− mice all the GP33-specific CD8 T cells became nonfunctional by day 30 and these mice were unable to control the viral infection (Fig. 3 B). These results show that CD4 T cells are essential to sustain effector functions of virus-specific CD8 T cells during chronic infection.
Figure 3.
Kinetics of LCMV-specific CD8 T cell responses during chronic infection. Splenocytes were prepared from either +/+ or CD4−/− mice at various days after infection with LCMV-clone 13 (2 × 106 PFU, i.v.). The total numbers of GP33- (□) and NP396- (○) specific CD8 T cells were enumerated by staining with recombinant MHC H-2Db tetramers, and IFN-γ ELISPOT assays were performed to determine the functional responsiveness of these cells (GP33–41, ▪; NP396–404, •). Serum virus titers were also determined and are represented by the gray area. Mean values are shown for two to four mice at each time point and the limit of detection is represented by the dashed line. Standard deviations were <10%.
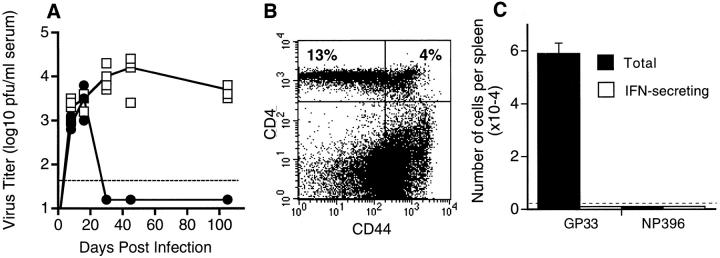
Lack of viral clearance and persistence of unresponsive GP33-specific CD8 T cells was also seen when +/+ mice were made CD4 deficient at the time of infection by injecting them with a monoclonal antibody (GK1.5) to deplete CD4 T cells. +/+ mice were injected with GK1.5 1 d before and 3 d after LCMV-clone 13 or -t1b infection; this treatment depleted >95% of the CD4 T cells but within 2 mo these mice had reconstituted their CD4 T cell compartment and contained normal levels of CD4 T cells (Fig. 4). However, GP33-specific CD8 T cells remained unresponsive in these mice and there was no control of the virus infection (Fig. 4). It is particularly noteworthy that although these mice had recovered their total number of CD4 T cells, they did not contain any detectable LCMV-specific CD4 T cells as measured by IFN-γ and IL-2 production (data not shown). These results show that the crucial factor in maintaining CD8 T cell function during chronic infection is not the total number of CD4 T cells but the presence of antigen-specific helper cells. This finding has obvious implications towards understanding the regulation of HIV-specific CTL responses (16, 21).
Figure 4.
Chronic LCMV infection and CD8 T cell unresponsiveness ensue after transient loss of CD4 T cells. +/+ mice were transiently depleted of CD4 T cells by injection with the anti-CD4 antibody, GK1.5, 1 d before and 3 d after infection with LCMV-t1b. (A) Serum virus titers were determined in +/+ (•) and GK1.5 treated (□) +/+ mice at various days after infection. (B) Reconstitution of CD4 T cells in GK1.5-treated mice was determined at 60 d after infection with LCMV-t1b. Splenocytes were costained for CD4 and the activation marker CD44. The percentage of CD4+ cells are indicated in the upper quadrants. By this time point, both +/+ and GK1.5-treated mice contained comparable numbers of CD4 T cells. (C) Splenocytes from GK1.5-treated mice were prepared at 60 d after infection with LCMV-t1b and both the total number (measured by tetramer staining) and the number of IFN-γ–secreting (measured by ELISPOT) GP33- or NP396-specific CD8 T cells were determined. Greater than 98.5% of GP33-specific T cells were unresponsive and NP396-specific T cells were not detectable. In A and C the limit of detection is indicated by the dashed line. At least three mice were analyzed at each time point.
Expression of Activation Markers and Turnover of Unresponsive T Cells.
We next asked whether these effector function–negative CD8 T cells express activation markers associated with TCR signaling. These T cells were CD44hi (Fig. 2 D) and also expressed low levels of CD62L (data not shown). This phenotype, CD44hiCD62Llo, is characteristic of activated/memory T cells and shows that these functionally “unresponsive” cells were antigen experienced (35, 38). However, neither of these two markers is indicative of recent TCR stimulation. To examine this we checked expression of the very early T cell activation marker, CD69. CD69 is rapidly upregulated after TCR-mediated signaling but its expression is transient and dependent upon continuous TCR activation (39–41). We found that the vast majority (∼80%) of effector-function–negative GP33-specific CD8 T cells were CD69hi (Fig. 5 A). This rather striking result shows that these CD8 T cells are cognizant of the continued presence of viral antigen and are receiving signals through their TCR but fail to elaborate downstream effector functions. Even after treatment with PMA and ionomycin, agents that activate protein kinase C and elevate intracellular Ca2+ levels, respectively (42, 43), GP33-specific CD8 T cells in chronically infected CD4−/− mice were unable to secrete IFN-γ (Fig. 5 B). Since these virus-specific CD8 T cells are maintained indefinitely in persistently infected CD4−/− mice, we checked whether these cells were turning over in vivo by BrdU incorporation (26, 32). Chronically infected CD4−/− and +/+ mice (100 d after infection) were given BrdU in the drinking water for 8 d and the percentage of cells that had replicated during this period was determined by staining for BrdU incorporation in the nucleus, in combination with cell surface stains (CD8 and tetramer) to identify the T cells. A representative analysis is depicted in Fig. 5 C showing that the “unresponsive” GP33-specific CD8 T cells do proliferate in vivo.
Figure 5.

GP33-specific CD8 T cells in chronically infected CD4−/− mice are activated and proliferate, but are refractive to stimulation with PMA and ionomycin. (A) CD69 expression on freshly explanted splenocytes from +/+ and CD4−/− mice at 78 d after infection was assessed using flow cytometry. (B) IFN-γ production by GP33-specific CD8 T cells after PMA and ionomycin stimulation. Splenocytes were isolated from +/+ and CD4−/− mice at 108 d after infection with LCMV-t1b. (C) The in vivo proliferation of GP33-specific CD8 T cells in LCMV-t1b infected +/+ and CD4−/− mice (day 100 after infection) was assessed by in vivo BrdU labeling. Mice were fed BrdU in their drinking water for a total of 8 d. All histograms show Db(GP33–41)-positive cells and the percentage of GP33-specific T cells within each region is indicated.
Discussion
The main point of this study is that virus-specific CD8 T cells can persist indefinitely in chronically infected hosts. These T cells express activation/memory markers (CD44hi CD62Llo), are receiving signals through the TCR (CD69hi), and can proliferate in vivo, but are unable to elaborate antiviral effector functions and thus fail to control the infection. Although T cell unresponsiveness (anergy) has been extensively studied, cells of such a phenotype have not been described previously (44–51). Moreover, the identification of these activated but effector-function–negative T cells reveals a new mechanism of silencing antiviral immune responses during chronic infections. These CD8 T cells seem to be engaged in a continual and ineffective effort much like the Greek king, Sisyphus, who was condemned forever to perform the fruitless task of pushing a large stone up a hill only to see it roll down again. Our studies show that these “Sisyphean” CD8 T cells are generated under conditions of chronic antigenic stimulus and are especially prevalent when there is inadequate CD4 help. Until now, deletion (exhaustion) of virus-specific CD8 T cells has been considered as a more general mechanism of tolerizing virus-specific immune responses during chronic infections (13, 22–24). Our studies documenting persistence of effector-function–negative antigen-specific CD8 T cells open new possibilities of therapeutic intervention for enhancing CD8 T cell immunity in chronically infected hosts. In addition, this study shows that within the same persistently infected host different mechanisms can operate to silence antiviral T cell responses; LCMV NP396-specific cells were deleted, whereas GP33-specific cells were maintained indefinitely but without any detectable CTL or cytokine secretion activity. Thus, strategies to enhance immune function during chronic infection need to be tailored towards individual epitopes.
In contrast to the strong correlation between CD4 help and CD8 T cell effector function, we found an inverse correlation between antigen persistence and responsiveness of virus-specific CD8 T cells. Of the three LCMV strains used in this study, clone 13 causes the most disseminated and persistent infection with the highest antigen load, followed by LCMV-t1b and then Armstrong, which only causes an acute infection (Figs. 1 and 3 and reference 19). During the course of acute LCMV Armstrong infection (which only lasts about a week) and after viral clearance, all of the GP33-specific CD8 T cells remained functional (Fig. 2 and reference 26). In LCMV-t1b–infected +/+ mice (viral clearance in ∼2 mo), ∼60% of the GP33-specific CD8 T cells were unresponsive during the first month after infection, but by day 90 all of these cells had become functional (Fig. 2 and data not shown). However, in clone 13– infected +/+ mice, ∼80% of the GP33-specific T cells remained unresponsive for an extended period. Thus, the duration of exposure to viral antigen is a critical parameter that influences T cell responsiveness. Clone 13 causes the most prolonged infection and results in a pronounced “functional deficit” within the GP33-specific CD8 T cell population. Unlike LCMV-t1b infection, even after clone 13 infection has been controlled the GP33-specific T cells do not rapidly regain their capacity to elicit antiviral effector functions. None of the LCMV strains used in this study infect CD8 T cells; therefore, the unresponsiveness of GP33-specific cells is not due to a direct effect of LCMV replication within these cells. However, LCMV does replicate within professional APCs and the presentation of viral antigen by these cells can target them for destruction by virus-specific CTL (52, 53). Loss of professional APCs is least apparent during Armstrong infection and most widespread during clone 13 infection. Consequently, during systemic LCMV infection professional APCs are destroyed and large numbers of “nonprofessional” APCs may present viral antigen. Such conditions are likely to favor the induction of CD8 T cell unresponsiveness. Moreover, professional APCs may be aberrantly activated in the absence of CD4 T cell help and this may further promote the functional unresponsiveness of CD8 T cells (54–57). Thus, a chronic antigenic stimulus in the absence of adequate CD4 help appears to be the worst possible scenario resulting in complete loss of CD8 effector functions and uncontrolled virus infection. Although such regulatory mechanisms are not well suited to deal with chronic infections and tumors, they may have evolved to prevent autoimmunity.
The degree of T cell activation is likely to be an important parameter that determines whether CD8 T cells are deleted or become functionally unresponsive (48). The extent of T cell activation can be influenced by the abundance of antigen that is presented and also by the affinity of the TCR for the given MHC–peptide complex. Virus- infected cells will display both GP33 and NP396 epitopes to responding T cells. One possibility is that different amounts of GP33 and NP396 epitopes are presented in the periphery of chronically infected adult mice (58, 59). Consequently, GP33- and NP396-specific T cells may not be similarly activated and therefore the fate of these T cells could be differentially regulated. Alternatively, the on and off rates of GP33- and NP396-specific TCRs may differ. In this case even if both epitopes are present in similar concentrations on the infected cells, the responding T cells may undergo different activation programs and consequently succumb to different fates (i.e., deletion versus nonresponsiveness). Interestingly, GP33-specific T cells are not intrinsically resistant to deletion, and both GP33- and NP396-specific T cells are undetectable by tetramer staining in congenitally infected LCMV carrier mice (data not shown). These results suggest that GP33-specific CD8 T cells can undergo deletion during central (thymic) tolerance.
Our findings are clearly relevant for understanding how virus-specific CD8 T cell responses determine the outcome of persistent viral infections. We have shown that virus-specific T cells that express activation markers, such as CD44 and CD69, can be maintained in an unresponsive state during chronic infections. Therefore, simply detecting the physical presence of activated virus-specific CD8 T cells, which turn over in vivo, does not necessarily mean that these cells are operating to control the infection (60). However, our data also demonstrate that not all virus-specific T cells need to be functionally competent in order to resolve a chronic infection. For example, even though NP396-specific T cells are deleted and only 10–20% of GP33-specific T cells are functional, infection with LCMV clone 13 is eventually resolved in +/+ mice. This suggests that for the control of persistent viral infections a threshold of functional antiviral T cells needs to be maintained. The number of functional antiviral CD8 T cells could fall below this threshold if too many virus-specific CD8 T cells are either deleted or become unresponsive, as occurs in the absence of CD4 T cell help. Such dampening of virus-specific CD8 T cell responses would result in a more protracted or even an uncontrollable infection.
The most striking finding of this study is that “activated” but effector-function–negative virus-specific CD8 T cells can persist indefinitely in chronically infected mice at remarkably high frequencies (1–2% of total CD8 T cells). It is likely that such effector-function–negative virus-specific CD8 T cells will also be found in chronic viral infections of humans. During HIV infection, CD8 CTLs play an important role in controlling the viral burden and both deletion and functional unresponsiveness may impair the efficacy of these responses (16). Moreover, as progression to AIDS occurs, the numbers of CD4 T cells decline, and Rosenburg et al. (21) reported that weak HIV-specific CD4 T cell responses correlate with higher viral loads. This is consistent with our data showing that CD4 T cells are required to maintain the effector functions of virus-specific CD8 T cells, and also suggests that if the number of functional virus-specific CD8 T cells falls below a threshold level, then an uncontrollable chronic infection can ensue. Since CD8 T cells are potent mediators of viral clearance, it is important to elucidate how to restore and sustain the effector functions of virus-specific T cells. Antiviral therapies that are designed to turn on the effector activity of preexisting virus-specific CD8 T cells may be successful at both clearing persistent infections and enhancing protective immunity to reinfection.
Acknowledgments
We wish to thank M. Large, J. Miller, and K. Madhavi-Krishna for technical assistance, and M. Jabbar, J. Grayson, D. Quinn, and L. Harrington for helpful discussion.
This work was supported in part by National Institutes of Health grants AI30048 and NS21496 (to R. Ahmed). A.J. Zajac was supported in part by fellowship DRG-1421 from the Damon Runyon-Walter Winchell Foundation. M. Suresh was supported in part by a Fellowship from the Multiple Sclerosis Society.
Abbreviations used in this paper
- +/+ mice
C57BL/6J mice
- BrdU
5-bromo-2′-deoxyuridine
- CD4−/−
CD4-deficient
- GP
glycoprotein
- LCMV
lymphocytic choriomeningitis virus
- NP
nucleoprotein
References
- 1.Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 2.Walsh CM, Matloubian M, Liu C-C, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10848. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MBA. An essential role for type 1 interferon-γ in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 5.Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. doi: 10.1007/978-3-642-85208-4_1. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 7.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 8.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 9.Chisari, F.V., and C. Ferrari. 1997. Viral hepatitis. In Viral Pathogenesis. N. Nathanson, editor. Lippincott-Raven, New York. 745–778.
- 10.Rickinson AB, Moss DJ. Human cytotoxic T-lymphocytes responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 11.Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, Steven N, McMichael AJ, Rickinson AB. Direct visualization of antigen-specific CD8+T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekaly RP, Fauci AS. Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G, Demarest JF, Schacker T, Vaccarezza M, Cohen OJ, Daucher M, Graziosi C, Schnittman SS, Quinn TC, Shaw GM, et al. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nature Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 15.Klein MR, van Baalen CA, Holwerda AM, Kerkhof SR, Garde, Bende RJ, Keet IP, Eeftinck-Schattenkerk JK, Osterhaus AD, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein MR, van der Burg SH, Pontesilli O, Miedema F. Cytotoxic T lymphocytes in HIV-1 infection: a killing paradox. Immunol Today. 1998;19:317–324. doi: 10.1016/s0167-5699(98)01288-2. [DOI] [PubMed] [Google Scholar]
- 17.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 18.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell- deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8065. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardin RD, Brooks JW, Sarawar SW, Doherty PC. Progressive loss of CD8+ T cell–mediated control of a γ-herpesvirus in the absence of CD4+T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 22.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 23.Doherty PC. Immune exhaustion: driving virus-specific CD8+ T cells to death. Trends Microbiol. 1993;1:207–209. doi: 10.1016/0966-842x(93)90133-c. [DOI] [PubMed] [Google Scholar]
- 24.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman JD, Moss PAH, McHeyzer-Williams MG, Bell JI, McMicheal AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 27.McMicheal A, O'Callaghan CA. A new look at T cells. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitton, J.L., and M.B.A. Oldstone. 1996. Immune response to viruses. In Fields Virology. B.N. Fields, editor. Lippincott-Raven, New York. 345–374.
- 29.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MBA. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 32.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed R, Butler LD, Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of anti-viral cytotoxic T cell and antibody responses. J Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King C-C, deFries R, Kolhekar SR, Ahmed R. In vivo selection of lymphocyte-tropic and macrophage-tropic variants of lymphocytic choriomeningitis virus during persistent infection. J Virol. 1990;64:5611–5616. doi: 10.1128/jvi.64.11.5611-5616.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budd RC, Cerottini J-C, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes: stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 36.Cobbold SP, Adams E, Marshall SE, Davies JD, Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev. 1996;149:5–33. doi: 10.1111/j.1600-065x.1996.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 37.Röcken M, Shevach EM. Immune deviation— the third dimension of nondeletional T cell tolerance. Immunol Rev. 1996;149:175–194. doi: 10.1111/j.1600-065x.1996.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 38.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 39.Yokoyama WM, Koning F, Kehn PH, Pereira GMB, Stingl G, Coligan JE, Shevach EM. Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J Immunol. 1988;141:369–375. [PubMed] [Google Scholar]
- 40.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering: requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 41.Testi R, D'Ambrosio A, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 42.Truneh A, Albert F, Goldstein P, Schmitt-Verhulst AM. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- 43.Isakov N, Mally MI, Scholz W, Altman A. T-lymphocyte activation: the role of protein kinase C and the bifurcating inositol phospholipid signal transduction pathway. Immunol Rev. 1987;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz, R.H. 1993. Immunological tolerance. In Fundamental Immunology. W.E. Paul, editor. Raven Press, New York. 677–731.
- 46.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JG, Jenkins MK. The role of anergy in peripheral T cell unresponsiveness. Life Sci. 1994;55:1767–1780. doi: 10.1016/0024-3205(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 48.Dillon ST, MacKay VL, Fink PJ. Functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity. 1995;3:321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 49.Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, Trautmann A, Rocha B. Modification of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- 50.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- 51.Parijs LV, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 52.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrow P, Evans CF, Oldstone MB. Virus- induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 55.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic responses is mediated by CD40 signaling. Nature. 1998;393:476–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 56.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 57.Schoenberger SP, Toes REM, van der Voort EIM, Offinga R, Meilief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:474–478. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 58.Oldstone MB, Buchmeier MJ. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982;300:360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- 59.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]