Abstract
The endothelial cell–derived peptide endothelin 1 (ET1) stimulates cell proliferation and differentiated functions of human osteoblastic cells (HOC), and HOC constitutively express the endothelin A receptor (ETRA). Therefore, ET1 may play an important role in the regulation of bone cell metabolism. As glucocorticoids (GC) exert a profound influence on bone metabolism and increase the effects of ET1 on bone cell metabolism in vitro, the effects of GC on ETRA expression in HOC were investigated. Dexamethasone (DEX) increased ETRA mRNA levels in a dose- and time-dependent fashion. The effects of dexamethasone, prednisolone, and deflazacort on the increase of ETRA mRNA levels correlate positively with their binding affinity to the GC receptor. Scatchard analysis of ET1 binding data to HOC revealed that DEX increased the binding capacity for ET1 from 25,300 to 62,800 binding sites per osteoblastic cell, leading to an enhanced mitogenic effect of ET1 on HOC after preincubation with DEX. Transiently transfected primary HOC with a reporter gene construct, containing the 5′-flanking region of the ETRA gene fused to luciferase gene, showed a promoter-dependent expression of the reporter gene and the induction of reporter gene expression by DEX treatment. Total RNA extracts of femoral head biopsies with osteonecrotic lesions from GC-treated patients showed threefold higher ETRA mRNA levels compared with extracts of bone biopsies from patients with traumatically induced osteonecrosis and coxarthrosis. Furthermore, GC treatment increased plasma ET1 levels by 50% compared with pretreatment values. These findings suggest that GC induced upregulation of ETRA, and ET1 plasma levels enhance ET1's anabolic action on bone cell metabolism. Increased ET1 concentrations may also impair bone perfusion by vasoconstriction in a metabolically activated skeletal region.
Keywords: endothelin 1, endothelin A receptor, glucocorticoids, bone, osteoblasts
Bone growth and metabolism are dependent on vascular supply. The ingrowth of blood vessels in the epiphyseal growth zone and in fracture callus formation precedes bone formation (1). Restriction of bone perfusion impairs bone metabolism, which may be associated with a loss of bone mass (2). The most severe form of an impaired vascular supply of the bone compartment is bone infarction leading to the clinical diagnosis of osteonecrosis (3). Frequently, osteonecrosis occurs in patients after long-term treatment with pharmacological doses of glucocorticoids, but the mechanism whereby glucocorticoid treatment causes osteonecrosis is not understood (4). Several studies demonstrate that glucocorticoids directly affect osteoblastic cell functions. Thus, physiological concentrations of glucocorticoids have a stimulatory effect on type I collagen synthesis, whereas pharmacological concentrations of glucocorticoids decrease type I collagen synthesis by osteoblasts. Alkaline phosphatase activity (ALP)1 increases in short-term experiments but decreases in long-term cultures of rat calvariae in the presence of glucocorticoids. DNA content and cell replication are not affected in short-term incubation but are decreased after long-term glucocorticoid treatment (5). Glucocorticoids stimulate endothelin 1 (ET1) secretion from vascular smooth muscle cells (6) and elevate plasma ET1 levels (7), which may contribute to increased blood pressure (8) and possibly to impaired bone perfusion in glucocorticoid-treated patients (9) because ET1 is a potent vasoconstrictor (10). ET1 also stimulates human osteoblastic cell (HOC) proliferation and differentiated functions (11), suggesting that a change in the local or systemic ET1 concentration may also affect bone metabolism. Therefore, both glucocorticoids and ET1 exert direct effects on osteoblastic cell metabolism. It is unclear how glucocorticoids and ET1 interact on osteoblastic cell metabolism, and how the direct effects of glucocorticoids and ET1 on bone cell metabolism are related to the frequent clinical observation of an osteonecrotic destruction, e.g., of the femoral head, in glucocorticoid-treated patients. To better understand the perturbation of human bone cell metabolism in glucocorticoid-treated patients, the interaction of glucocorticoids and ET1 on human bone cell metabolism in vitro was investigated. Glucocorticoids elevate circulating ET1 serum levels in vivo and enhance the mitogenic effect of ET1 on HOC in vitro by upregulating the expression of the osteoblastic endothelin A receptor (ETRA) in vitro and in vivo.
Materials and Methods
HOC Culture.
Human bone biopsies were obtained from adult healthy patients (35–75 yr old) undergoing selective orthopedic surgery of the femur (approval for this study was given by the ethics commission of the University of Heidelberg). HOC were obtained from femur biopsies as described previously (12). Freshly removed bone biopsies were placed in DME (GIBCO BRL, Gaithersburg, MD) supplemented with 10% calf serum (CS) from Hyclone (Logan, UT), 10 U/ml penicillin-G, and 10 μg/ml streptomycin (PS; GIBCO BRL). The biopsies were minced into small pieces (0.5 cm3), rinsed, and cleaned thoroughly of contaminating connective, erythropoietic, and fat tissue. The resulting bone explants were incubated in culture medium at 37°C in a humidified atmosphere with 5% CO2 until human bone cells had attached to the culture dishes (Costar Corp., Cambridge, MA). Cells were removed from dishes with 0.05% trypsin solution (GIBCO BRL) and plated in culture medium as stated above. Cells of the first and second passage were used for the experiments. HOC were identified as osteoblastic cell populations on the basis of 1,25(OH)2D3-stimulated ALP, osteocalcin secretion, type I procollagen peptide secretion, and in vitro mineralization as described previously (13). Dexamethasone, ET1, prednisolone, actinomycin D, and cycloheximide were obtained from Sigma Chemical Co. (St. Louis, MO); deflazacort was a gift from Dr. Görlich (Marion Merrell Dow, Rüsselsheim, Germany); and RU486 was provided by Roussel Uclaf (Paris, France).
Human Femoral Head Biopsies.
Femoral head biopsies for total RNA extractions were obtained from 40–65-yr-old patients with no history of cardiovascular diseases or metabolic disorders (e.g., diabetes mellitus) who underwent surgery for total hip replacement. Three groups of patients with three different diagnoses were examined: (a) coxarthrosis, (b) glucocorticoid-induced osteonecrosis, and (c) traumatically induced osteonecrosis. After removal at surgery, intact femoral heads were frozen immediately in liquid nitrogen and maintained in −80°C until use.
Cloning and Sequence Analysis of 5′-flanking Region.
Cloning of the 5′-flanking region of the human ETRA gene was performed with the human Genome Walker Kit (Clontech, Palo Alto, CA). A gene-specific primer and an adaptor-specific primer were used for amplification of a genomic DNA fragment upstream from the known ETRA gene sequence, followed by reamplification using a second set of nested primers (one gene-specific and one adaptor-specific primer). A 3.1-kb amplification product was cloned in a PCR cloning vector (pT-Adv vector; Clontech) and automatically sequenced. After computer-aided restriction enzyme mapping, a SacI-XhoI fragment containing the 5′-flanking region of the human ETRA gene from −3050 to +48 (plus one transcription start site) was subcloned into the pGL3 Basic luciferase reporter gene plasmid (Promega Corp., Madison, WI). The 3.1-kb ETRA promoter region was analyzed with regard to the presence of steroid hormone response elements by performing a computer-aided search using the HUSAR Factor search utility (German Cancer Research Center, Heidelberg, Germany).
Transient Transfection and Reporter Gene Assays.
HOC were seeded with 104 cells per well in six-well plates and grown in DME supplemented with 10% CS and 1% PS to 60–75% confluence. Immediately before transfection, the cultures were washed twice in serum and phenol red–free DME. Transfections were performed with Lipofectin transfection reagent (GIBCO BRL). Plasmids used for transfection were purified with CsCl gradient centrifugation. Before transfection, calculated for each well of a six-well plate, 10 μl Lipofection reagent was incubated for 10 min at room temperature with 1.5 μg of reporter gene construct, which contains the human ETRA 5′-flanking fragment (−3050 to +48) fused to luciferase gene, 0.5 μg glucocorticoid receptor expression plasmid when cotransfection experiments were performed (American Type Culture Collection, Rockville, MD), and 1.0 μg β-galactosidase expression plasmid (pSV-βgal; Promega Corp.). Lipofectin/plasmid solution was added to washed cells in serum and phenol red DME (end vol 1 ml/well) for 4 h at 37°C. After plasmid incubation, medium was changed to DME supplemented with 5% CS and 1% PS for 44 h at 37°C. To determine glucocorticoid-dependent expression of luciferase reporter gene, cells were incubated for a further 24 h in the absence or presence of 100 nM dexamethasone in serum and phenol red–free DME. Cells were harvested in 250 μl/well 1× reporter lysis buffer (Promega Corp.) and centrifuged to pellet the debris, and the luciferase activity in the supernatant was quantitated in a luminometer (model LB9507; EG&G Berthold, Wildbad, Germany) using a Luciferase Assay System (Promega Corp.). To correct for variations in transfection efficiencies, luciferase activities were normalized on the basis of β-galactosidase activity assayed by the Galacto Light Plus System (Tropix, Inc., Bedford, MA) following the instructions of the manufacturer.
RNA Isolation and Northern Blot Analysis.
48 h before RNA extraction, HOC were cultured in serum and phenol red–free DME for 24 h, after which the medium was replaced by serum and phenol red–free DME with or without additional glucocorticoids (as detailed below). After 24 h continuous treatment with glucocorticoids, total RNA was isolated for Northern blot analyses. Culture dishes were rinsed in ice-cold PBS, and total RNA was extracted by the guanidinium thiocyanate method (14) with subsequent CsCl gradient centrifugation for 16 h.
Frozen femoral head biopsies were also used for RNA isolation. These frozen biopsies were ground under liquid nitrogen, and the resulting bone powder was suspended in 4 M guanidinium thiocyanate solution. This suspension was centrifuged at low speed twice to separate insoluble fractions, and the supernatant was loaded onto a CsCl gradient.
Total RNA was quantitated by absorption at 260 nm, and equal RNA samples were denatured with 2.2 M formaldehyde, 50% formamide, 5 μg/ml ethidium bromide, and loading dye in 1× MOPS buffer (40 mM morpholinolpropanesulfonic acid, pH 7.0, 10 mM sodium acetate, and 0.5 mM EDTA) at 65°C for 15 min. 15 μg total RNA was fractionated on a 1.0% formaldehyde-MOPS agarose gel. RNA was transferred overnight to Hybond N (Nycomed Amersham plc, Little Chalfont, Buckinghamshire, UK) by capillary action with 20× SSC (1× = 0.15 M sodium chloride and 0.015 M sodium citrate, pH 7.0) and immobilized by heating for 2 h at 80°C.
Complementary DNA probes for 28S RNA (Ambion Inc., Austin, TX), ETRA (provided by Dr. Haendler, Schering AG, Berlin, Germany), and ET1 (American Type Culture Collection) were labeled with [32P]dCTP by the random priming method using the Prime-a-gene labeling kit (Promega Corp.). The RNA filters were prehybridized in 50% formamide, 5× SSPE (1× = 150 mM NaCl, 10 mM NaH2PO4, 1 mM EDTA, pH 7.4), 5× Denhardt's (1× = 0.2% of Ficoll, BSA, and polyvinyl pyrrolidone), 100 μg/ml denatured herring sperm DNA, and 0.1% SDS for 3 h at 42°C. Hybridizations were performed for 24 h at 42°C in the prehybridization solution containing 5 × 106 cpm/ml of the radiolabeled denatured cDNA probe. Filters were washed at 42°C four times for 5 min with 2× SSC/0.1% SDS and twice for 10 min with 0.2× SSC/0.1% SDS before exposure to Agfa curix HT 1.000G films at −80°C for 24 h (28S) and 4 d (ETRA), respectively. The autoradiographs were quantitated using a densitometer (Bio-Rad Laboratories, Hercules, CA) and Molecular Analyst software. The mRNA signals were normalized against 28S RNA signal and expressed as relative units.
Endothelin Enzyme Immunoassay.
ET1 concentrations in culture supernatants and in human plasma were measured by an ELISA (Biomedica, Vienna, Austria). 200 μl of undiluted supernatant from control and dexamethasone-treated cultures was assayed in five replicates and corrected for protein contents. For detection of plasma ET1, 200 μl of human EDTA-plasma was assayed in three replicates. ET1, if present in the sample, binds to the precoated polyclonal capture rabbit antiendothelin antibody and forms a sandwich with antiendothelin mAbs. After a washing step, horseradish peroxidase–conjugated anti–mouse IgG antibody detects the presence of detection mAb. Tetramethylbenzidine is added as substrate, and ET1 is quantitated by an enzyme-catalyzed color change on an ELISA reader at 450 nm. The measuring range of the assay is 0.05–15.6 fmol/ml. The cross-reaction with endothelin 2, endothelin 3, and big endothelin was 100, 5, and 1%, respectively.
Measurement of Cell Proliferation.
HOC were plated for 24 h in DME, 10% CS, 1% PS in 48-well plates with 2 × 104 cells/ well. After 24 h preincubation with vehicle and 10 nM dexamethasone in DME supplemented with 1% CS and 1% PS, medium was changed and cells were treated for an additional 24 h in DME containing 1% CS/1% PS in the presence of increasing concentrations of ET1 (1, 10, 100, and 1,000 pg/ml). Cells were trypsinized, and each well was counted separately using a hemacytometer. Mean values from six wells of two separate experiments were calculated and expressed as percentage of control ± SD. Cultures pretreated with vehicle and 10 nM dexamethasone, respectively, without subsequent addition of ET1 were used as control values. The Student's t test was applied, and statistical significance was accepted at P < 0.05.
125I-ET1 Binding Analysis.
HOC were plated in culture dishes (78 cm2) with DME containing 10% CS and 1% PS. After growing to 90% confluence, cultures were maintained for 24 h in serum-free, phenol red–free DME before treatment with 100 nM dexamethasone or vehicle control for 24 h. Cells were rinsed with ice-cold PBS containing 1.25 mg/ml BSA and incubated for 2 h at 4°C in serum-free, phenol red–free DME containing 1.25 mg/ml BSA with increasing concentrations of 125I-ET1 (5–1,000 pM, specific activity 2,200 Ci/mmol; DuPont-NEN, Dreieich, Germany). Nonspecific 125I-ET1 binding was determined in the presence of 1 μM unlabeled ET1 for all concentrations. At the end of the 2-h incubation period, the cultures were rinsed in ice-cold PBS/BSA and solubilized in 0.5 M NaOH before counting the adherent activity with a gamma counter. Measurements were obtained in six replicates and evaluated by Scatchard analysis (15).
Results
Effect of Dexamethasone on ET1-induced HOC Proliferation.
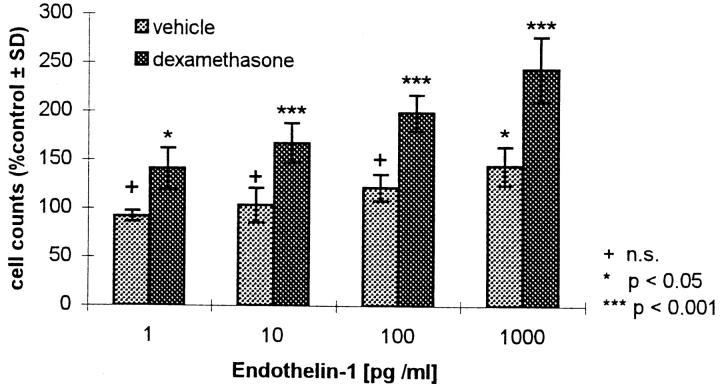
ET1 stimulates HOC proliferation in a dose- and time- dependent fashion (11). To determine the effect of dexamethasone on ET1-induced HOC proliferation, cells were counted in the absence and presence of dexamethasone. The cultures were pretreated with vehicle and 10 nM dexamethasone, respectively, in DME supplemented with 1% CS/1% PS. After 24 h incubation, medium was changed to DME containing 1% CS/1% PS, and HOC were incubated for 24 h in the presence of 1, 10, 100, and 1,000 pg/ml ET1. ET1 stimulated HOC proliferation in a dose-dependent manner, and pretreatment with 10 nM dexamethasone enhanced the mitogenic effect of ET1 (Fig. 1). This suggests a link between the systemically acting steroid hormones and the mechanism of action of the paracrine regulator of bone cell metabolism, ET1.
Figure 1.
Effect of dexamethasone on ET1-induced HOC proliferation. Subconfluent HOC were pretreated for 24 h with vehicle and 10 nM dexamethasone, respectively, and subsequently incubated for 24 h with various concentrations of ET1 (1, 10, 100, and 1,000 pg/ml). Cells were counted in six replicates and calculated as percent of control ± SD. n.s., Not significant (control = preincubation with vehicle and dexamethasone, respectively, without subsequent addition of ET1; vehicle control 24,500 ± 710 and dexamethasone control 16,000 ± 1,410 cells/ well).
To examine whether the positive effect of dexamethasone on the ET1-stimulated HOC proliferation is mediated by the induction of ET1, ET1 expression in HOC was determined. After pretreating HOC cultures with various concentrations of dexamethasone, the ET1 concentration in the culture medium was measured by ELISA and the ET1 mRNA expression after dexamethasone treatment was analyzed by Northern blots using an ET1 cDNA probe. ET1 mRNA and ET1 protein could not be detected in HOC or in HOC culture supernatants before or after dexamethasone treatment. Dexamethasone did not affect ET1 mRNA levels or ET1 concentrations in HOC cultures (data not shown).
Effects of Prednisolone on ET1 Plasma Concentrations In Vivo.
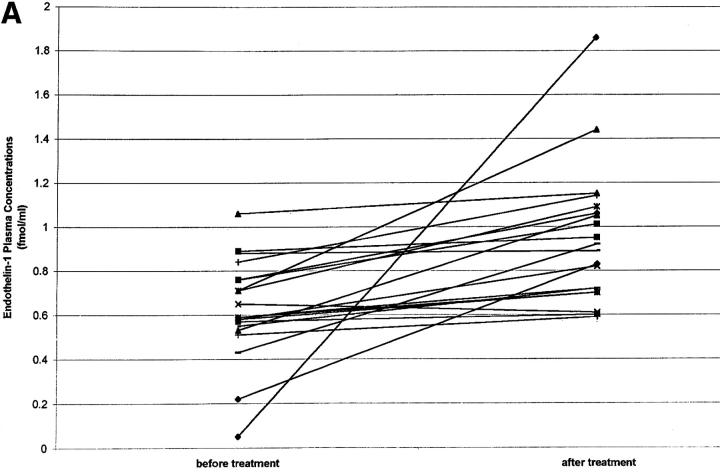
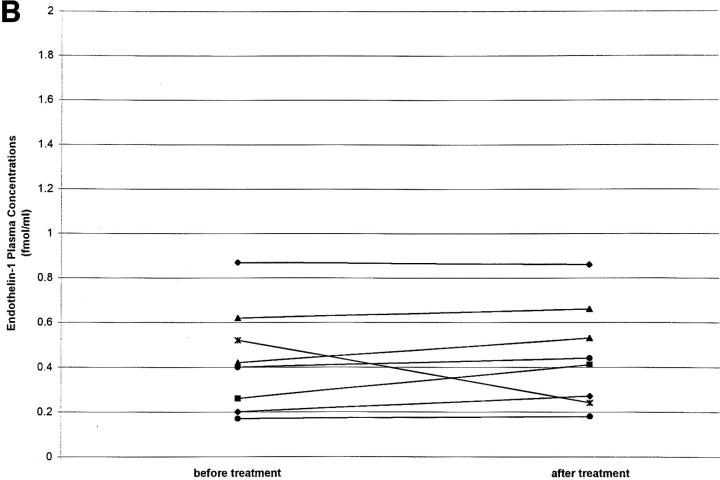
Glucocorticoids increase ET1 plasma concentrations in a rat model (7). To determine whether glucocorticoid treatment also increases ET1 in humans, plasma ET1 concentrations were measured in 17 patients with back pain (arising from spondylosis) before and 5 d after treatment with intravenous prednisolone (50 mg prednisolone on day 1, 25 mg on days 2 and 3, and 10 mg on days 4 and 5). Patients also received intravenous diclofenac (75 mg), tetrazepam (12.5 mg), and tramadol (50 mg) and had no other acute or chronic disease. It was found that prednisolone treatment increased ET1 plasma levels by 50% (Fig. 2 A), whereas the control group of patients similarly infused but not receiving prednisolone showed no significant change in plasma ET1 levels after 5 d treatment (Fig. 2 B).
Figure 2.
(A) ET1 plasma concentrations (fmol/ml) before and after a 5-d intravenous treatment with prednisolone (n = 17; 10 males and 7 females; mean age 51.3 yr). Before glucocorticoid treatment (mean ± SE): 0.58 ± 0.05; after 5 d glucocorticoid treatment: 0.89 ± 0.08; P < 0.014. (B) ET1 plasma concentrations before and after a 5-d intravenous treatment with diclofenac, tetrazepam, and tramadol but without prednisolone (n = 8; four males and four females; mean age 44.2 yr): pretreatment 0.43 ± 0.08 fmol/ml (mean ± SE); ET1 plasma concentration after 5 d treatment 0.44 ± 0.07 fmol/ml (P not significant).
Regulation of ETRA mRNA Expression in HOC by Glucocorticoids.
To determine whether dexamethasone enhances the mitogenic effect of ET1 by increasing ETRA expression, the effect of dexamethasone on the ET1-specific ETRA mRNA level in HOC was evaluated.
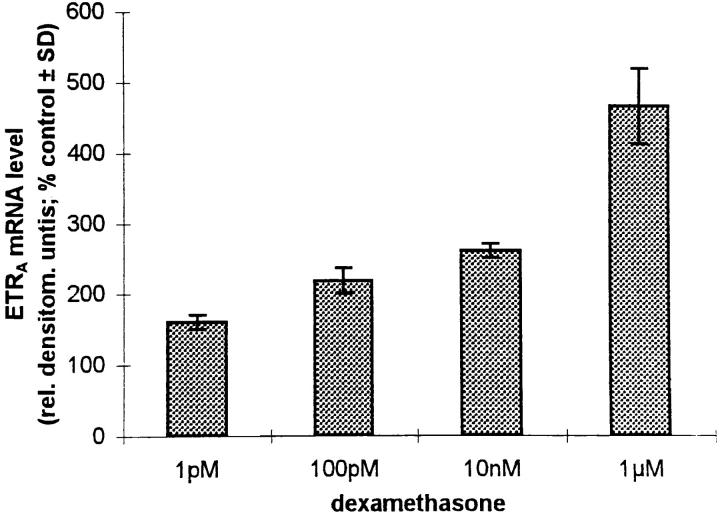
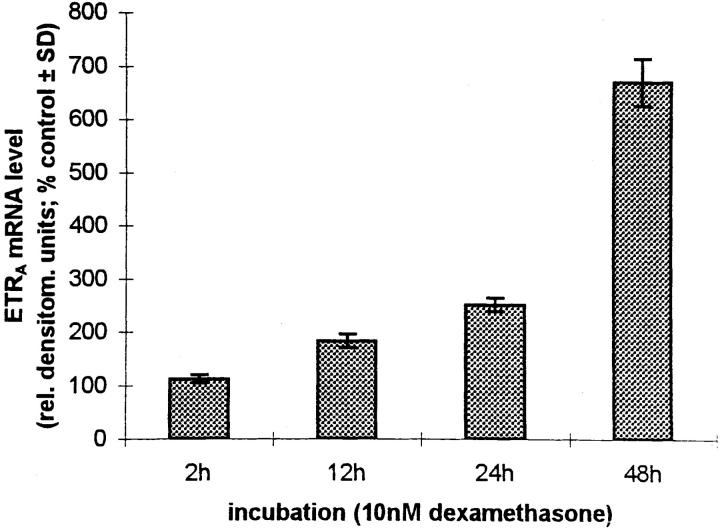
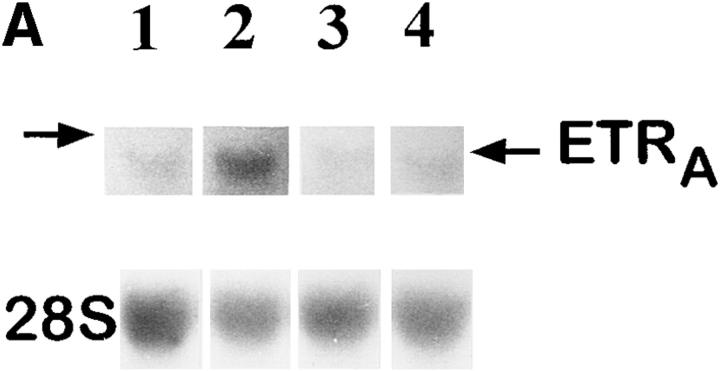
Culture of HOC in the presence of 1 pM, 100 pM, 10 nM, and 1 μM dexamethasone for 24 h resulted in an increase of ETRA mRNA transcripts, with greatest response at 1 μM dexamethasone (4.7-fold over control, as determined by densitometry; Fig. 3 A). The effect was observed as early as 12 h after addition of 10 nM dexamethasone (Fig. 3 B) and continued to increase at least until 48 h. Therefore, dexamethasone upregulates ETRA mRNA levels in a dose- and time-dependent fashion.
Figure 3.
Dose response (A) and time course (B) of ETRA mRNA upregulation in HOC by dexamethasone. HOC were incubated for 24 h with increasing concentrations of dexamethasone (1 pM–1 μM) (A). HOC were also treated for 2–48 h with 10 nM dexamethasone (B). 15 μg/lane of total RNA from control and treated cells was loaded on MOPS agarose gel, transferred on nylon membrane, and hybridized to 32P-labeled human ETRA mRNA cDNA probes. To assess loading differences, the filters were hybridized with a 28S probe. Arrows, left, Position of 28S and 18S RNA. The corrected ETRA mRNA signal in relative densitometry units expressed as percentage of control is plotted against dexamethasone concentration (A) and incubation time (B). The shown data are representative of the repeats of three identical experiments.
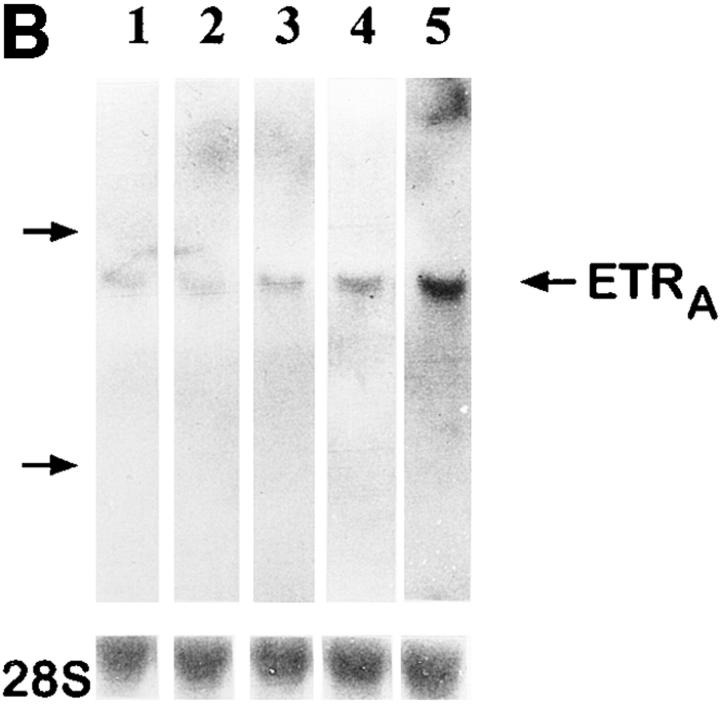
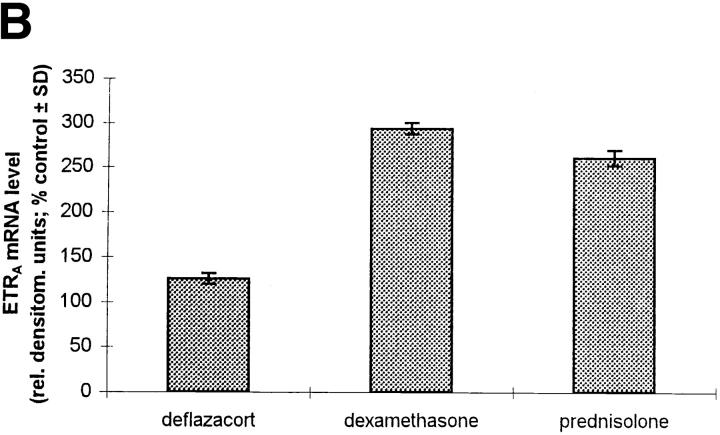
To evaluate the effect of dexamethasone on the ETRA mRNA level in comparison with other glucocorticoids which possess a lower affinity to the glucocorticoid receptor, the effects of equal concentrations (10 nM) of dexamethasone, deflazacort, and prednisolone on ETRA mRNA expression were determined after 24 h treatment (Fig. 4, A and B). Equal glucocorticoid concentrations produced similar effects on ETRA mRNA expression after 24 h treatment but of different magnitude. The effects of the used glucocorticoids on the ETRA mRNA expression correspond to the affinity of the examined glucocorticoids to the glucocorticoid receptor, i.e., prednisolone has a lower affinity than dexamethasone to the glucocorticoid receptor and elicits a smaller increase of ETRA mRNA levels than dexamethasone, whereas deflazacort, which has the lowest affinity to the glucocorticoid receptor, shows the smallest effect on the ETRA mRNA levels compared with prednisolone and dexamethasone (16).
Figure 4.
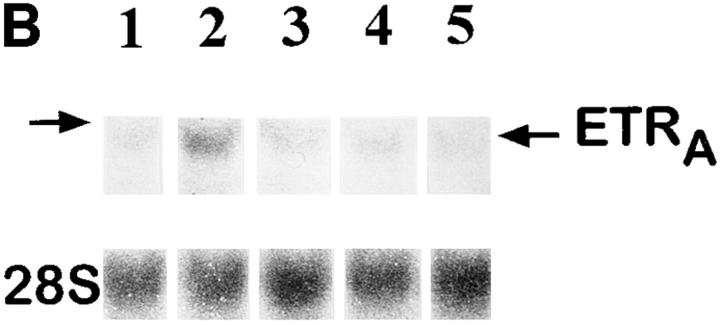
Comparison of the effects of different glucocorticoids on ETRA mRNA expression. HOC were treated for 24 h with 10 nM deflazacort, dexamethasone, and prednisolone (lanes 2–4). Total RNA was harvested from control and treated cultures, subjected to Northern blot analysis (A), and hybridized with 32P-labeled ETRA cDNA probes. Loading differences were determined by rehybridizing with a 28S probe. Arrow, left, Position of 28S rRNA. The corrected ETRA mRNA signal is shown in relative densitometry units expressed as percent of control for the used glucocorticoids (B). The shown data are representative of the results of two identical experiments.
Effect of Glucocorticoid Treatment on ETRA mRNA in Ex Vivo Human Bone Biopsies.
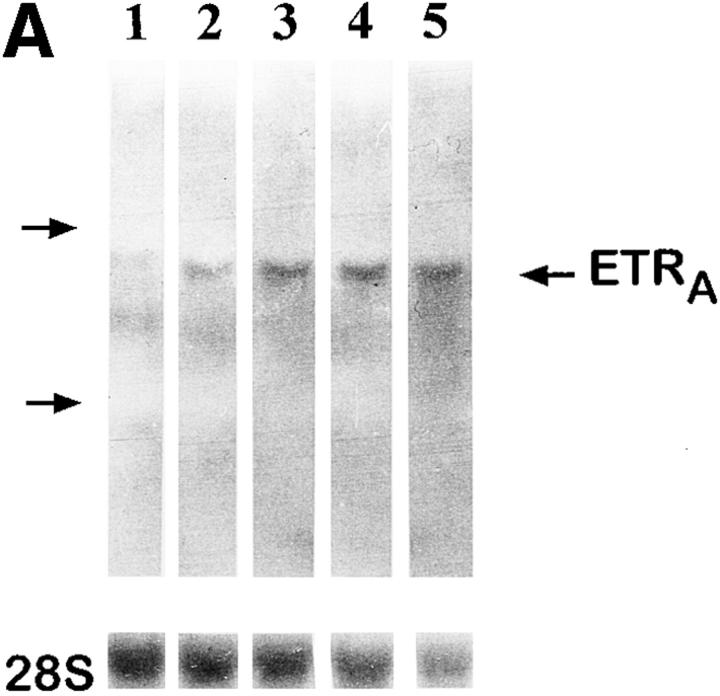
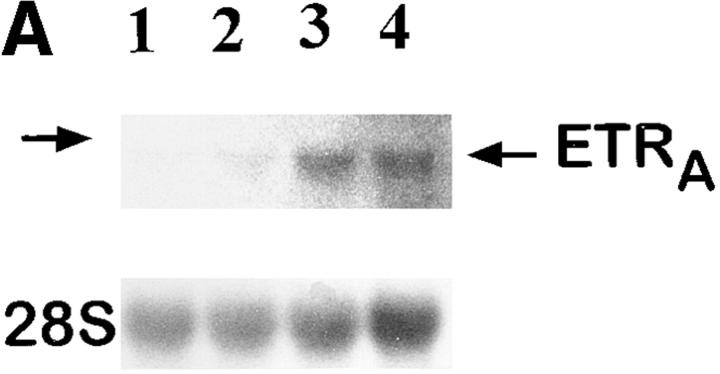
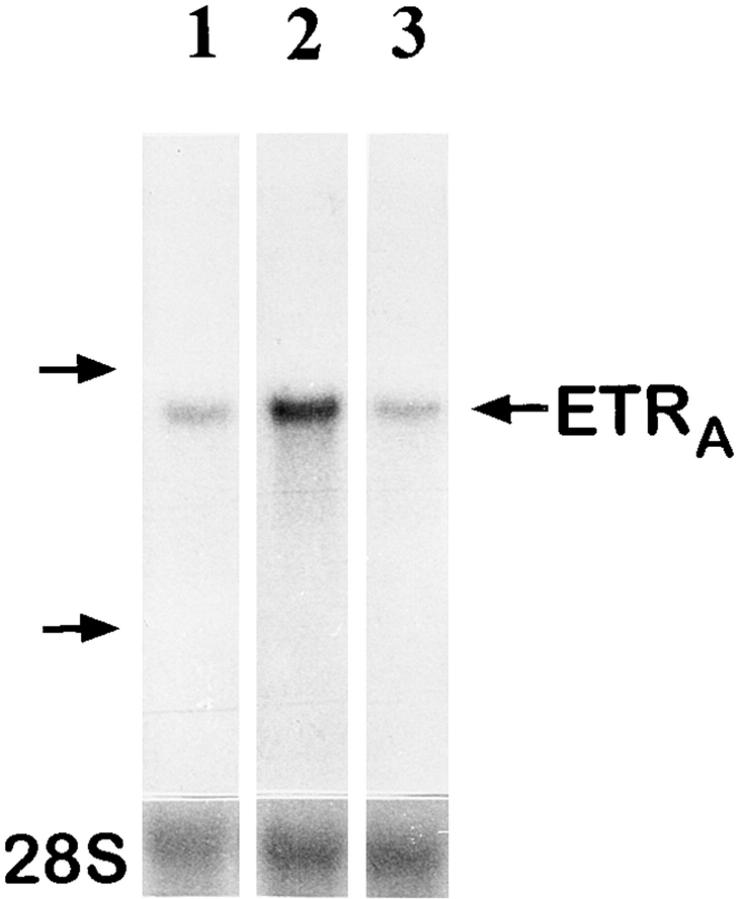
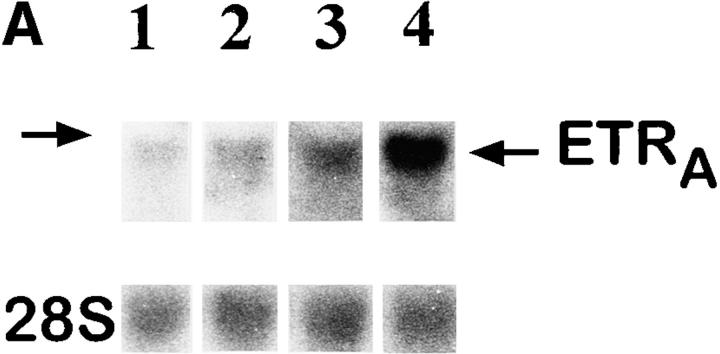
To investigate whether the effect of glucocorticoids on ETRA mRNA expression in HOC can also be observed in vivo, total RNA extracts were prepared from femoral head biopsies of asthmatic and polymyalgia patients with osteonecrosis of the hip due to several months of treatment with high doses of glucocorticoids. Total RNA extracts of femoral head biopsies from glucocorticoid-treated patients showed threefold higher ETRA mRNA levels compared with extracts of femoral head biopsies from patients with traumatically induced osteonecrosis and coxarthrosis (Fig. 5).
Figure 5.
Ex vivo studies of ETRA mRNA expression in human femoral head biopsies. Total RNA was extracted from human femoral head biopsies. Northern blot analyses were performed with 15 μg/lane total RNA and hybridized with 32P-labeled ETRA cDNA and 28S probes. In lanes 1–3, total RNA extracts of femoral head biopsies from patients with coxarthrosis (1), glucocorticoid-induced osteonecrosis (2), and traumatically induced osteonecrosis (3) were loaded. Arrows, left, 28S and 18S rRNA position. The analyzed RNA in each lane is the total RNA extract of one bone biopsy. The data in each lane are representative of two separate bone biopsies of different patients. Patients with coxarthrosis and traumatically induced osteonecrosis did not receive glucocorticoids at any time. Patients with glucocorticoid-induced osteonecrosis of the femoral head had been treated with 10–20 mg of prednisolone per day for 5 and 6 mo, respectively.
Binding Characteristics of 125I-ET1 in HOC after Dexamethasone Treatment.
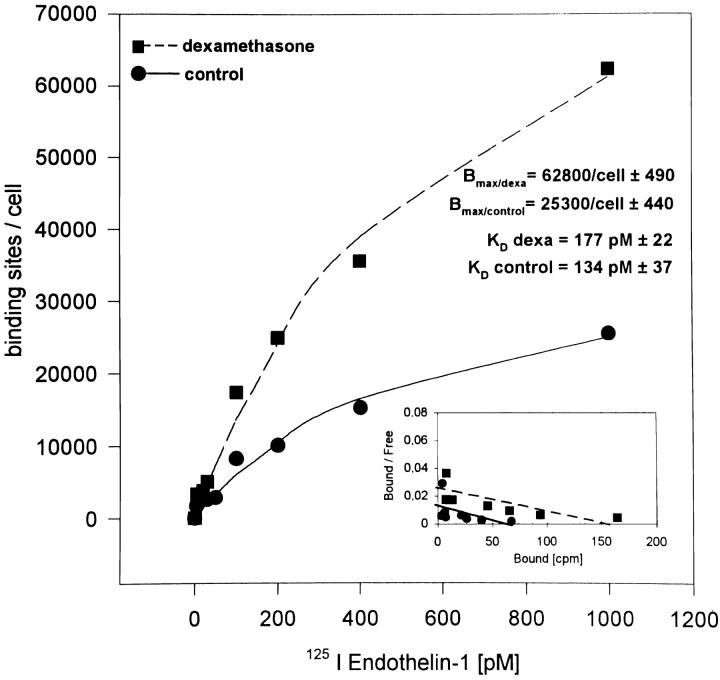
The effects of dexamethasone on the binding characteristics of 125I-ET1 to HOC were also studied. Saturation of ET1 binding capacity was observed at a concentration of 1,000 pM. A 24-h pretreatment with 100 nM dexamethasone increased the ET1 binding sites per osteoblastic cell from ∼25,300 to 62,800, i.e., 2.4-fold over control (Fig. 6). Scatchard transformation of the binding data was performed to calculate the K D for ET1 binding. There was no significant change in the binding affinity of ET1 to the osteoblastic ETRA with and without dexamethasone treatment (134 vs. 177 pM).
Figure 6.
Saturation binding and Scatchard plot of 125I-ET1 in control and dexamethasone-treated HOC cultures. HOC were grown to 90% confluence and treated for 24 h with 100 nM dexamethasone. Control and treated cultures were incubated at 4°C for 2 h in serum-free medium containing 1.25 mg/ml BSA with increasing concentrations of 125I-ET1 (5–1,000 pM). Cells were washed with PBS/BSA, solubilized in NaOH, and counted in a gamma counter. Data represent the mean of six determinants. Curve fittings were performed by regression analyses.
Effect of RU486 on Dexamethasone-induced ETRA mRNA Expression.
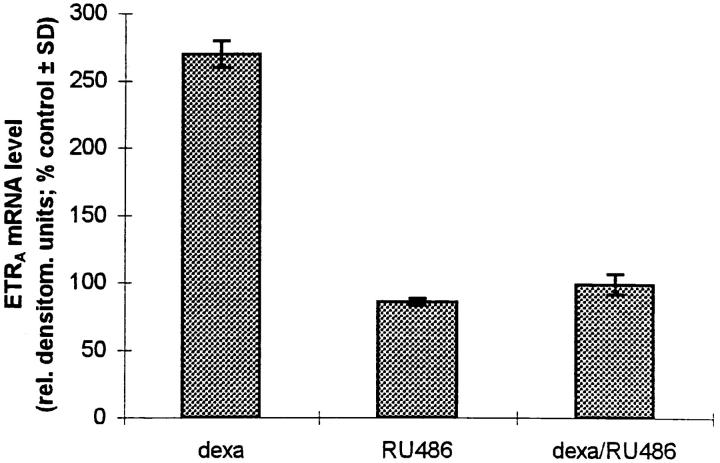
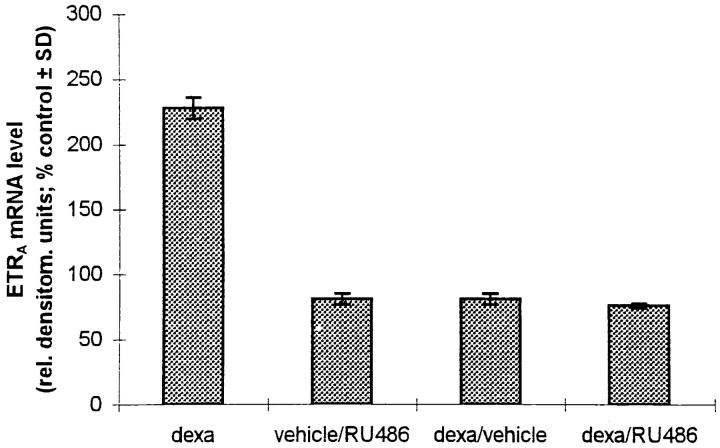
The stimulation of ETRA expression by dexamethasone, prednisolone, and deflazacort corresponds to the binding affinity of the glucocorticoids to the glucocorticoid receptor, i.e., the higher the receptor affinity of the glucocorticoid the greater the positive effect of the glucocorticoid on ETRA mRNA levels. To further demonstrate that this positive effect of glucocorticoids on the ETRA mRNA expression is mediated by a mechanism involving glucocorticoid receptors, the receptor binding sites were blocked with the nonspecific steroid hormone antagonist RU486. 100 nM RU486 showed no positive effect on ETRA mRNA level when given alone but completely blocked the induction of ETRA mRNA expression by 10 nM dexamethasone. There was no difference between the ETRA mRNA levels of the dexamethasone plus RU486– treated group and the control group (Fig. 7 A). To study whether the effect of dexamethasone on ETRA mRNA expression is mediated through a mechanism which requires only short-term incubation with the glucocorticoid present to start a postreceptor cascade leading to the observed increase of ETRA mRNA levels, combination experiments were performed. After 6 h preincubation in the presence of 10 nM dexamethasone and subsequent addition of 100 nM RU486 for the following 18 h, there was no difference between the ETRA mRNA levels of the dexamethasone/RU486-treated and the control cultures (Fig. 7 B). These results demonstrate that the induction of ETRA mRNA by dexamethasone in HOC requires the continuous presence of the glucocorticoid dexamethasone in the culture medium.
Figure 7.
Effect of the steroid hormone receptor antagonist RU486 on ETRA mRNA expression. (A) HOC were treated for 24 h with vehicle (lane 1), 10 nM dexamethasone (lane 2), 100 nM RU486 (lane 3), and dexamethasone/RU486 coincubation (lane 4). (B) HOC were incubated for a total experimental period of 24 h (changing medium after 6 h) with vehicle (lane 1) and 10 nM dexamethasone (lane 2). The other experimental groups (lane 3–5) were incubated for 6 h vehicle/18 h 100 nM RU486 (lane 3), 6 h 10 nM dexamethasone/ 18 h vehicle (lane 4), and 6 h 10 nM dexamethasone/18 h 100 nM RU486 (lane 5). Total RNA was extracted from control and treated cells. Northern blot analyses were performed with 32P-labeled ETRA and 28S cDNA probes. Arrows, left, 28S rRNA position. Data are representative of three experiments. Lower panels show corrected ETRA mRNA signals in relative densitometry units expressed as percent of control.
Effect of Dexamethasone on ETRA mRNA Half-life.
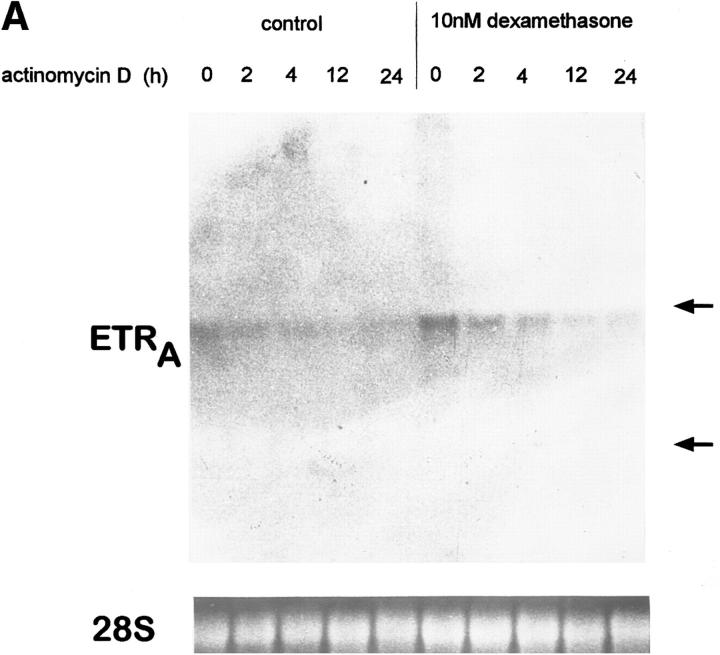
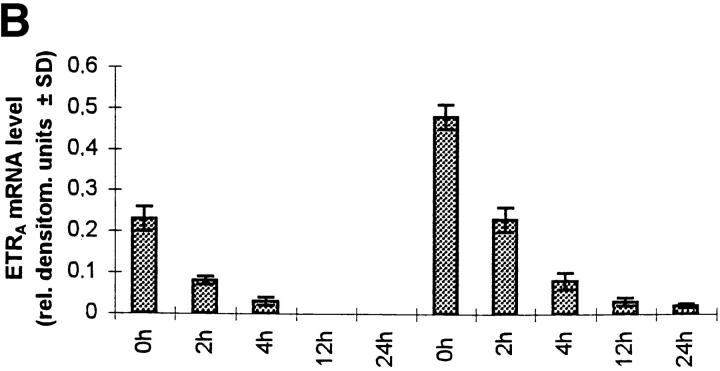
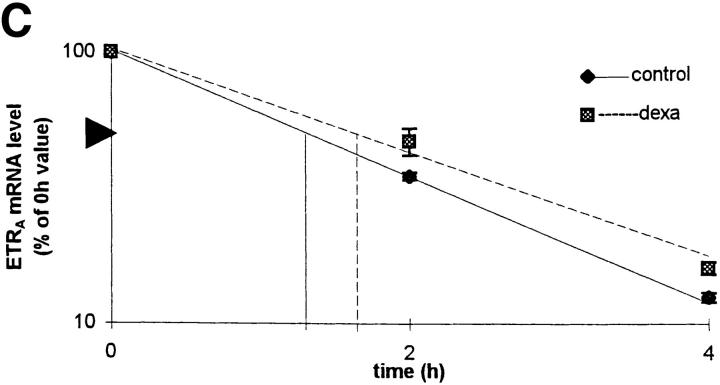
To examine whether the effect of dexamethasone on ETRA mRNA levels is due to changes in transcript stability, the half-life of ETRA mRNA in transcriptionally arrested HOC was determined. HOC were incubated for 24 h in medium containing vehicle or 10 nM dexamethasone, respectively, followed by an addition of 1 μg/ml actinomycin D for 0, 2, 4, 12, and 24 h, which inhibits RNA transcription by intercalating in double-stranded DNA. There was no consistent difference with regard to the half-life of ETRA mRNA in control and dexamethasone-treated cultures (Fig. 8). These results indicate that dexamethasone does not affect the stability of ETRA mRNA and therefore that the increased ETRA mRNA levels after dexamethasone treatment are not due to changes of the ETRA mRNA stability.
Figure 8.
Effect of 10 nM dexamethasone on ETRA mRNA decay in transcriptionally blocked HOC by actinomycin D. HOC were treated for 24 h with 10 nM dexamethasone before the addition of actinomycin D. Total RNA was extracted from control and treated cultures after 0, 2, 4, 12, and 24 h 1 μg/ ml actinomycin D treatment. RNA was analyzed by Northern blot and hybridized with 32P-labeled ETRA cDNA probes. Data are representative of three experiments. Ethidium bromide staining of rRNA was used to check equal RNA loading. Arrows, right, 28S and 18S rRNA positions (A). The signals were analyzed by densitometry (B), and 0-, 2-, and 4-h ETRA mRNA levels were plotted in logarithmic scale with linear regression as a percentage of the 0-h value against time (C).
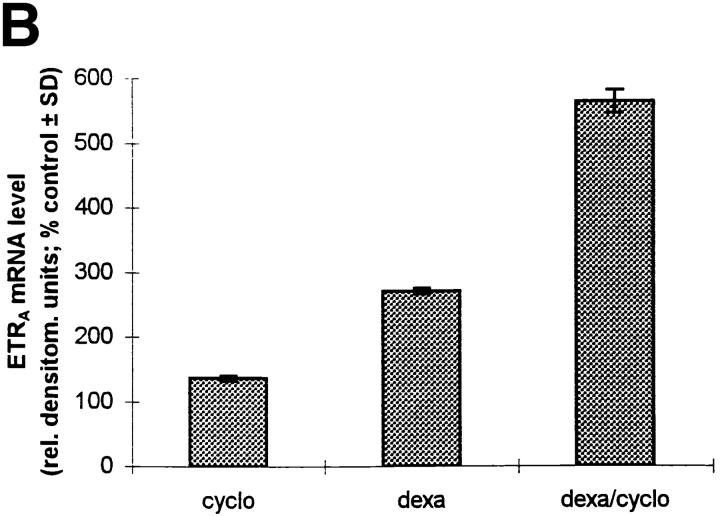
Effect of Cycloheximide on Dexamethasone-induced ETRA mRNA Expression.
HOC were incubated with 10 μg/ml cycloheximide for 24 h in control and dexamethasone-containing medium (Fig. 9) to determine whether the effect of dexamethasone on ETRA mRNA expression depends on de novo protein synthesis. Cycloheximide alone had a small inductive effect on the ETRA mRNA level. Coincubation of 10 nM dexamethasone in the presence of cycloheximide led to a superinduction of ETRA mRNA expression. These data demonstrate that the effect of dexamethasone on ETRA mRNA is not dependent on de novo protein synthesis. Moreover, cycloheximide in combination with dexamethasone in fact increases ETRA mRNA levels in HOC more than dexamethasone treatment alone.
Figure 9.
Effect of cycloheximide on ETRA mRNA regulation in HOC by dexamethasone. Cultures were treated for 24 h with vehicle (lane 1), 10 μg/ml cycloheximide (lane 2), 10 nM dexamethasone (lane 3), and 10 nM dexamethasone in the presence of 10 μg/ml cycloheximide (lane 4). Total RNA was extracted, and Northern analyses were performed with 32P-labeled ETRA and 28S cDNA probes. Arrow, left, Position of 28S rRNA (A). (B) The corrected ETRA mRNA signal is plotted as percent of control for the experimental groups. The shown data are representative of three identical experiments.
5′-flanking Region of the Human ETRA Gene and Promoter Activity of Reporter Gene Constructs in HOC.
A 3.1-kb fragment of the 5′-flanking region of the human ETRA gene was amplified by nested PCR using a gene-specific primer pair and a manufacturer-provided adaptor primer pair. The PCR product was cloned in a PCR cloning vector and automatically sequenced. The nucleotide sequence analysis of the 5′-flanking region from −3050 to +48 (sequence data available from EMBL/GenBank/DDBJ under accession no. AF005637) extends our knowledge about the sequence of the promoter region of the ETRA gene by 2.2 kb (41; Fig. 10 A).
Figure 10.

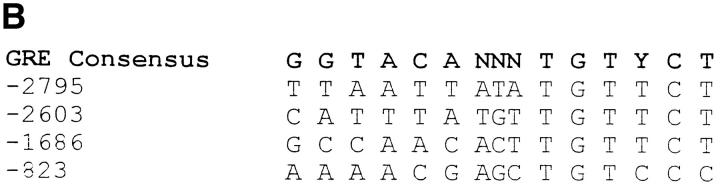
Nucleotide sequence of the human ETRA gene 5′-flanking region from −3050 to +48 relative to the transcription start site. Arrow, Overlapping region of the promoter sequence reported by Hosoda et al. (reference 41). (B) The 3.1-kb ETRA 5′-flanking region was analyzed by computer-aided search for GRE.
A basic analysis of the 3.1-kb ETRA 5′-flanking region was performed by using a computer-aided search for glucocorticoid response elements (GRE). The sequence analysis revealed the presence of four putative GRE sequences at positions −2795, −2603, −1686, and −823 (Fig. 10 B).
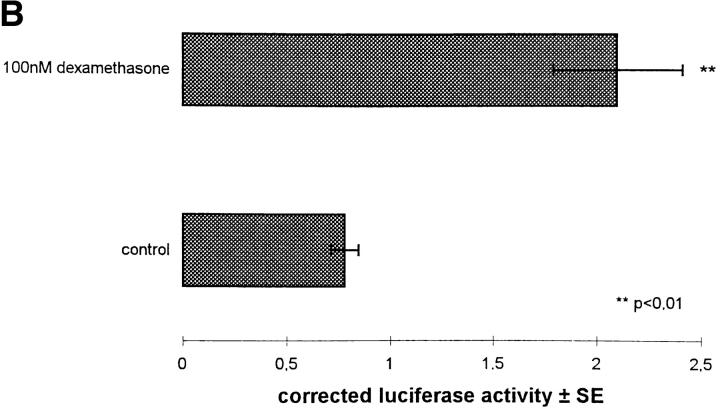
To demonstrate promoter activity of the sequenced 5′-flanking region of the human ETRA gene, the DNA fragment from −3050 to +48 was cloned upstream of a luciferase reporter gene in the pGL3 Basic vector without a promoter (Fig. 11 A). The chimeric construct was cotransfected with a glucocorticoid receptor expression plasmid into primary HOC and treated with 100 nM dexamethasone and control medium for 24 h to determine the effect of a glucocorticoid treatment on the reporter gene activity (Fig. 11 B). Significant luciferase activity was detected in cells transfected with the ETRA promoter/reporter gene construct compared with cells transfected only with the promoter-deficient vector pGL3 Basic (data not shown). A treatment with 100 nM dexamethasone for 24 h led to an induction of the luciferase reporter gene activity of ∼2.6-fold over untreated osteoblastic control cultures. Experiments without cotransfection of glucocorticoid receptor expression plasmid showed no significant difference in the luciferase reporter gene activity between control and dexamethasone-treated cultures (data not shown).
Figure 11.
(A) Schematic demonstration of the chimeric construct of the 5′-flanking region of the ETRA gene fused to luciferase gene in pGL3 Basic vector. (B) Reporter gene activity was assayed in HOC cotransfected with reporter gene construct and glucocorticoid receptor expression plasmid and β-galactosidase expression plasmid, treated for 24 h in parallel with 100 nM dexamethasone and control medium, and expressed in corrected luciferase activity ± SE.
Discussion
Glucocorticoid hormones modulate differentiated bone cell functions and osteoclastogenesis to regulate the balance of bone resorption and bone formation in the skeleton (17). Supraphysiological concentrations of synthetic glucocorticoids disturb bone metabolic balance directly by antiproliferative effects on bone-forming cells and indirectly by stimulating bone resorption (17–20). In vitro studies have also shown that glucocorticoids have potent antiproliferative effects (21–23) on mesenchymal cells, including bone cells at high concentrations in long-term experiments (24), whereas at low concentrations short-term experiments have shown that glucocorticoids stimulate osteoblastic proliferation (21–23). Thus, the effects of glucocorticoids may be related not only to dose and treatment duration but also to the degree of cellular differentiation. At physiological concentrations, glucocorticoids provide a basal stimulus for differentiation, ensuring not only a continous stream of mesenchymal stem cells maturing into proliferating osteoblastic precursor cells but also their differentiation into osteoblasts (25–27). The detrimental effects of supraphysiological glucocorticoid concentrations on bone metabolism result from the inhibition of precursor cell growth by interference with paracrine signaling mechanisms by mitogenic bone growth factors (17, 24), leading to a reduction in the number of terminally differentiated bone cells.
This study provides evidence that long-term exposure to pharmacological doses of systemically circulating glucocorticoid hormones may interfere with paracrine signaling between bone tissue and the vascular system by affecting the osteoblastic ETRA gene expression and increasing ET1 plasma concentrations. ET1 is a paracrine factor of the vascular system secreted by vascular smooth muscle and endothelial cells and was initially identified as a potent vasoconstrictor (28, 10). ET1 does also exert biological activity on various cell types (29), including osteoblastic cells (30). ET1 stimulates type I collagen secretion and ALP expression in primary HOC and exerts a potent mitogenic action on human bone cells (11). These effects on osteoblastic cells are mediated by specific ETRAs, which are constitutively expressed in HOC. The action of ET1 on differentiated osteoblastic functions (e.g., type I collagen synthesis and ALP expression) and the mitogenic effect of ET1 on osteoblastic cells suggest a regulatory role of ET1 in human bone formation and bone growth.
To investigate how glucocorticoids may interact with the regulatory effect of ET1 on human bone cell metabolism, HOC were pretreated with glucocorticoids and the mitogenic effect of a subsequent ET1 treatment was determined. A glucocorticoid treatment enhanced the mitogenic action of ET1. There are several possibilities for how glucocorticoids may affect ET1-mediated actions.
(a) Glucocorticoids may induce the secretion of other peptides, exerting a proliferative effect on bone cells, which has been shown for other steroid hormones, e.g., progesterone, which increases insulin-like growth factor (IGF)-II expression in bone cells (31). Growth factor release may enhance ET1-induced cell proliferation in a concerted action as observed in rat kidney fibroblasts for transforming growth factor (TGF)-α and ET1 (32). However, there are also reports demonstrating a downregulation of the osteoblastic secretion of bone cell mitogens by glucocorticoids (33). A second mechanism by which glucocorticoids may affect ET1-induced cell proliferation is a glucocorticoid- induced increase in ET1 secretion, leading to higher ET1 concentrations in the culture medium which may increase the cell proliferation of HOC in a dose-dependent manner. However, there was no ET1 measurable in HOC cultures, and ET1 expression was not stimulated by glucocorticoid treatment in vitro. Because the culture medium was changed after glucocorticoid pretreatment, it appears unlikely that glucocorticoid-stimulated growth factor or ET1 release by osteoblastic cells contributes to the observed enhancement of ET1 action by glucocorticoids in vitro. However, in vivo glucocorticoid treatment elevated plasma ET1 concentrations significantly. This observation is consistent with a stimulatory action of glucocorticoids on ET1 production by vascular cells, which was demonstrated in a rat model (6). The significant interpatient variability in ET1 plasma level changes after glucocorticoid treatment (Fig. 2 A) is reminiscent of the variability of changes in lymphocyte response, insulin resistance, and bone density among glucocorticoid-treated patients (34–36).
(b) Glucocorticoids may also stimulate ET1-induced human bone cell proliferation by affecting postreceptor mechanisms. An interaction between glucocorticoids and growth factors on the postreceptor level was demonstrated recently in dexamethasone-treated fibroblastic cultures where the activation of the glucocorticoid receptor interferes with postreceptor processes of the IGF-I receptor (37), impairing IGF-I–mediated effects. To our knowledge, there are no reports demonstrating glucocorticoid-induced enhancement of growth factor actions by affecting postreceptor mechanisms.
(c) Finally, glucocorticoid treatment may enhance osteoblastic ETRA expression, because other steroid hormones (1,25(OH)2D3) have also been shown to modulate ETRA mRNA expression in HOC (11). This possibility was tested in a primary HOC system. The results demonstrate that ETRA mRNA expression is indeed upregulated by dexamethasone in a dose- and time-dependent fashion. Experiments with actinomycin D reveal that increased ETRA mRNA levels are not due to increased transcript stability. The slow time course of ETRA mRNA induction by glucocorticoids may be due to an unphysiologically low basal ETRA mRNA level in vitro after a 48-h period of serum deprivation, a short half-life of ETRA mRNA, or to the recruitment of transcription factors which are constitutively present at very low basal concentrations (after a 48-h culture period in the absence of serum) and are required for ETRA gene transcription.
Elevated ETRA mRNA expression by glucocorticoids translates into a greater number of ET1 binding sites in HOC, whereas no change in the binding affinity for ET1 after glucocorticoid treatment was found. The observation that the glucocorticoid-increased ETRA mRNA expression level correlates positively to the affinity of the used glucocorticoid to the glucocorticoid receptor supports the view that the glucocorticoid-stimulated ETRA upregulation is a receptor-mediated phenomenon that depends on the ligand-activated glucocorticoid receptor protein. In vivo experiments examining osteonecrotic femoral head biopsies from glucocorticoid-treated patients demonstrate higher ETRA mRNA levels compared with biopsies from patients with traumatically induced osteonecrosis of the femoral head and coxarthrosis. However, this observation of an increased ETRA mRNA level in total RNA extracts from femoral head biopsies of glucocorticoid-treated patients may in part also be a glucocorticoid effect on nonosteoblastic cells, which are always present in bone tisssue (e.g., endothelial or smooth muscle cells).
RU486, a nonspecific steroid hormone antagonist, blocked the effect of dexamethasone on ETRA mRNA expression in HOC, suggesting a direct glucocorticoid receptor–mediated effect of the used compounds on ETRA mRNA expression. Combination experiments with RU486 demonstrate that maximal upregulation of osteoblastic ETRA mRNA levels by glucocorticoids depends on the continuous presence of the glucocorticoid. An increase of ETRA mRNA in HOC by glucocorticoids does not require de novo protein synthesis, indicating that the mediation of the effect of glucocorticoids on ETRA mRNA expression does not require de novo synthesis of transcription factors (e.g., AP-1 [38]). Moreover, superinduction of ETRA mRNA levels in the presence of dexamethasone and cycloheximide suggests that cycloheximide stabilizes ETRA mRNA transcripts by inhibiting the synthesis of a protein responsible for mRNA degradation, thereby leading to an accumulation of ETRA mRNA. The necessity for a continuous presence of the glucocorticoid to observe the upregulation of the ETRA mRNA is consistent with a mechanism by which ETRA expression is upregulated by the presence of ligand-activated glucocorticoid receptors serving as transcription factors after translocation into the HOC nucleus. To test this hypothesis, a 3.1-kb fragment of the 5′-flanking region of the human ETRA gene was cloned and automatically sequenced. Computer-aided sequence analysis revealed the presence of four putative GRE (−2795, −2603, −1686, and −823); two of them showed high conservation to the GRE consensus sequence (39). Transient transfections of HOC cultures with a chimeric construct of the 5′-flanking region of the ETRA gene fused to luciferase reporter gene and a glucocorticoid receptor expression plasmid showed a promoter-dependent expression of the reporter gene and regulation by dexamethasone. With a 2.6-fold induction of luciferase activity by dexamethasone treatment, the results of the transfection experiments correspond to the observed induction of ETRA by dexamethasone on the mRNA and protein level. However, the dexamethasone-mediated induction of luciferase activity required cotransfection with the glucocorticoid receptor expression plasmid, demonstrating that the basal expression level of glucocorticoid receptor in transfection experiments without cotransfection of glucocorticoid receptor expression plasmid is not sufficient to mediate induction of reporter gene activity after dexamethasone treatment. A low level of endogenous glucocorticoid receptor gene expression may be due to the experimental design: before dexamethasone treatment, cells were incubated in the presence of serum, which could lead to downregulation of glucocorticoid receptor expression by serum-derived steroid compounds (40).
Some limitations of the presented work also arise from experimental design and the experimental model (HOC) used. Glucocorticoid treatment of HOC was not continued for more than 48 h; therefore, it is not clear when the maximum ETRA mRNA level can be observed after glucocorticoid treatment of primary HOC. Furthermore, binding affinity for ET1 in HOC was reduced fivefold, an effect which apparently conflicts with a higher previous estimate (11) of the ET1 binding affinity in HOC (K D of 35 pM). This difference is likely to be due to the heterogeneous nature of primary HOC cultures, which are contaminated to various degrees (20–50%) by nonbone cells such as fibroblastic and vascular cells (11). Thus, the secretion of an unlabeled competing ligand by an unusually high percentage of contaminating nonbone cells may explain the higher apparent K D.
These data provide evidence that glucocorticoid- induced upregulation of circulating ET1 plasma levels and osteoblastic ETRA gene expression stimulate the osteoblastic cell metabolism. However, ET1-induced potent vasoconstriction may increase blood pressure (8) and could impair bone perfusion (9) in metabolically activated skeletal sites, which may contribute to the pathogenesis of glucocorticoid-induced osteonecrosis.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft, Germany (Ka 682/2-2 and Ka 682/2-3).
Footnotes
Abbreviations used in this paper: ALP, alkaline phosphatase activity; CS, calf serum; ET1, endothelin 1; ETRA, endothelin A receptor; GRE, glucocorticoid response element(s); HOC, human osteoblastic cell(s); IGF, insulin-like growth factor; PS, 10 U/ml penicillin-G, 10 μg/ml streptomycin.
References
- 1.Jee, W.S.S. 1983. The skeletal tissues. In Histology, Cell and Tissue Biology. L. Weiss, editor. Elsevier Science Inc., New York. 200–255.
- 2.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–288. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 3.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 4.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E. Effect of glucocorticoids on type I collagen, alkaline phosphatase activity and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983;112:931–939. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- 6.Kanse SM, Takahashi K, Warren JB, Ghatei M, Bloom SR. Glucocorticoids induce endothelin release from vascular smooth muscle cells but not endothelial cells. Eur J Pharmacol. 1991;199:99–101. doi: 10.1016/0014-2999(91)90641-3. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Suda K, Lam H-C, Ghatei MA, Bloom SR. Endothelin-like immunoreactivity in rat models of diabetes mellitus. J Endocrinol. 1991;130:124–127. doi: 10.1677/joe.0.1300123. [DOI] [PubMed] [Google Scholar]
- 8.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 9.Coessens BC, Miller VM, Wood MB. Endothelin induces vasoconstriction in the bone vasculature in vitro: an effect mediated by a single receptor population. J Orthop Res. 1996;14:611–617. doi: 10.1002/jor.1100140416. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa M, Kurihara H, Kimura S, Tomombe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 11.Kasperk CH, Börcsök I, Schairer HU, Schneider U, Nawroth PP, Niethard FU, Ziegler R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif Tissue Int. 1997;60:368–374. doi: 10.1007/s002239900245. [DOI] [PubMed] [Google Scholar]
- 12.Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37:453–460. [PubMed] [Google Scholar]
- 13.Kasperk CH, Wergedal JE, Strong DD, Farley JR, Wangerin K, Gropp H, Ziegler R, Baylink DJ. Human bone cell phenotypes differ depending on their skeletal site of origin. J Clin Endocrinol Metab. 1995;80:2511–2517. doi: 10.1210/jcem.80.8.7629252. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 16.Luzzani F, Glässer A. Differential binding in vitro to glucocorticoid receptors of deflazacort and prednisolone. Eur J Pharmacol. 1981;76:427–430. doi: 10.1016/0014-2999(81)90115-1. [DOI] [PubMed] [Google Scholar]
- 17.Canalis E. Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–3447. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- 18.Rubin J, Biskobing DM, Jadhav L, Fan D, Nanes MS, Perkins S, Fan X. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology. 1998;139:1006–1012. doi: 10.1210/endo.139.3.5778. [DOI] [PubMed] [Google Scholar]
- 19.Kaji H, Sugimoto T, Kanatani M, Nishiyama K, Chihara K. Dexamethasone stimulates osteoclast-like cell formation by directly acting on hemopoietic blast cells and enhances osteoclast-like cell formation stimulated by parathyroid hormone and prostaglandin E2. J Bone Miner Res. 1997;12:734–741. doi: 10.1359/jbmr.1997.12.5.734. [DOI] [PubMed] [Google Scholar]
- 20.Dempster DW. Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res. 1989;4:137–141. doi: 10.1002/jbmr.5650040202. [DOI] [PubMed] [Google Scholar]
- 21.Kasperk CH, Schneider U, Sommer U, Niethard F, Ziegler R. Differential effects of glucocorticoids on human osteoblastic cell metabolism in vitro. Calcif Tissue Int. 1995;57:120–126. doi: 10.1007/BF00298432. [DOI] [PubMed] [Google Scholar]
- 22.Jonsson KB, Frost A, Larsson R, Ljunghall S, Ljunggren O. A new fluorometric assay for determination of osteoblastic proliferation: effects of glucocorticoids and insulin-like growth factor-1. Calcif Tissue Int. 1997;60:30–36. doi: 10.1007/s002239900182. [DOI] [PubMed] [Google Scholar]
- 23.Ishida Y, Tertinegg I, Heersche JN. Progesterone and dexamethasone stimulate proliferation and differentiation of osteoprogenitors and progenitors for adipocytes and macrophages in cell populations derived from adult rat vertebrae. J Bone Miner Res. 1996;11:921–930. doi: 10.1002/jbmr.5650110708. [DOI] [PubMed] [Google Scholar]
- 24.Hulley PA, Gordon F, Hough FS. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA15.4) by dexamethasone: role of protein phosphatases. Endocrinology. 1998;139:2423–2431. doi: 10.1210/endo.139.5.6020. [DOI] [PubMed] [Google Scholar]
- 25.Shalhoub V, Conlon D, Tassinari M. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem. 1992;50:425–440. doi: 10.1002/jcb.240500411. [DOI] [PubMed] [Google Scholar]
- 26.Kamalia N, McCulloch CAG, Tenenbaum HC, Limenack H. Dexamethasone recruitment of self-renewing osteoprogenitor cells in chick bone marrow stromal cell cultures. Blood. 1992;79:320–326. [PubMed] [Google Scholar]
- 27.Boden SD, Hair G, Titus L, Racine M, McCuaig K, Wozney JM, Nanes MS. Glucocorticoid- induced differentiation of fetal rat calvarial osteoblasts is mediated by bone morphogenetic protein-6. Endocrinology. 1997;138:2820–2828. doi: 10.1210/endo.138.7.5125. [DOI] [PubMed] [Google Scholar]
- 28.Kanse, S.M., K. Takahashi, J.B. Warren, T. Perara, M. Porta, M.A. Ghatei, and S.R. Bloom. 1991. Production of endothelin by smooth muscle cells. J. Cardiovasc. Pharmacol. 17(Suppl. 7):S113–S116. [DOI] [PubMed]
- 29.Simonson MS, Dunn MJ. The molecular mechanisms of cardiovascular and renal regulation by endothelin peptides. FASEB J. 1990;4:2989–3000. [PubMed] [Google Scholar]
- 30.Tatrai A, Foster S, Lakatos P, Shankar G, Stern PH. Endothelin-1 actions on resorption, collagen and noncollagen protein synthesis, and phosphatidylinositol turnover in bone organ cultures. Endocrinology. 1992;131:603–607. doi: 10.1210/endo.131.2.1639010. [DOI] [PubMed] [Google Scholar]
- 31.Lempert, U.G., D.D. Strong, S. Mohan, K. Demarest, and D.J. Baylink. 1992. Effect of progesterone on the mRNA levels of insulin-like growth factors, IGF-binding proteins and type I- and type II-IGF receptors in human osteoblastic cells. In Calcium Regulating Hormones and Bone Metabolism. Basic and Clinical Aspects. D.V. Cohen, C. Gennari, and A.H. Tashjian, Jr., editors. Excerpta Medica, Inc., New York. 239–243.
- 32.Yeh YC, Burns ER, Yeh J, Yeh HW. Synergistic effects of endothelin-1 and transforming growth factor alpha or epidermal growth factor on DNA replication and G1 to S phase transition. Biosci Rep. 1991;11:171–180. doi: 10.1007/BF01182486. [DOI] [PubMed] [Google Scholar]
- 33.Delany AM, Dong Y, Canalis E. Mechanisms of glucocorticoid action in bone cells. J Cell Biochem. 1994;56:295–302. doi: 10.1002/jcb.240560304. [DOI] [PubMed] [Google Scholar]
- 34.Hirano T, Oka K, Takeuchi H, Sakurai E, Matsuno N, Tamaki T, Kozaki M. Clinical significance of glucocorticoid pharmacodynamics assessed by antilymphocyte action in kidney transplantation. Transplantation (Baltimore) 1994;57:1341–1348. doi: 10.1097/00007890-199405150-00010. [DOI] [PubMed] [Google Scholar]
- 35.Pagano G, Bruno A, Cavallo-Perin P, Cesco L, Imbimbo B. Glucose intolerance after short-term administration of corticosteroids in healthy subjects. Prednisone, deflazacort, and betamethasone. Arch Intern Med. 1989;149:1098–1101. [PubMed] [Google Scholar]
- 36.Reid IR, Heap SW. Determinants of vertebral mineral density in patients receiving chronic glucocorticoid therapy. Arch Intern Med. 1990;150:2545–2548. [PubMed] [Google Scholar]
- 37.Hansson A, Hehenberger K, Thorén M. Long-term treatment of Swiss 3T3 fibroblasts with dexamethasone attenuates MAP kinase activation induced by insulin-like growth factor-I (IGF-I) Cell Biochem Funct. 1996;14:121–129. doi: 10.1002/cbf.656. [DOI] [PubMed] [Google Scholar]
- 38.Maroder M, Farina AR, Vacca A, Felli MP, Meco D, Screpanti I, Frati L, Gulino A. Cell-specific bifunctional role of jun oncogene family members on glucocorticoid receptor-dependent transcription. Mol Endocrinol. 1993;7:570–584. doi: 10.1210/mend.7.4.8388998. [DOI] [PubMed] [Google Scholar]
- 39.Beato M, Arnemann J, Chalepakis G, Slater E, Willmann T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27:9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- 40.Dong Y, Poellinger L, Gustafsson J-A, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol. 1988;2:1256–1263. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- 41.Hosoda K, Nakao K, Tamura N, Arai H, Ogawa Y, Suga S, Nakanishi S, Imura H. Organization, structure, chromosomal assignment and expression of the gene encoding the human endothelin-A receptor. J Biol Chem. 1992;267:18797–18804. [PubMed] [Google Scholar]