Abstract
The differentiation of T helper (Th) cells is regulated by members of the signal transducer and activator of transcription (STAT) family of signaling molecules. We have generated mice lacking both Stat4 and Stat6 to examine the ability of Th cells to develop in the absence of these two transcription factors. Stat4, Stat6 −/− lymphocytes fail to differentiate into interleukin (IL)-4–secreting Th2 cells. However, in contrast to Stat4 −/− lymphocytes, T cells from Stat4, Stat6 −/− mice produce significant amounts of interferon (IFN)-γ when activated in vitro. Although Stat4, Stat6 −/− lymphocytes produce less IFN-γ than IL-12–stimulated control lymphocytes, equivalent numbers of IFN-γ–secreting cells can be generated from cultures of Stat4, Stat6 −/− lymphocytes activated under neutral conditions and control lymphocytes activated under Th1 cell–promoting conditions. Moreover, Stat4, Stat6 −/− mice are able to mount an in vivo Th1 cell–mediated delayed-type hypersensitivity response. These results support a model of Th cell differentiation in which the generation of Th2 cells requires Stat6, whereas a Stat4-independent pathway exists for the development of Th1 cells.
Keywords: T cell differentiation, interleukin 4, interleukin 12, signal transducer and activator of transcription
Stimulation of naive T cells results in their differentiation into effector cells with either a Th1 or Th2 phenotype. As Th1 cells develop, a number of genetic changes occur, including the loss of expression of IFN-γRβ chain (1, 2), the induced expression of a modified form of P-selectin glycoprotein ligand 1 (3, 4), and the priming for subsequent secretion of high levels of IFN-γ and TNF-β after T cell receptor stimulation (5). Similarly, as Th2 cells develop, there is a loss of expression of IL-12Rβ2 chain (6, 7), the induced expression of CCR3 (8), and the priming to secrete a different panel of cytokines after activation, including IL-4, IL-5, IL-10, and IL-13 (5).
Several lines of evidence have led to a paradigm of Th cell differentiation in which IL-12–mediated activation of signal transducer and activator of transcription (Stat)4 and IL-4–induced activation of Stat6 are critical for the generation of Th1 and Th2 cells, respectively. Mice harboring a disrupted gene for either IL-4 (9, 10), IL-4Rα (11), or Stat6 (12–14) fail to develop Th2 cells. Similarly, mice lacking either IL-12 (15), IL-12Rβ1 (16), or Stat4 (17, 18) demonstrate impaired Th1 cell function and, moreover, a propensity for T cells to develop into Th2 cells (15, 17).
Although these observations suggest that STAT proteins are important for the differentiation of Th cell subsets, they do not rule out the possibility that STAT-independent pathways may also exist. Stat6−/− lymphocytes are completely impaired in their ability to generate IL-4–secreting Th2 cells, suggesting that this differentiation pathway may be entirely dependent on Stat6. In contrast, reduced levels of IFN-γ are produced by Stat4−/− lymphocytes when cultured under conditions that promote Th1 cell differentiation, suggesting that Stat4 is not absolutely required for IFN-γ expression. Additionally, the cytokine environment during commitment to a Th cell lineage is clearly important, since IL-4 confers a dominant differentiation signal which inhibits Th1 cell differentiation even in the presence of IL-12 (19–22). These observations suggest that although the impaired development of Th1 cells in Stat4−/− mice may be due to the absence of Stat4, it may also be due in part to the preferential outgrowth of Th2 cells in response to IL-4–activated Stat6. Since IL-4 both activates Stat6 and interferes with the development of Th1 cells, we generated double-deficient mice lacking Stat4 and Stat6 to examine the ability of Th1 cells to develop in the absence of these two signaling molecules. Although lymphocytes lacking both Stat4 and Stat6 fail to differentiate into IL-4–secreting Th2 cells, they do give rise to functional IFN-γ–secreting Th1 cells both in vitro and in vivo.
Materials and Methods
Mice.
Stat4, Stat6 −/− mice were generated by mating Stat4 −/− and Stat6 −/− mice at the third backcross generation to Balb/c and intercrossing the resulting compound heterozygotes. Controls were inbred BALB/c and 129/SvJ mice, both of which gave comparable results in each of the assays tested.
In Vitro Culture and Differentiation.
Differentiation of T cells and assay of cytokines were performed as described (12, 17). In brief, cells were activated with 1 μg/ml plate-bound anti-CD3. Where indicated, IL-12 was added at 1 ng/ml, and anti–IFN-γ, anti–IL-4, and anti–IL-12 were added at 10 μg/ml (Genzyme Corp., Cambridge, MA). After 1 wk in culture, cells were washed and restimulated with plate-bound anti-CD3 for 24 h. Supernatants were harvested, and IFN-γ and IL-4 production were quantified by ELISA. Purification of CD4+ and CD62L+ cells was done by positive selection using magnetic beads (Miltenyi Biotec, Inc., Auburn, CA) according to the supplier's instructions. CD4+ isolated cells were ∼90% CD4+. For the purification of naive cells, pooled spleens and lymph nodes were depleted before positive selection with 2.4G2 (anti-CD16/32), 2.43 (anti-CD8), and M5114 (anti–I-Ad) coupled to magnetic beads.
Enzyme-linked Immunospot.
Cells were activated and cultured as above. After 1 wk, cells were restimulated with plate-bound anti-CD3 for 6 h and plated in dilution on anti–IFN-γ (R4/6A2)-coated Immobilon-P membranes in a 96-well plate for 18 h. Cells were washed away, and bound cytokine was detected with biotinylated anti–IFN-γ (PharMingen, San Diego, CA), avidin–alkaline phosphatase (Sigma Chemical Co., St. Louis, MO), and a stable BCIP/NBT mix (GIBCO BRL, Gaithersburg, MD). Spots were developed for 30–60 min, washed with distilled water, and counted under a dissecting microscope.
Delayed-type Hypersensitivity Reaction.
Male mice were immunized with 100 μg DNP-KLH (Calbiochem Corp., La Jolla, CA) emulsified in CFA (Sigma Chemical Co.), at the base of the tail. After 6 d, footpad thickness was measured using a spring-loaded caliper, and mice were challenged with 50 μg DNP-KLH in one foot and PBS in the contralateral appendage. Footpad thickness was measured blindly at 24 and 48 h after challenge. Specific swelling was determined by subtracting nonspecific swelling in the PBS-injected foot from antigen-induced swelling. At killing, popliteal and inguinal lymph nodes were removed and stimulated with 500 μg/ml DNP-KLH at a cell concentration of 106/ml for IFN-γ production and 2 × 105 for proliferation assay. Proliferation assays were pulsed with [3H]thymidine for the last 18 h of a 72-h incubation.
Results and Discussion
To further examine the mechanism by which Stat4 and Stat6 regulate the differentiation of Th cell subsets, we generated Stat4, Stat6 −/− mice by intercrossing compound heterozygotes. Double-deficient mice were produced at the expected mendelian frequency and were grossly indistinguishable from their control littermates. All lymphoid organs in Stat4, Stat6 −/− mice had normal cellularity and contained percentages of CD3+, CD4+, CD8+, and B220+ cells comparable to that seen in control littermates (data not shown).
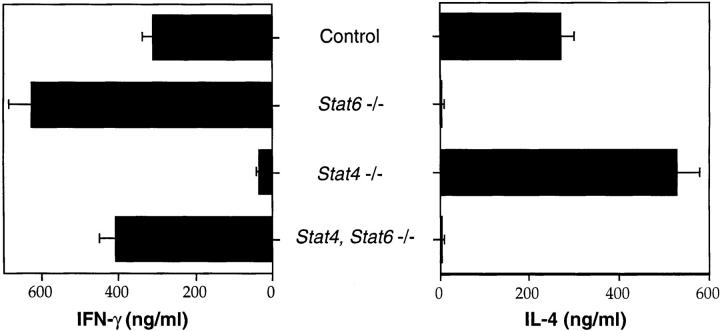
Spleen cells from control, Stat4 −/−, Stat6 −/−, and Stat4, Stat6 −/− mice were activated in vitro with plate-bound anti-CD3 in the absence of exogenous cytokines or antibodies to determine their developmental potential. After 1 wk in culture, cells were washed and restimulated with plate-bound anti-CD3 for 24 h. Supernatants were harvested, and IFN-γ and IL-4 production were quantified by ELISA. As expected, lymphocytes from control mice secrete both IFN-γ and IL-4 when activated in vitro under neutral conditions (Fig. 1). Consistent with previous results (12–14, 17, 18), Stat4 −/− lymphocytes produce high levels of IL-4 but greatly reduced levels of IFN-γ, whereas Stat6 −/− lymphocytes secrete high levels of IFN-γ but no IL-4. Stat4, Stat6 −/− lymphocytes also do not produce IL-4 when activated in vitro, supporting the notion that Stat6 is essential for the differentiation of Th2 cells. However, in contrast to Stat4 −/− lymphocytes, Stat4, Stat6 −/− lymphocytes secrete levels of IFN-γ comparable to that produced by control or Stat6 −/− lymphocytes cultured under identical conditions. Similar results were seen when cultures were either initiated with purified naive T cells obtained by selection of CD4+ CD62L+ cells or when CD4+ T cells were purified after the initial 7-d culture period and subsequently restimulated with anti-CD3 for 24 h (data not shown).
Figure 1.
Th cell differentiation of STAT-deficient lymphocytes. Spleen cells from mice with the indicated genotypes were activated with plate-bound anti-CD3 in the absence of exogenous cytokines or antibodies for 1 wk. Supernatants were collected 24 h after restimulation with plate-bound anti-CD3, and cytokine levels were quantified by ELISA.
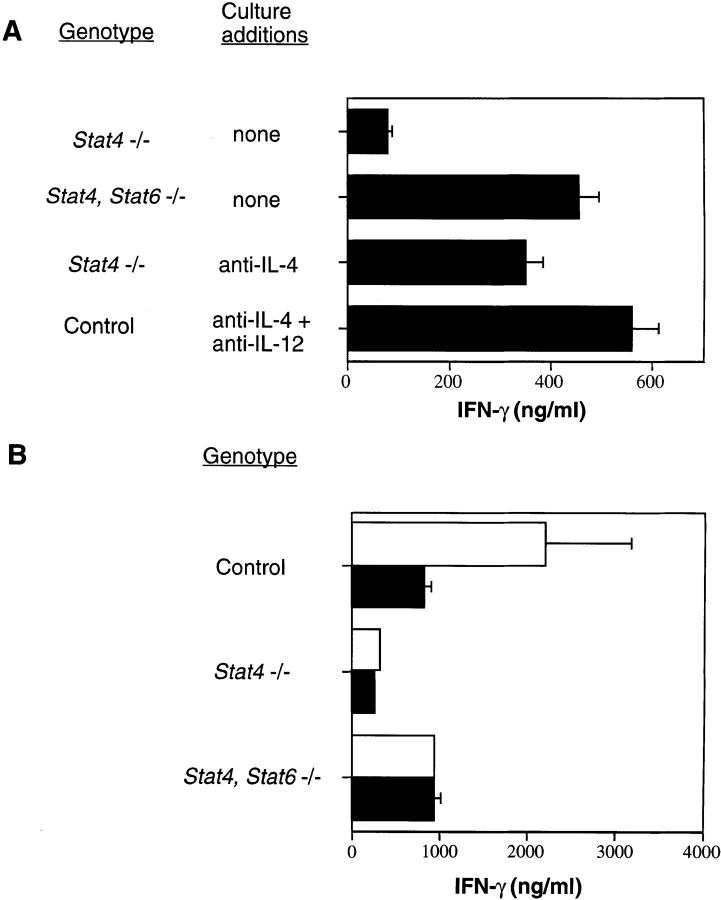
IL-4 signaling has a dominant effect over IL-12 stimulation in determining the outcome of Th cell differentiation (21), and the results shown in Fig. 1 suggest that the presence of IL-4 signaling through Stat6 may impair the ability of cells to develop the Th1 cell phenotype. To test this hypothesis, we attempted to recapitulate the IFN-γ–secreting phenotype of Stat4, Stat6 −/− lymphocytes by activating Stat6-expressing cells in vitro in the presence of cytokine-specific antibodies to eliminate STAT activation. In the absence of exogenous cytokines and antibodies, in vitro–activated Stat4, Stat6 −/− lymphocytes secrete more IFN-γ than Stat4 −/− lymphocytes (Figs. 1 and 2 A). When Stat4 −/− lymphocytes are activated in the presence of anti–IL-4, the levels of IFN-γ produced are nearly equal to those seen in cultures of activated Stat4, Stat6 −/− lymphocytes (Fig. 2 A). Similarly, when control cells are activated in the presence of anti–IL-4 plus anti–IL-12, the levels of IFN-γ produced are indistinguishable from those seen in cultures of activated Stat4, Stat6 −/− lymphocytes. These results are consistent with a recent report demonstrating IFN-γ production in the presence of neutralizing antibodies to both IL-4 and IL-12 (23). Thus, substantial amounts of IFN-γ can be produced by activated cells lacking Stat4 as long as Stat6 is not activated in response to IL-4 stimulation.
Figure 2.
IFN-γ production by STAT-deficient lymphocytes. (A) Spleen cells from mice with the indicated genotypes were activated with plate-bound anti-CD3 in the presence of antibodies as indicated for 1 wk. Cells were restimulated with plate-bound anti-CD3, and supernatants were collected 24 h later to quantify IFN-γ levels by ELISA. (B) Cells were activated as in A in the absence (white bars) or presence (black bars) of anti–IFN-γ. Cells were restimulated with plate-bound anti-CD3, and supernatants were collected 24 h later to quantify IFN-γ levels by ELISA.
IFN-γ has been suggested to have an important role in priming cells for subsequent IFN-γ production, although it remains controversial whether it does so by a direct or indirect mechanism. Inclusion of anti–IFN-γ to primary cultures of Stat4, Stat6 −/− lymphocytes activated under neutral conditions did not affect the amount of IFN-γ produced by these cells after restimulation with anti-CD3 (Fig. 2 B). This is in contrast to the lower amount of IFN-γ produced by control cells after restimulation when anti–IFN-γ was added in the primary culture. These results demonstrate that the ability of Stat4, Stat6 −/− lymphocytes to acquire an IFN-γ–secreting phenotype is not driven by endogenous IFN-γ, and are consistent with studies demonstrating that the role of IFN-γ in Th1 cell differentiation is indirect, upregulating IL-12Rβ2 expression so that cells become responsive to IL-12 stimulation (7).
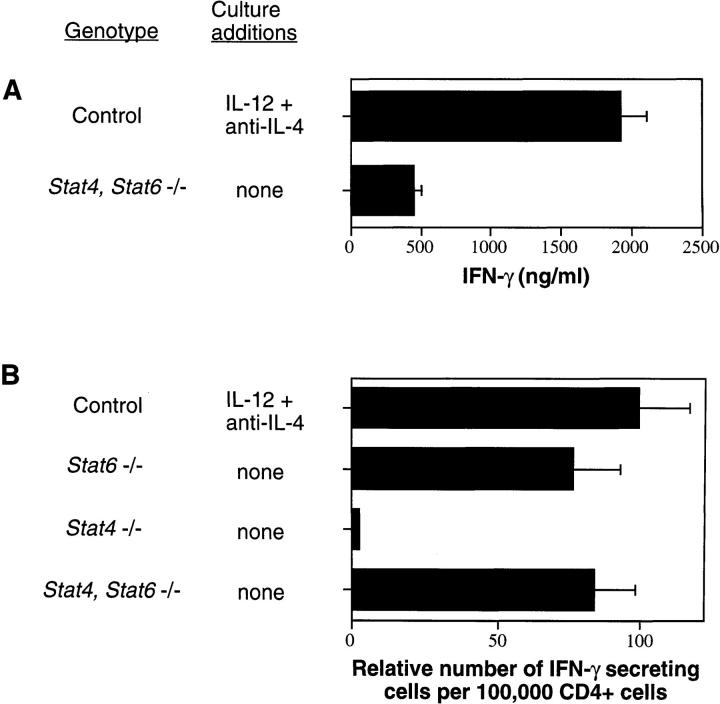
IL-12 stimulation of activated T cells has been shown to prime cells for high levels of IFN-γ production after subsequent restimulation (24). When compared with control lymphocytes cultured under Th1 cell–promoting conditions (IL-12 plus anti–IL-4), the levels of IFN-γ produced by activated Stat4, Stat6 −/− lymphocytes were decreased four- to fivefold (Fig. 3 A). The amount of IFN-γ produced by Stat4, Stat6 −/− lymphocytes cultured in the presence of IL-12 does not increase over that seen when no exogenous cytokines are added (data not shown). The increased amounts of IFN-γ seen in cultures of Th1 cell–skewed control lymphocytes relative to those seen in cultures of Stat4, Stat6 −/− lymphocytes could be the result of an increase either in the number of differentiated Th1 cells or in the amount of IFN-γ produced on a per cell basis. To distinguish between these possibilities, the number of IFN-γ–secreting lymphocytes in these cultures was quantified by enzyme-linked immunospot (ELISPOT). Spleen cells from control mice were differentiated into Th1 cells in vitro by activation with plate-bound anti-CD3 in the presence of IL-12 and anti–IL-4 while spleen cells from Stat4 −/−, Stat6 −/−, and Stat4, Stat6 −/− mice were activated in the absence of exogenous cytokines and antibodies. After 1 wk in culture, CD4+ T cells were purified and restimulated with plate-bound anti-CD3 for 6 h. Cells were then transferred in dilution to plates containing anti–IFN-γ (R4/6A2)- coated Immobilon-P membranes at the bottom of the wells and incubated overnight. In contrast to cultures of Stat4 −/− lymphocytes where very few IFN-γ–secreting cells were detected, large numbers were seen in cultures of Th1 cell– skewed control and unskewed Stat6 −/− lymphocytes (Fig. 3 B). Strikingly, cultures of Stat4, Stat6 −/− lymphocytes had numbers of IFN-γ–secreting cells comparable to that seen in cultures of Th1 cell–skewed control and unskewed Stat6 −/− lymphocytes. The spots produced in wells containing Th1 cell–skewed control cells were both larger and darker than those seen in wells containing Stat4, Stat6 −/− lymphocytes, supporting the conclusion that IL-12 stimulation and Stat4 activation lead to higher levels of IFN-γ production on a per cell basis rather than a substantial increase in the number of differentiated Th1 cells.
Figure 3.
Quantification of IFN-γ–secreting cells. Spleen cells from mice with the indicated genotypes were activated with plate-bound anti-CD3 in the presence of exogenous cytokines or antibodies as indicated for 1 wk. (A) Cells were restimulated with plate-bound anti-CD3, and supernatants were collected 24 h later to quantify IFN-γ levels by ELISA. (B) Cells were restimulated with plate-bound anti-CD3 for 6 h and then transferred to plates containing anti–IFN-γ (R4/6A2)-coated membranes for 18 h. ELISPOTs were developed with a stable BCIP/NBT reagent and counted under a dissecting microscope. Th1-skewed control cultures had 750–1,000 IFN-γ–secreting cells/105 CD4+ cells.
Given that IFN-γ–secreting cells could be generated from Stat4, Stat6 −/− mice in vitro, we sought to determine whether Stat4, Stat6 −/− mice could mount a Th1 cell response in vivo. To investigate this possibility, we examined a classic Th1 cell–mediated immune response, the delayed-type hypersensitivity (DTH) reaction. Control, Stat4 −/−, and Stat4, Stat6 −/− mice were immunized with 100 μg DNP-KLH in CFA. 6 d later, mice were challenged by footpad injection with either aqueous antigen or PBS as control. All animals were killed 48 h after challenge, and draining popliteal and inguinal lymph node cells were removed and restimulated in vitro with antigen. Lymphocytes from all three genotypes of mice produced a similar in vitro proliferative response to antigen (Fig. 4 A), demonstrating comparable in vivo generation of antigen-specific T cells. As expected, the levels of IFN-γ produced after antigen stimulation in vitro varied among the groups. Stat4 −/− lymphocytes produced <10% of the IFN-γ secreted by control cells (Fig. 4 B). Stat4, Stat6 −/− lymphocytes also secreted less IFN-γ than control cells, although the levels were fourfold higher than that seen in cultures of Stat4 −/− lymphocytes (Fig. 4 B). Finally, footpad thickness was measured before and 48 h after challenge, and specific swelling was calculated (Fig. 4 C). Footpad swelling was significantly different in all three groups using the Kruskal-Wallis test (P < 0.005). Furthermore, pairwise comparisons using the Wilcoxon-Mann-Whitney test showed that swelling in Stat4 −/− mice was significantly reduced compared with control (P < 0.005) and Stat4, Stat6 −/− mice (P < 0.05). Thus, functional Th1 cells capable of eliciting a DTH response can be generated in vivo in Stat4, Stat6 −/− mice.
Figure 4.
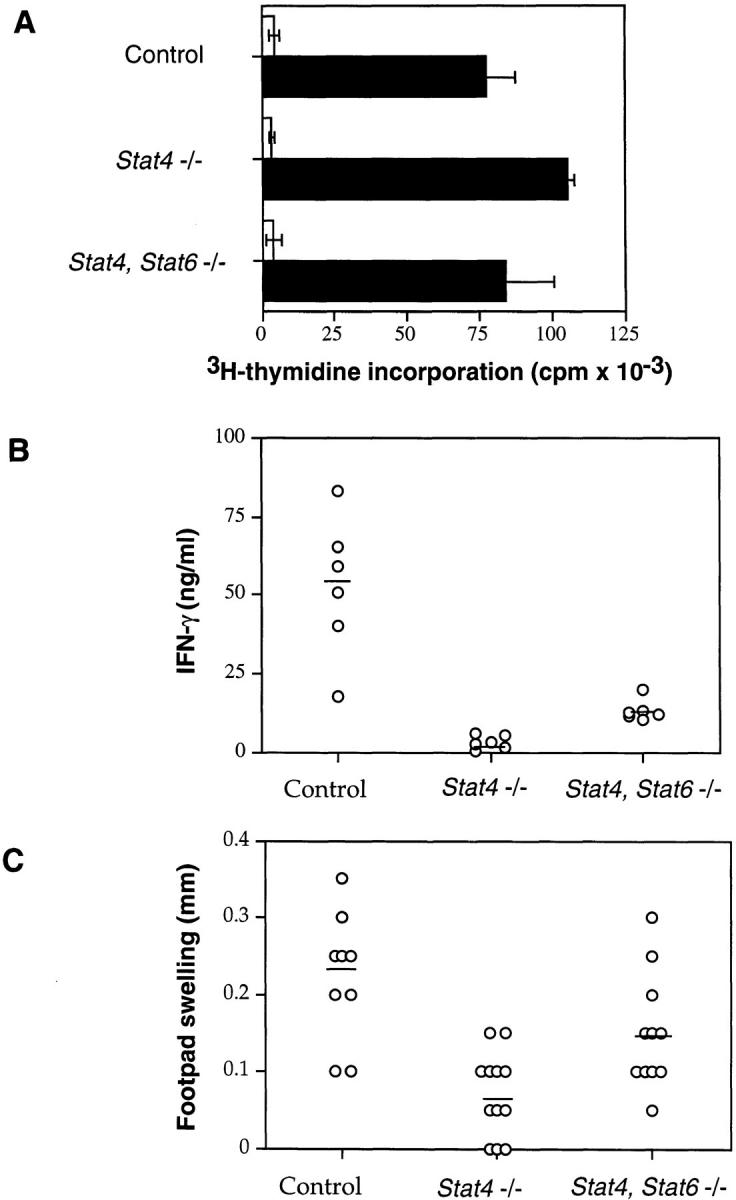
DTH responses in STAT-deficient mice. (A) Draining lymph node cells from immunized mice were stimulated in vitro in the absence (white bars) or presence (black bars) of 500 μg/ml DNP-KLH. Cells in round-bottomed 96-well plates were pulsed with [3H]thymidine for the last 18 h of a 72-h incubation. (B) Supernatants from DNP-KLH– stimulated lymph node cells were analyzed for IFN-γ levels by ELISA. (C) Specific footpad swelling of each mouse was determined by subtracting nonspecific swelling in the PBS-injected foot from the DNP-KLH– induced swelling. Each data point represents one mouse; horizontal lines indicate averages, and results are pooled from two independent experiments.
Our results demonstrate that the mechanisms that regulate the differentiation of Th1 and Th2 cells are not the same. Previous studies have shown that Stat6 is essential for the development of Th2 cells (12–14), presumably through the transcriptional regulation of as yet unidentified genes involved in the differentiation process. As IL-4–secreting Th2 cells are not generated from Stat4, Stat6 −/− lymphocytes, Stat6 is required for their generation even in the absence of Stat4. In contrast, both Stat4-dependent and -independent pathways appear to exist for the development of IFN-γ–producing Th1 cells. IL-12 stimulation and Stat4 activation lead to the differentiation of Th1 cells capable of secreting large amounts of IFN-γ. However, in the absence of IL-4 and IL-12–mediated signals, the natural tendency of CD4+ Th cells is to develop into IFN-γ–secreting cells. Thus, IL-4–mediated activation of Stat6, but not IL-12– induced activation of Stat4, results in a differentiative signal to Th cells. Mechanistically, this would explain the observation that IL-4 signaling has a dominant effect over IL-12 stimulation in determining the outcome of Th cell differentiation (21). Interestingly, a paradigm whereby the decision between two possible developmental pathways is dictated by the presence or absence of one signaling cascade occurs in other differentiative processes, including sex determination. Sry expression activates a genetic program which results in male gonadal development; female gonads develop in its absence (25).
Although our results suggest that the ability of IL-12 to promote Th1 differentiation may be indirect, by priming for high levels of IFN-γ production which would inhibit Th2 development and allow the outgrowth of Th1 cells, they do not minimize the role of IL-12 and Stat4 activation in normal Th cell development and function. Stat4 activation is clearly involved in regulating the production of IFN-γ and potentially other factors such as TNF-β that are important in many Th1 cell–mediated responses. Nevertheless, the demonstration that functional Th1 cells can develop in the absence of Stat4 has important implications for the design of therapeutic strategies that aim to alter immune function by targeting STAT proteins in vivo.
Acknowledgments
We thank M. Gately for IL-12, Y. Han for technical assistance, J. Whalen for advice on DTH responses, R. Bosch for help with statistical analysis, and L. Glimcher and members of the laboratory for critical reading of the manuscript.
Footnotes
M.H. Kaplan is a Special Fellow and M.J. Grusby is a Scholar of the Leukemia Society of America. This work was supported by a grant from the National Institutes of Health to M.J. Grusby (RO1 AI-40171), and by a gift from the Mathers Foundation.
References
- 1.Bach EA, Szabo SJ, Dighe AS, Ashkenazi A, Aguet M, Murphy KM, Schreiber RD. Ligand-induced autoregulation of IFN-γ receptor β chain expression in T helper cell subsets. Science. 1995;270:1215–1218. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 2.Pernis A, Gupta S, Gollob KJ, Garfein E, Coffman RL, Schindler C, Rothman P. Lack of interferon-γ receptor β chain and the prevention of interferon-γ signaling in Th1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 3.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 4.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 6.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 7.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 10.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 11.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)- independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DAA, et al. Lack of IL-4 induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S-I, Nakanishi K, Yoshido N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 15.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu C-Y, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 16.Wu C-Y, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)- deficient mice. IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- 17.Kaplan MH, Sun Y-L, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 18.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DAA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12 mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 19.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 20.Hsieh C-S, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Hu-Li J, Seder RA, De St. Groth BF, Paul WE. Interleukin 4 suppresses interleukin 2 and interferon γ production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc Natl Acad Sci USA. 1993;90:5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu-Li J, Huang H, Ryan J, Paul WE. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon γ production is cytokine-dependent. Proc Natl Acad Sci USA. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. . Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]