Abstract
To generate a vaccine to protect against a variety of human pathogenic fungi, we conjugated laminarin (Lam), a well-characterized but poorly immunogenic β-glucan preparation from the brown alga Laminaria digitata, with the diphtheria toxoid CRM197, a carrier protein used in some glyco-conjugate bacterial vaccines. This Lam-CRM conjugate proved to be immunogenic and protective as immunoprophylactic vaccine against both systemic and mucosal (vaginal) infections by Candida albicans. Protection probably was mediated by anti-β-glucan antibodies as demonstrated by passive transfer of protection to naive mice by the whole immune serum, the immune vaginal fluid, and the affinity-purified anti-β-glucan IgG fractions, as well as by administration of a β-glucan–directed IgG2b mAb. Passive protection was prevented by adsorption of antibodies on Candida cells or β-glucan particles before transfer. Anti-β-glucan antibodies bound to C. albicans hyphae and inhibited their growth in vitro in the absence of immune-effector cells. Remarkably, Lam-CRM–vaccinated mice also were protected from a lethal challenge with conidia of Aspergillus fumigatus, and their serum also bound to and markedly inhibited the growth of A. fumigatus hyphae. Thus, this novel conjugate vaccine can efficiently immunize and protect against two major fungal pathogens by mechanisms that may include direct antifungal properties of anti-β-glucan antibodies.
Fungal diseases are a major infectious threat that demands increasing medical attention. A few invasive mycoses (e.g., histoplasmosis and coccidiomycosis) are geographically limited, but of special concern are the worldwide infections caused by opportunistic fungal agents in immunocompromised hosts. In particular, candidiasis and aspergillosis are common in hospitalized patients and carry a high mortality toll even in the presence of effective therapy (1). Early and accurate diagnosis of these systemic infections is often difficult because of the nonspecificity of clinical symptoms and lack of standardized diagnostic tools. Moreover, antifungal therapy may be frustrated by toxicity and emergence of resistance (2).
A prophylactic and/or therapeutic vaccine would be the safest and most effective means to meet this pressing medical need. Thus, different approaches to antifungal vaccination are being developed that, in analogy with bacterial and viral vaccines, are based on the use of one or more antigens, including whole inactivated cells, specific for each particular fungus (3, 4). However, no such vaccine is currently available. In none of the vaccine formulations under study has β-glucan, a polysaccharide that is critical for fungal viability and is present in all human pathogenic fungi, been considered as vaccine component.
Previous circumstantial evidence indicates that intact cells of Candida albicans (5) and Saccharomyces cerevisiae (unpublished data) treated to expose glucan rather than mannoprotein on the cell surface confer a substantial degree of anti-Candida protection. Therefore we have considered that a vaccine composed of β-glucan would best fit the medical need, possibly allowing simultaneous immunization against more than one fungal infection. To test the strength of this novel approach and to avoid any likely contamination with other fungal antigens, a β-glucan of nonfungal source, i.e., laminarin (Lam), a rather well-characterized β(1, 3) glucan preparation from the brown alga Laminaria digitata (6), was selected as the immunizing antigen. Because laminarin, like most free polysaccharides, is a poor immunogen, it was conjugated with the diphtheria toxoid CRM197, a protein carrier safely used in other human vaccines (7, 8). This novel glyco-conjugate has been tested extensively as an immunoprophylactic vaccine against experimental systemic and mucosal infections by C. albicans, a fungal pathogen of particular medical relevance. Initial investigations with Aspergillus fumigatus support the concept that our conjugate vaccine may prove indeed effective against multiple agents of opportunistic fungal infections.
Results
Generation and characterization of the Lam-CRM conjugate
Several batches of Lam-CRM and other CRM conjugates with a soluble β-glucan extract of C. albicans (glucan ghosts [GG]–zymoliase [Zym]) were prepared by the three-step reaction described in the Methods section. The saccharide/protein ratio of the Lam-CRM conjugate ranged between 0.57 and 0.75; for the GG-Zym conjugate it was ∼0.20, indicating a low conjugation efficiency. All the experiments with the Lam-CRM conjugate were performed using the most glycosylated batch.
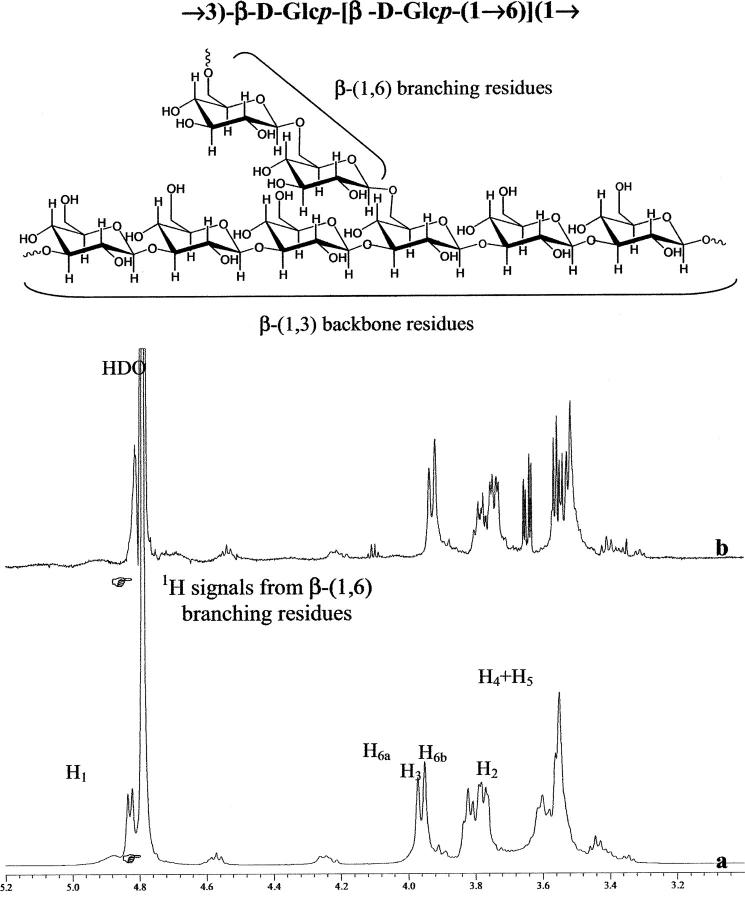
Nuclear magnetic resonance (NMR) spectroscopic techniques were used to evaluate the integrity of the linked saccharide chains in the bulk conjugate vaccines. Proton NMR spectra of the Lam-CRM conjugate showed a superimposition of relatively sharp signals originating from the saccharide component and broader peaks arising from the protein carrier. The assignment of the saccharide peaks, situated between 3.0 and 5.5 ppm (ppm), was accomplished with two-dimensional correlation spectroscopy experiments and by comparison with published data (9–11). Signals originating from the carrier protein were observed at <2.8 ppm (aliphatic region) and at >6.5 ppm (aromatic region) and were broader because the protein has less mobility than the saccharide moieties. For a direct comparison, the proton NMR spectra of unconjugated laminarin and Lam-CRM conjugate are shown in Fig. 1, a and b. A simple visual observation of the relevant part of the spectra demonstrates the presence of intact, saccharide-attributable signals in the Lam-CRM conjugate.
Figure 1.
Partial 600 MHz NMR proton spectra of (a) laminarin polysaccharide and (b) laminarin-CRM197 conjugate recorded at 25°C. Molecular structure of laminarin and labels are shown for the NMR peak assignment.
The Lam-CRM conjugate is immunogenic and protective against systemic candidiasis in mice
Initial experiments served to optimize dose, route, and schedule of vaccine administration. In the typical experiment (repeated three times), CD2F1 mice were immunized with the Lam-CRM conjugate through a priming s.c. injection of 7 μg of conjugate (as polysaccharide equivalent) in complete Freund adjuvant, followed 1 wk later by an i.p. booster of the same amount of adjuvant-free conjugate. Mice injected adjuvant and the carrier protein or Lam only, or adjuvant and saline only (hereafter referred to as nonimmunized or adjuvant-treated), served as controls. 2 wk after the booster, some animals were bled to measure serum antibodies, and others were challenged with 2 LD50 of C. albicans and monitored for mortality and fungus burden in the kidney for a period of 60 d.
After administration of the Lam-CRM conjugate, IgM and IgG antibodies against β(1, 3), laminarin-like, or β(1, 6), pustulan-like, glucans were generated (Fig. 2 a). No antibodies against mannoproteins of C. albicans were detected (Fig. 2 a). No anti-β-glucan antibodies were present in any control animals, with the exception of inconsistent, low-titer antibodies (never > than 1:100 serum dilution) exclusively of IgM class in mice given laminarin only (unpublished data). Comparable, high levels of anti-CRM antibodies (titers always in excess of 5 log serum dilution) were detected in both Lam-CRM– and in CRM-immunized mice (unpublished data).
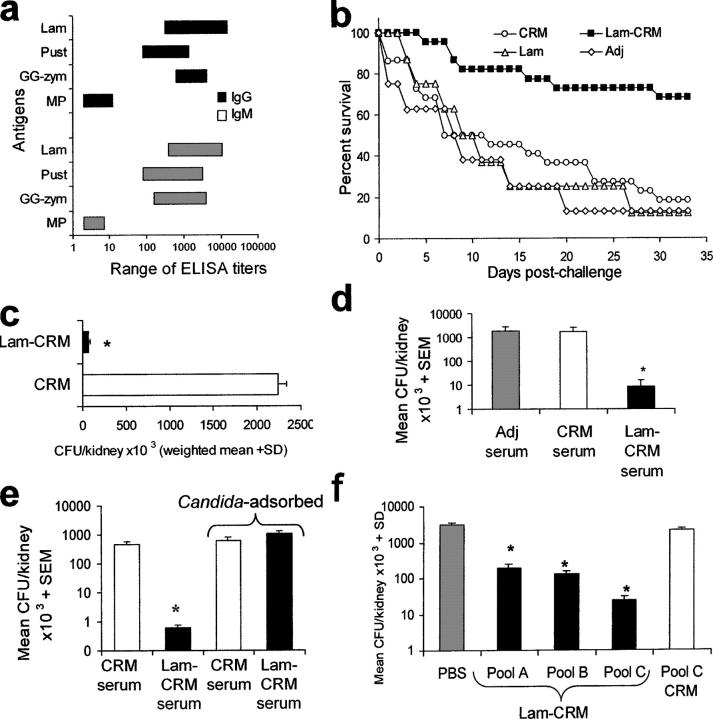
Figure 2.
Vaccination with the Lam-CRM conjugate induces antibody-mediated anti-Candida protection in a murine experimental model of disseminated infection. (a) Anti-β-glucan IgG and IgM titers in Lam-CRM–vaccinated mice. The graph shows the ranges of ELISA titers against the indicated antigens measured in five groups of 6–12 mice (for a total of 46 animals) independently immunized with Lam-CRM. MP, mannoproteins; Pust, pustulan. (b) Survival rates of mice immunized with Lam or CRM or with the Lam-CRM conjugate, as compared with nonimmunized mice (Adj), after a lethal systemic challenge with C. albicans (cumulative data from three independent experiments and 28 mice per group). (c) Fungal burden in the kidneys from four Lam-CRM–vaccinated or four control CRM-vaccinated mice on day 2 after i.v. infection with C. albicans. (d) Number of fungal CFU in kidneys from naive mice given a single administration of anti-Lam-CRM, anti-CRM, or nonimmune (Adj) serum 2 h before an i.v. challenge with C. albicans. Data are from three independent experiments with a total of nine mice per group. (e) Reversal of the passive protection after serum adsorption with Candida cells. The experiment was performed with three mice per group. (f) Effect of the passive vaccination with Protein A affinity–separated fractions of the Lam-CRM serum on Candida kidney load. Data are from three mice per group. Some details on isotype and subclass of anti-β-glucan immunoglobulin in pool A, B and C are given in Fig. S2.
Because in all three experiments performed with this immunization schedule the protection against the lethal fungal challenge was extremely consistent, with a lack of statistical heterogeneity, all the data are pooled in the Kaplan-Meyer graph of Fig. 2 b. The figure shows that the Lam-CRM–immunized mice, but not the CRM- or Lam-immunized ones, were significantly protected from the lethal systemic challenge by C. albicans, as compared with the nonimmunized (adjuvant-treated) mice. Protection in Lam-CRM–vaccinated mice was verified both in terms of median survival time (P < 0.001) and overall mortality (P < 0.001; Fig. 2 b), as well as in terms of dramatic reduction of the fungal cells in the kidney (P < 0.001; Fig. 2 c).
We therefore asked whether the anti-β-glucan antibodies generated by the Lam-CRM vaccine were instrumental in the observed protection. To this aim, a single dose of the serum from Lam-CRM–immunized mice or, as control, the serum from nonimmunized or CRM-immunized mice, was given i.p. to naive animals 2 h before Candida challenge, and the fungus burden in the kidney was assessed on day 2 after challenge. As shown in Fig. 2 d, only the serum from Lam-CRM–immune animals was capable of reducing the fungal load (nearly 3 logs) in the mouse kidney compared with the infectious burden in nonimmunized or CRM-immunized mice. Moreover, this rather dramatic reduction in the fungus burden was abolished totally by serum adsorption on Candida cells (Fig. 2 e).
Because immune sera contain other nonimmunoglobulin constituents that may affect Candida in vivo, the immunoglobulin fraction was separated and purified by affinity chromatography on Protein A, which resulted into three pooled serum fractions (pools A, B and C) with differential content of anti-β-glucan IgM and IgG classes. In particular, pools A and B were composed almost exclusively of IgM and IgG1 antibodies, respectively, whereas pool C contained IgG1 as well as IgG2a, IgG2b, and IgG3 (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20050749/DC1). When these fractions were used for passive protection experiments, all were able to cause some reduction of the fungus burden in the kidney (Fig. 2 f). However, pool C, which was the only one containing appreciable IgG2 and IgG3 isotypes and a much lower IgG1 titer than pool B, was the most protective, causing an approximate 2-log reduction in fungal burden (Fig. 2 f). As a further control, an IgG2- and IgG3-enriched pool C fraction prepared from the serum of animals immunized with CRM only did not provide any reduction of the fungal load in the kidney (Fig. 2 f). Overall, these data support the concept that the protection conferred upon mice by the Lam-CRM vaccine was mediated to a great extent, if not entirely, by anti-β-glucan antibodies, particularly, although not exclusively, those belonging to the IgG class (see also the following sections of Results).
The Lam-CRM conjugate is immunogenic and protective against estrogen-dependent vaginal candidiasis in rats
Systemic infections by Candida usually occur in settings quite different from those typical of mucosal infections by the same fungus, which, besides the AIDS patients, may also affect to a large extent nonpatently immunodepressed subjects, as, for instance, women with chronic vaginal candidiasis (12). The fungus virulence and the immune mechanisms of the fungus are different in the two settings, so protection against mucosal infection cannot be anticipated by the results of a protective systemic vaccination (13). Therefore, we asked whether the Lam-CRM vaccine was endowed with immunogenic and protective potential in a model of rat vaginal candidiasis, which has some similarities to the human vaginal disease and in which the rate of fungal clearance from the vaginal cavity can be measured. As shown in Fig. 3 a, rats intravaginally immunized with the Lam-CRM vaccine and a mucosal adjuvant exhibited a significantly accelerated rate of Candida clearance from the vagina as compared with the controls (P < 0.01) at any time point except day 0, with full clearance 3 wk after challenge, several days before the clearance observed in controls. In the vaginal fluid of Lam-CRM–vaccinated rats, IgG against laminarin, against the Candida glucan digest GG-Zym, and, at lower titer, against pustulan, were present (Fig. 3 b). Intranasal instead of intravaginal immunization with the vaccine induced a similar pattern of anti-β-glucan antibodies in vaginal fluids and a comparable degree of protection against the experimental vaginal infection (not depicted). Immune vaginal fluids were able to transfer protection to nonimmunized rats, as shown by the significant acceleration of the fungus clearance as compared with the animals given normal vaginal fluid (Fig. 3 c). The accelerated clearance of the fungus conferred by the administration of Lam-CRM–immune vaginal fluids was remarkably similar to that achieved in this model by treatment with fluconazole, an active anticandidal drug (Fig. 3 c), and was almost completely abolished by fluid adsorption on insoluble β-glucan particles from C. albicans. Thus, the Lam-CRM vaccine confers protection against the experimental vaginal candidiasis, and this protection seems to be mediated by vaginal anti-β-glucan antibodies of the IgG class.
Figure 3.
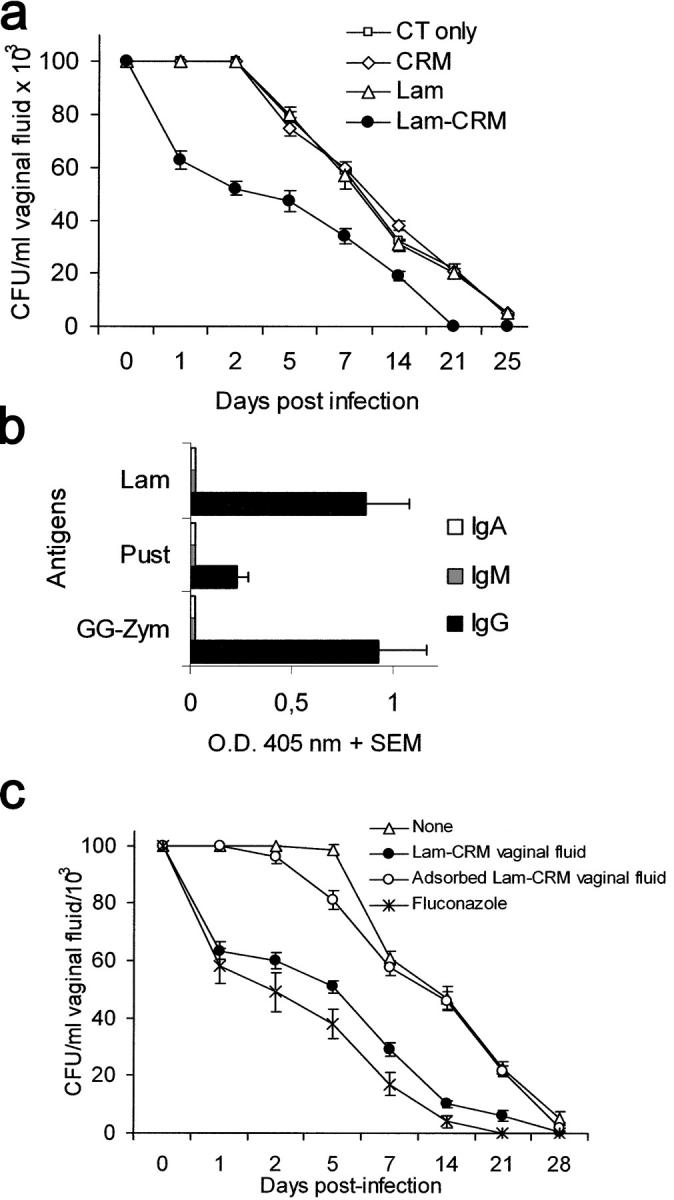
Antibodies raised by the vaccination with the Lam-CRM conjugate significantly accelerate the resolution of a rat vaginal infection with C. albicans. (a) Vaginal fungal clearance in rats vaccinated intravaginally with the Lam-CRM conjugate, with unconjugated CRM or Lam, or left immunized (CT) were intravaginally infected with C. albicans. Figure shows mean values plus SD of CFU counts from individual animals, measured in two independent experiments with a total of 10 animal per group (b) Anti-β-glucan antibodies in vaginal fluids from Lam-CRM–vaccinated rats. Results are expressed as mean OD 405 nm plus SEM readings of ELISA assays against the indicated solid-phase β-glucan antigens by two pooled vaginal fluids, obtained in two independent experiments from a total of 10 rats. OD values measured from CRM-vaccinated control rats (usually <0.15) have been subtracted. Pust, pustulan. (c) Course of the experimental vaginal infection in rats administered whole or β-glucan particle–adsorbed vaginal fluids from Lam-CRM–vaccinated animals, as compared with rats given fluconazole therapy. Results are expressed as mean CFU plus SD measured from five animals per group.
Passive protection with an anti-β-glucan mAb
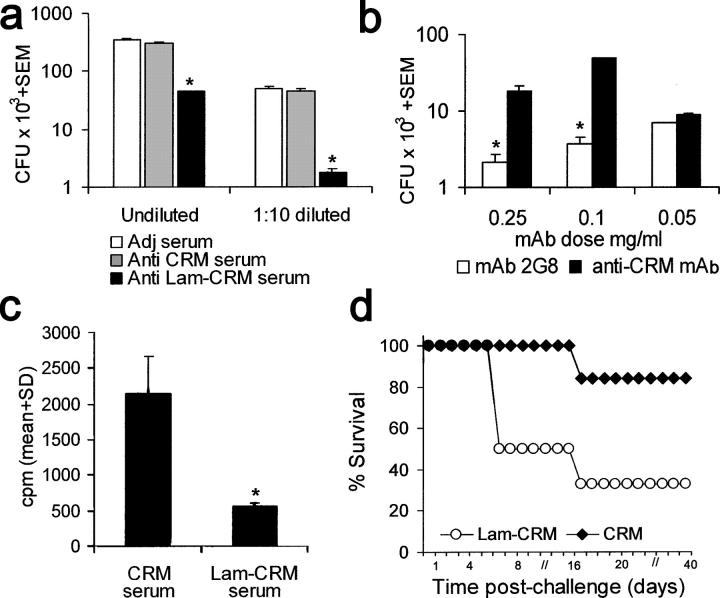
These data pointed out that anti-β-glucan antibodies, in particular those of the IgG class, were instrumental in the protection conferred by the Lam-CRM vaccine in both systemic and mucosal candidiasis. In an attempt to support this notion further, mAbs directed against β-glucan and, as control, against the carrier CRM197 protein were generated. One (mAb 2G8; IgG2b isotype) recognized both laminarin and, with lower reactivity, pustulan, as well as β-glucans from either C. albicans and S. cerevisiae, but not α(1,6) glucan (dextran) configurations or mannan (Fig. 4 a). This mAb was tested for its capacity to confer passive protection against Candida infection. In the systemic model, a single i.p. administration of the mAb 2G8 2 h before an i.v. challenge with the same fungal dose as the one used for testing passive protection by Lam-CRM serum conferred significant protection, averaging a >1-log decrease in the fungus kidney burden, as compared with that measured in control mice given the irrelevant, isotype-matched anti-CRM mAb (P < 0.01, Fig. 4 b). Similarly, in the rat model of experimental vaginal candidiasis, a single intravaginal administration of mAb 2G8 caused a significant acceleration of the fungus clearance, with resolution of the infection on day 21, in comparison with control animals treated with the irrelevant anti-CRM mAb (Fig. 4 c).
Figure 4.
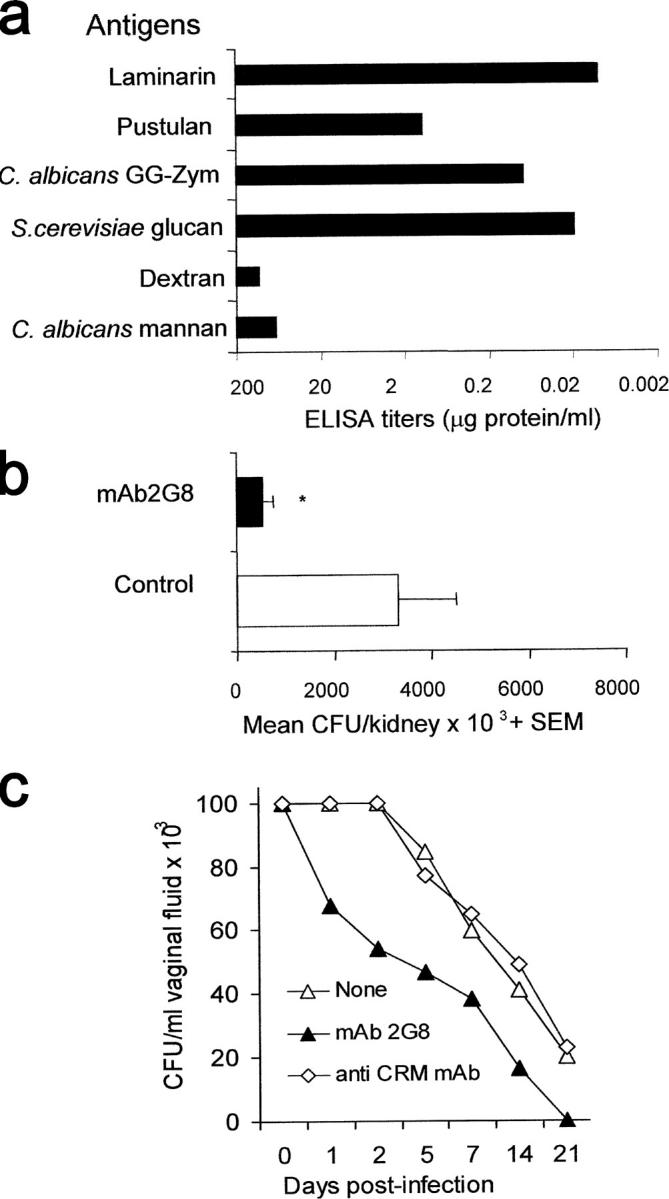
Specificity and anti-Candida protective activity by mAb 2G8, a murine anti-β-glucan, mAb. (a) ELISA reactivity of mAb 2G8 with various β-glucan or non–β-glucan polysaccharides. (b) Candida load in the kidney of mice given as a single i.p. administration of the mAb 2G8 or of the irrelevant anti-CRM mAb (Control) and subjected to a systemic challenge with C. albicans. Results are from three independent experiments with a total of 10 mice/group. (c) Vaginal clearance of C. albicans in rats (five per group) administered the anti-β-glucan mAb 2G8, the irrelevant anti-CRM mAb, or none and intravaginally infected with the fungus. Data are mean values of CFU counts from individual vaginal fluids. SD was always <15%.
Overall, the reduction in fungus burden in the systemic infection conferred by treatment with mAb 2G8 was lower than that conferred by an equal volume of the whole immune serum or its pool C IgG-rich fraction (compare the relevant parts of Figs. 3 and 4), even though mAb 2G8 had higher anti-laminarin titer (>1:20,000) than either serum (1:800) or pool C fraction (1:3000). Conversely, the passive protection conferred by the same mAb in the model of mucosal infection was substantially comparable to the protection conferred by transfer of the immune vaginal fluid.
Antibody binding and growth inhibition of C. albicans and A. fumigatus hyphae
A variety of mechanisms have been shown to be involved in the protective effects of antifungal antibodies, substantially matching similar mechanisms of antibacterial protection (14, 15). Because β-glucan is a viability-critical cell wall component of pathogenic fungi (16), and there is evidence that some β-glucan–binding toxins are fungicidal (17, 18), we asked whether the anti-β-glucan antibodies generated by Lam-CRM vaccination could exert a direct anti-Candida effect. Thus, we first examined the pattern of antibody binding to fungal cells and then tested for any growth inhibition exerted by the antibodies during in vitro assays.
Microscopic observations after immunofluorescence staining with both the anti-Lam-CRM serum and mAb 2G8 showed that the serum, which expectedly was strongly reactive with isolated β-glucan fungal particles (Fig. 5 a), bound preferentially to the germ tubes and hyphae of C. albicans and particularly to hyphal tips and septa (Fig. 5, b and c and b1 inset). Essentially the same pattern of C. albicans fluorescence was observed with mAb 2G8 (Fig. 5, d–f). Conidial wall of short and long hyphal threads of A. fumigatus also fluoresced equally when reacting with either the Lam-CRM serum (Fig. 5, g and h) or mAb 2G8 (Fig. 5 i). In both fungi, the fluorescence was not distributed uniformly along the hyphae (Fig. 5, f, h, and i), and, particularly in C. albicans, some septal regions were strongly fluorescent with both antibodies (Fig. 5, b and e). Weak and cell-to-cell variable fluorescence was observed on C. albicans yeast cells, with some exceptions in discrete cell wall areas, probably corresponding to cell wall scars, where abundant, β-glucan-rich new cell wall accumulates (Fig. 5 b, inset b1). No reaction at all was observed in either fungus upon treatment with CRM-immune serum or anti-CRM mAb (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20050749/DC1).
Figure 5.
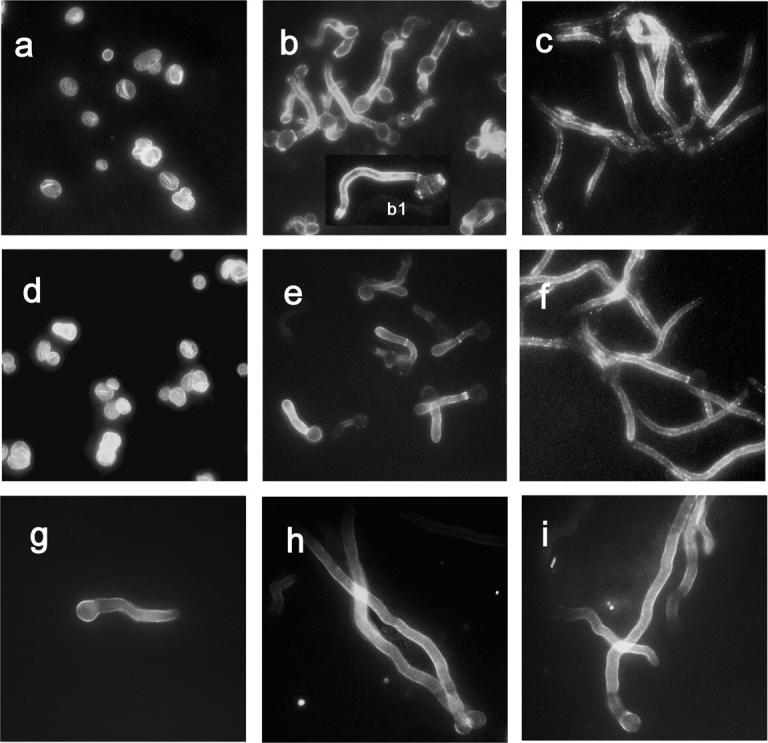
Expression of anti-Lam-CRM serum– and mAb 2G8– reactive epitopes on the cell surface of C. albicans and A. fumigatus. (a and d) Indirect immunofluorescence staining of isolated β-glucan cell wall ghosts of C. albicans with the anti-Lam-CRM serum (a) or the mAb 2G8 (d). (b and e) Pattern of immunofluorescence reactivity in C. albicans germ tubes stained with the anti-Lam-CRM serum (b) or the mAb 2G8 (e). The b1 inset shows the preferential staining of the hyphal cell. (c and f) Hyphal filaments of C. albicans reacted with anti-Lam-CRM serum (c) or mAb 2G8 (f). (g) Immunofluorescence staining by the anti-Lam-CRM serum on a germinated conidium of A. fumigatus. (h and i): Reactivity of the anti-Lam-CRM serum (h) and of the mAb 2G8 (i) with A. fumigatus hyphae. Negative control staining of fungal cells is shown in Fig. S2.
Therefore, both the Lam-CRM serum and mAb 2G8 were tested in growth-inhibition experiments in vitro. Because of the difficulties in measuring CFU of growing Aspergillus hyphae, a growth-inhibition assay based on radioactive glucose incorporation was used for this fungus, as previously reported for Fusarium spp (19). As shown in Fig. 6 a, the Lam-CRM immune serum (but none of the irrelevant antibody-containing sera) was able to inhibit hyphal growth of C. albicans significantly, and the growth inhibition seemed to be greater when the immune serum was slightly diluted. Hyphal growth inhibition was also observed with the use of mAb 2G8, apparently in a dose-dependent fashion (Fig. 6 b). The Lam-CRM immune serum also strongly inhibited the growth of A. fumigatus, (averaging ∼75% inhibition in four independent experiments; Fig. 6 c). The inhibitory effect on hyphal growth of A. fumigatus was evident both on addition of immune serum to conidia and on its addition to preformed hyphae (Table S1, available at http://www.jem.org/cgi/content/full/jem.20050749/DC1). Direct microscopic observations (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20050749/DC1) confirmed the functional inhibitory data presented here.
Figure 6.
In vitro growth of C. albicans and A. fumigatus is significantly restricted by the anti-β-glucan antibodies, and Lam-CRM vaccination significantly prolongs the survival of mice subjected to a systemic challenge with A. fumigatus. (a) CFU number in C. albicans cultures grown overnight in the presence of whole or 1:10 diluted anti-Lam-CRM or control sera. Data are from one representative experiment out of three performed with similar results and represent mean values from triplicate independent determinations. (b) Dose–response C. albicans CFU reduction by the anti-β-glucan, 2G8 and the irrelevant anti-CRM, mAbs. The figure shows a representative experiment as for Fig. 6 a. (c) Effect of anti-Lam-CRM and control anti-CRM serum on the in vitro growth of A. fumigatus, as evaluated by 3H-glucose incorporation assays. Mean count per minute values from four independent experiments, each performed in triplicate, are shown. For additional experimental details, see Fig. S3 and Table S1. (d) Survival of mice vaccinated with the Lam-CRM conjugate or with CRM only and infected i.v. with A. fumigatus. Data are from a single experiment with eight mice per group.
On the basis of the growth inhibition described here, we performed an initial investigation asking whether immunization with the Lam-CRM vaccine could protect mice from a lethal systemic challenge by A. fumigatus. An immunization schedule similar to that used to immunize against systemic candidiasis was employed, and the mice were challenged i.v. with a lethal inoculum of conidia. As shown in Fig. 6 d, mortality was lower and was significantly delayed in Lam-CRM–vaccinated mice when compared with controls immunized with CRM alone.
DISCUSSION
A novel glyco-conjugate vaccine, composed of a simple β-glucan polysaccharide such as laminarin conjugated with the diphtheria toxoid CRM197, has been shown here to confer protection, when used as preventive vaccine, against both a lethal systemic infection in mice and against a self-healing vaginal C. albicans infection in rats. Conjugation of laminarin, which by itself is a poor immunogen, with the protein carrier made this polysaccharide immunogenic, as demonstrated by the induction in both rats and mice of anti-β-glucan antibodies, most of which are of the IgG class. No antibodies directed against non–glucan-containing fungal materials, including cell surface mannoproteins, were generated, matching the nonfungal source and purity of both laminarin and carrier protein. High levels of anti-CRM antibodies were induced equally by vaccination with Lam-CRM or with CRM alone, making the use of this latter protein a particularly good control.
Antibody production was essential for anti-Candida protection, as was demonstrated by experiments involving passive transfer of the whole immune serum or its IgG-enriched fraction, which conferred protection to nonimmune animals. Such protection was not conferred by any control serum or when the antibodies present in the Lam-CRM serum were adsorbed on fungal cells or β-glucan particles. Loss of protective capacity on absorption on β-glucan particles and the passive protection conferred by mAb 2G8, an anti-β-glucan mAb, constitute additional although indirect evidence that anti-β-glucan antibodies are largely responsible for the vaccine-induced protection. The degree of passive protection apparently ranked in the order: whole immune serum → IgG-rich serum fraction pool C → mAb, suggesting that a number of saccharide epitopes and antibody isotypes participate in the protection. Supporting this possibility, some degree of passive protection in the systemic model was also detected with the transfer of Lam-CRM antiserum pool A and B, which contained mostly IgM and IgG1 isotypes. No anti-β-glucan IgM were detected in the vaginal fluid of Lam-CRM–immunized rats after mucosal vaccination; here, the protection was fully associated with anti-β-glucan IgG. Anti-β-glucan antibodies were shown to bind preferentially to the hyphae of C. albicans and to cause inhibition of their growth in vitro in the absence of any host cellular factor. Remarkably, the same vaccine also protected the mice from a lethal infection of A. fumigatus, a fungal organism quite distinct from C. albicans but also expressing β-glucan polysaccharides on its cell wall. Although these latter data must be taken cautiously because of their preliminary nature, they nonetheless contribute to the proof of principle that a single immunizing tool can indeed be developed against multiple fungal infections. Moreover, they match the consistent observations made here demonstrating the efficiency of the binding and growth inhibition of A. fumigatus hyphae by the Lam-CRM immune serum.
Fungal β-glucan has various molecular and supramolecular configurations (11, 16). Although laminarin is commonly considered a prototypal β(1, 3) glucan, we were not surprised to find both anti-laminarin and anti-pustulan (which is a classic β[1, 6] glucan) antibodies in the serum of Lam-CRM–vaccinated mice. Laminarin has very short β(1, 6)–bound side residues (6). In addition, a degree of cross-reactivity between these two linear glucose polymers is a distinct possibility that is indirectly supported by the fact that the anti-β-glucan mAb 2G8 recognizes both configurations (although it recognizes the β[1, 3] glucan more intensely). Overall, given the heterogeneous distribution and cell surface expression of the different β-glucan configurations in the different fungi, cross-reactivity would increase the possibility that Lam-CRM might be a useful anti-fungal vaccine (16).
Other glyco-conjugate vaccines against C neoformans and against Candida itself have been generated (reviewed in [3, 4]). For both fungi, the conjugated polysaccharide is the external capsular or cell wall material (glucuronoxylomannan for C neoformans or mannan for Candida). Besides the antigenic variability of mannan (13, 20, 21), one concern with these vaccines is the demonstrated capacity of surface polysaccharides to induce the production of both protective and inhibitory antibodies (5, 21). β-glucan is a much less variable molecule, and, to our knowledge, no inhibitory or competing anti-β-glucan antibodies have been described. Moreover, β-glucan has some advantages for vaccine formulation. It is an abundant and stable molecule and, given its strong resistance to hot alkali and acid solutions, it can be deprived rather easily of protein and other saccharide contaminants. Also, laminarin is a widely marketed, available compound, devoid of chitin, which covalently associates with glucan in some fungi (11).
Our data do not establish the immunologic mechanism(s) whereby anti-β-glucan antibodies may confer protection. However, our findings show that β-glucan constituents are present on fungal cell surfaces and are accessible to antibodies, which therefore could opsonize the cells and facilitate complement deposition. The importance of this process (e.g., the rapid opsonization of Blastomyces dermatitidis cells, which notoriously expose β-glucans on their surface) for antifungal protection has been highlighted in several studies (22). Antibodies to cell surface components of C. albicans have been shown to favor both intracellular and extracellular killing of the fungus (21), and, quite recently (23) anti-β-glucan antibodies have been shown to increase the candidacidal activity of macrophages in vitro. In a more subtle fashion, these antibodies may alter the host inflammatory responses, of which β-glucans may be a strongly inductive component (24), and shift the cytokine profile toward the protective Th1 pattern, as demonstrated in experimental cryptococcosis (25). Importantly, anti-β-glucan IgG have been shown here to bind efficiently Candida and Aspergillus hyphae (i.e., those forms of growth that are considered particularly invasive in host tissues). Antibodies binding the hyphal form of growth could easily perturb adherence and tissue invasion, as has been demonstrated recently with a mAb directed against a stress-mannoprotein of C. albicans (26). All factors seem to be relevant for host response to Candida, possibly collaborating with cell-mediated immunity (27).
In addition to the mechanisms already discussed, our data suggest that an additional mechanism for protection by anti-β-glucan antibodies could operate in vivo. In fact, the immune serum from vaccinated mice exerted a marked inhibition of Candida (and Aspergillus) hyphal growth in vitro, an effect that was also exerted by the affinity-purified IgG-rich fraction and that is in keeping with the preferential antibody binding to the hyphae. Together with previous data obtained with other yeast killer toxin–mimicking, anti-idiotypic antibodies (17, 18), our present findings suggest that certain anti-β-glucan antibodies may be endowed with direct inhibitory activity on fungi through some sort of interaction with such viability-critical molecules.
The presence of β-glucan on cell surfaces of C. albicans has been a matter of controversy (28). Recently, Gantner et al. (29) have shown that soluble Dectin-1, a β-glucan receptor important for host response to Candida (24), did not appreciably bind the hyphae of C. albicans. We show here quite clearly that β-glucan–specific antibodies bind to the hyphal cell surface and inhibit their growth, indicating that functional β-glucan is accessible to antibodies on hyphal cell surfaces. Possibly, epitope number, affinity, fine molecular configuration, and steric hindrance features differ for Dectin-1 and antibody binding. Nonetheless, it is of interest that the patched pattern of Dectin-1 binding to the yeast cell surface of C. albicans has some resemblance to that of anti-β-glucan antibodies.
As recently discussed by Deepe (3) and by Mochon and Cutler (4), the development of antifungal vaccines requires overcoming several major obstacles, one of which could be the need to identify and vaccinate persons at risk before they become so immunocompromised as to be unresponsive to immunization. Several categories of patients at risk of invasive candidiasis could benefit from a safe and effective immunoprophylactic vaccine (30, 31). There are also mucosal pathologies (e.g., Candida vaginitis) that affect millions of apparently nonimmunocompromised women for whom a vaccine inducing protective antibodies would be of utmost usefulness. Our Lam-CRM vaccine elicits highly protective, specific IgG vaginal antibodies both by intravaginal and intranasal immunization. Previously, other antibodies against some defined virulence traits proved to confer protection in this model (32, 33).
The anti-β-glucan antibodies described here could also be exploited for immunotherapy, particularly given the recent biotechnological developments making more feasible the in vitro production of human antibodies with preselected specificity (34). A particular preparation of anti-HSP90 antibody complexed with amphotericin B has recently completed phase III trials of efficacy for systemic candidiasis (3, 35). In particular, fungal hyphae are a potent means to evade protective host response (36–38); thus the possibility of inhibiting the hyphal growth of major fungal pathogens with anti-β-glucan antibodies deserves consideration in future preclinical and clinical studies.
MATERIALS AND METHODS
Glucan antigens and other reagents
Laminarin, a linear β(1, 3)-linked glucan backbone with occasional β(1, 6)-linked branching of a single glucosyl residue (6) (average mol wt, 4.5 kD), was purchased from Sigma-Aldrich. Pustulan, a β(1, 6)–linked glucan (mol wt, 20 kD) was obtained from Calbiochem. A β-glucan preparation from Saccharomyces cerevisiae (G5011; Sigma-Aldrich) and dextran, an α(1, 6)-linked polysaccharide (Dextran Standard 1000; Sigma-Aldrich) were also used throughout this study. Soluble C. albicans β-glucan (GG-Zym), a mixture of β(1, 3) and β(1, 6) glucan, was obtained by limited β(1, 3) glucanase (Zymoliase 100T, Seikagaku Corporation) digestion of particulate glucan ghosts of C. albicans (5). Mannoproteins were extracted and purified as previously described (39). The nontoxic mutant protein of diphtheria toxin CRM197 (7), herein referred to as CRM, was manufactured by Chiron Corp.
Glyco-conjugate preparation and analytical determinations
Laminarin and C. albicans GG-Zym were used to prepare glyco-conjugates with the CRM protein. To this aim, terminal reducing groups of polysaccharides (2 mg/ml) were subjected to reductive amination by incubation for 5 d at 50°C in 300 mg/ml ammonium acetate (Sigma-Aldrich) and 0.2 M sodium cyanoborohydride (pH 7.5; Sigma-Aldrich). Aminated laminarin was purified from the reaction mixture by diafiltration on regenerated cellulose membrane (cut-off 3.0 kD; Millipore), whereas aminated GG-Zym was purified by gel filtration on Sephadex G-10 column (Amersham Biosciences). The amount of primary amino groups introduced by the reaction was determined by the method of Habbeb (40). The aminated polysaccharides were vacuum dried, resolubilized in DMSO/H2O mixture, and then reacted with N-hydroxysuccinimide diester of adipic acid (Ferro Pfanstiehl; 12-fold molar excess as compared with primary amino groups) in the presence of triethylamine (fivefold molar excess) to introduce an active spacer molecule in the conjugate (41). After selective precipitation with dioxane and drying under vacuum, the content of N-hydroxysuccinimide ester groups introduced in the activated polysaccharide was estimated according to Miron and Wilchek (42). For conjugation to CRM, a 20-fold molar excess of activated polysaccharide was reacted overnight with the protein, at room temperature, in 10 mM phosphate buffer, pH 7.2. The unbound polysaccharide was separated from the glyco-conjugate by ultrafiltration, using Amicon 10-kD filter devices (Millipore). High-performance anionic exchange chromatography coupled with pulsed amperometric detection was used to quantify total and free (unconjugated) polysaccharide content in the conjugate preparations (8). Molecular size of conjugate preparations and absence of unconjugated CRM protein was assessed by HPLC analysis onto a Toso Haas TSK 4000SWXL guard and analytical columns (HPLC system, Waters Corporation), with 0.1 M Na2PO4/0.1 M NaCl pH 7.2 as the mobile phase, as well as by standard SDS-PAGE analysis followed by Comassie staining.
Polysaccharide content was controlled repeatedly throughout the entire procedure by the phenol-sulfuric acid method, with glucose as reference compound (43). To estimate the conjugation and polysaccharide/protein ratio in final conjugate products, this analysis was coupled to MicroBCA Protein assay (Bio-Rad Laboratories), following the manufacturer's specifications
NMR spectroscopy
The glyco-conjugate samples were freeze-dried and redissolved in 700 μl of deuterated water (2H2O, 99.9% deuterium; Sigma-Aldrich). Proton spectra were recorded with a 14.1-T Brucker Avance 600 MHz spectrometer, equipped with a 5-mm PFG triple resonance probe and operating under controlled temperature (25 ± 0.1°C). XWINNMR 2.6 software (Brucker) was used for acquisition and processing data. Standard pulse sequences were used throughout. For the one-dimensional spectra, the spectral width was 6,000 Hz and 32k data points were acquired. The spectra were referenced relative to the signal of monodeuterated water, 4.79 ppm.
Microorganisms
C. albicans strains BP and SA-40 (type collection of the Istituto Superiore di Sanità) were used in the models of disseminated and vaginal Candida infections, respectively. For experimental infections, cells from stock cultures in Sabouraud-dextrose agar (Difco Laboratories) were grown in Winge medium (strain BP[5]) or in YEPD medium (1% yeast extract, 2% peptone, 2% glucose, all w/v) (strain SA-40 [32]) at 28°C for 24 h, then harvested by centrifugation, washed, counted in an hemocytometer, and resuspended to the desired concentration in PBS. Germ tube and hyphal forms were obtained by culturing yeast cells for 1.5 h or 5 h, respectively, in Lee's medium or in RPMI 1640 (Euroclone Ltd.), supplemented with FCS (1 mM glutamine and 2% FCS; HyClone) at 37°C, as previously described (5). A. fumigatus 495, from the type collection above, was routinely maintained on Sabouraud-dextrose agar slants. Suspensions of conidia were prepared by gently flushing the surface of fungal colonies with sterile PBS. After filtration of hyphal debris through a BD Falcon 100-μm cell straining device (BD Biosciences), the conidia were thoroughly washed with PBS by centrifugation, counted in a hemocytometer, and resuspended at suitable concentrations in PBS. Hyphal growth was obtained by incubation in RPMI-FCS for 18 h at 37°C.
mAbs
Two stable hybridoma secreting anti-β-glucan (2G8) or anti-CRM IgG mAb were generated after fusion of spleen cells of GG-Zym-CRM–immunized Balb/c mice (Harlan Nossan) and myeloma cells of the murine line X63-Ag8 653, using standard in-house protocols (44). Indirect ELISA assays using different glucan molecules, mannoprotein preparations, and the unconjugated CRM protein as solid-phase antigens were used for selecting and assessing mAb specificity. Hybridoma were routinely cultured in RPMI 1640 (Euroclone Ltd.) supplemented with 10% FCS (Hyclone), 100 U penicillin/ml, 100 μg streptomycin/ml, 1 mM sodium pyruvate, and 2 mM L-glutamine (Hyclone). 2G8 and anti-CRM mAbs were precipitated from culture supernatants by ammonium sulfate (44) and affinity purified on Protein A Sepharose resin (Amersham Biosciences), following the manufacturer's instructions.
Immunogenicity and protection assays
Disseminated candidiasis and aspergillosis in mice.
Female 4-wk-old CD2F1 mice (Harlan) were immunized with two doses of the Lam-CRM vaccine, each consisting of 7 μg polysaccharide/0.1 ml PBS/mouse. The priming was given s.c. in complete Freund's adjuvant (Sigma-Aldrich); the booster was given i.p. 15 d later, without adjuvant. Control animals were injected with unconjugated laminarin or CRM, with Freund's adjuvant only, or with PBS, as specified in single experiments. Vaccinated and control animals were bled by retroorbital puncture 2 wk after the last injection, and their sera were pooled and stored at −20°C. In some experiments, sera from Lam-CRM–or CRM-only–immunized mice, as well as control animals, were separated by affinity chromatography on Protein A Sepharose (Amersham Biosciences), with 0.1 M glycine buffer pH 3.0 as the eluant. Unbound (pool A), weakly bound (pool B, eluted from the column by buffer washing), and strongly bound (pool C, eluted with glycine buffer, pH 3) immunoglobulin fractions were extensively dialyzed against PBS by ultrafiltration in 10-kD cut-off centrifuge filter devices (Millipore) and concentrated to 1 ml. Pools were characterized for their immunoglobulin concentration (by protein assay) and composition by ELISA with laminarin as the coating antigen. Some lots of immune sera were also subjected to adsorption with intact C. albicans cells or insoluble GG particles (5). The fungal challenge was made by i.v. administration of 2 LD50 (106 cells/0.2 ml/mouse) of yeast cells of C. albicans strain BP or of conidial cells (5 × 106/0.2 ml/mouse) of A. fumigatus. Protection end-points were mortality (median survival time and ratio of dead/total challenged mice) and, for C. albicans, enumeration of fungal CFU in the left kidney 2 d after the challenge (5).
For passive immunization experiments, mice were administered a single i.p. injection of 0.5 ml of serum (or Protein A–separated serum fractions) or equivalent doses (200–250 μg protein/0.5 ml/mouse) of the anti β-glucan, or anti-CRM mAb. 2 h later, the animals received a sublethal challenge with 5 × 105 C. albicans cells i.v. Protection was evaluated on day 2 after challenge by measuring the extent of kidney invasion by the fungus (5).
Rat vaginal candidiasis.
Oophorectomized Wistar rats (Charles River Laboratories) were immunized three times at weekly intervals with 50 μg Lam-CRM conjugate (polysaccharide)/rat by either the intravaginal or the intranasal route, using 3 μg/rat of cholera toxin (CT, provided by Swiss Serum and Vaccine Institute) as the adjuvant, as described previously (32). Control rats were given unconjugated laminarin plus CT, unconjugated CRM plus CT, or CT only. All rats were maintained under pseudoestrus by the s.c. administration of estradiol benzoate (Benzatrone; Samil). For experimental infection, rats were inoculated intravaginally with C. albicans (107 cells/0.1 ml of saline/rat) as described elsewhere (32). Before infection, samples of vaginal fluids (PBS vaginal washes) were taken from immunized and control animals and were pooled and assayed for vaginal anti-β-glucan antibodies. Other aliquots were stored at −20°C.
Groups of naive animals were also passively transferred vaginal fluids from Lam-CRM–vaccinated or control rats (0.5 ml intravaginally/rat, at equivalent protein concentrations), anti-β-glucan 2G8, or the irrelevant anti-CRM mAb (each 200 μg protein/rat) and, after 30 min, were infected. In some experiments, vaginal fluids were preadsorbed with insoluble GG particles to remove anti-β-glucan antibodies (5). As a positive control, rats were infected with C. albicans and subjected to effective fluconazole (Pfizer Inc.) therapy, 10 μg of fluconazole/rat at 1, 24, and 48 h after challenge. Active and passive protection were evaluated through the estimation of fungal CFU in vagina until day 21–28 after infection, as described in previous reports (32). All animal studies were approved by the Istituto Superiore di Sanità intramural Institutional Review Committee.
Serological assays
Immune sera, vaginal fluids, and mAbs were assayed for their reactivity with various β-glucan or control antigens by indirect ELISA, according to previously described protocols (5). Immunoglobulin classes and isotypes of murine anti-β-glucan antibodies were evaluated using alkaline phosphatase–conjugated goat anti–mouse IgM or IgG antibodies (Sigma-Aldrich) or with biotin-conjugated anti–mouse IgG1, IgG2a, IgG2b, or IgG3 antibodies (BD Biosciences) and alkaline phosphatase–conjugated ExtrAvidin reagent (Sigma-Aldrich). Rat vaginal fluids were reacted with alkaline phosphatase-conjugated sheep anti–rat IgG, IgM, or IgA (Serotec Ltd.). Vaginal fluids were considered positive for a determined antibody when their ELISA readings (OD, 405 nm) were at least twofold those from control wells reacted with fluids from mock-vaccinated rats. Serum titers were defined as the highest dilution of sera producing an OD of 405 nm, at least twofold that obtained from sera of mock-vaccinated animals assayed at the same dilution.
For immunofluorescence assay, fungal cells (2 × 105 C. albicans germ tubes in Lee's medium or 104 A. fumigatus conidia in RPMI 1640-FCS) were cultured on microscope chamber slides (Nalge Nunc International). After incubation for 1 or 5 h (Candida) or 18 h (Aspergillus) at 37°C, the culture medium was removed, the slides were blocked with BSA (3% w/v in PBS containing 0.05% v/v Tween 20), then reacted with various dilutions of murine sera, serum fractions, or mAbs in PBS-Tween20 (1 h at 37°C). After extensive washings with PBS, slides were treated with fluorescein isothiocyanate–conjugated anti–mouse IgG or IgM antibody (Sigma-Aldrich), washed again, and observed with a Leitz Diaplan fluorescence microscope. Negative controls with sera from CRM-immunized mice or with the anti-CRM mAb were always included in the experiments.
Growth inhibition assays
150 C. albicans germ tubes or Aspergillus conidia were incubated in 200 μl of RPMI-FCS, in the presence of sera from Lam-CRM–vaccinated mice, with their pool C fractions (at 1:2–1:10 final dilutions), or with 250–20 μg/ml 2G8 mAb. Control cultures were prepared with un-fractionated sera or pool C fractions from CRM-vaccinated mice or with the irrelevant anti-CRM mAb at the same protein concentration. Cultures were incubated overnight at 37°C. Candida growth was evaluated by classic CFU counts (5), whereas growth of A. fumigatus was quantitated by measuring 3H-glucose incorporation over a 3-h period, as described elsewhere (19). The inhibitory activity of anti-β-glucan sera or mAb was always confirmed by microscopic examination of the fungal cultures. In two experiments, A. fumigatus conidia were allowed to produce elongated hyphae by incubation in medium for 18 h at 37°C, then were treated with 1:5 diluted anti-Lam-CRM or anti-CRM serum or with medium only for additional 18 h, and finally were labeled for 3 h with 3H-glucose.
Statistical analysis
Differences in survival rates and overall 30- or 60-d survival in C. albicans–challenged mice were analyzed by Fisher's exact test and by nonparametric two-tailed Mann-Whitney U test, respectively. Data from CFU counts, in both in vitro and in vivo experiments, were analyzed by two-tailed Student's t test. Multiple comparisons were made by analysis of variance (one-way ANOVA) followed by Bonferroni's multiple t test.
Online supplemental material
Fig. S1 shows isotype and IgG subclass composition of anti-Lam antibodies in serum fractions (pool A, B, and C) of animals immunized with the Lam-CRM vaccine. Fig. S2 shows parallel bright field and immunofluorescence staining of A. fumigatus or C. albicans hyphae reacted with the control anti-CRM serum or anti-CRM mAb. Fig. S3 depicts the microscopic aspect of A. fumigatus cultures generated from an equal number of conidial cells which were incubated overnight with the anti–Lam-CRM or the control anti-CRM serum, showing inhibition of hyphal growth by the former serum. Table S1 displays the results of 3H-glucose incorporation experiments, showing inhibition of hyphal growth of A. fumigatus. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050749/DC1.
Acknowledgments
The authors wish to express their gratitude to Alessandro Spurio for help in preparation of the figures.
This work was supported in part by institutional grants and by special grants from the AIDS National Project (Istituto Superiore di Sanità, Ministero della Salute, Italy) under contracts 50B/F and 50F.27.
The authors have no conflicting financial interests.
Abbreviations used: CT, cholera toxin; GG, glucan ghosts; Lam, laminarin; NMR, nuclear magnetic resonance; ppm, parts per million; Zym, zymoliase.
References
- 1.Edmond, M.B., S.E. Wallace, D.K. McClish, M.A. Pfaller, R.N. Jones, and R.P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three years analysis. Clin. Infect. Dis. 29:239–244. [DOI] [PubMed] [Google Scholar]
- 2.Sanglard, D. 2002. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5:379–385. [DOI] [PubMed] [Google Scholar]
- 3.Deepe, G.S. Jr. 2004. Preventative and therapeutic vaccines for fungal infections: from concept to implementation. Expert Rev. Vaccines. 3:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Mochon, A.B., and J.E. Cutler. 2005. Is a vaccine needed against Candida albicans? Med. Mycol. 43:97–115. [DOI] [PubMed] [Google Scholar]
- 5.Bromuro, C., A. Torosantucci, P. Chiani, S. Conti, L. Polonelli, and A. Cassone. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70:5462–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read, S.M., G. Currie, and A. Bacic. 1996. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 281:187–201. [DOI] [PubMed] [Google Scholar]
- 7.Giannini, G., R. Rappuoli, and G. Ratti. 1984. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 12:4063–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho, M.M., B. Bolgiano, and M.J. Corbel. 2001. Assessment of the stability and immunogenicity of meningococcal oligosaccharide C-CRM197 conjugate vaccines. Vaccine. 19:716–725. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura, T., C. Bignon, J. Allouch, M. Czjzek, H. Darbon, T. Watanabe, and B. Henrissat. 2001. Streptomyces matensis laminaripentaose hydrolase is an ‘inverting’ beta-1,3-glucanase. FEBS Lett. 499:187–190. [DOI] [PubMed] [Google Scholar]
- 10.Petersen, B.O., M. Krah, J.Ø. Duus, and K.K. Thomsen. 2000. A transglycosylating 1,3 (4)-beta-glucanase from Rhodothermus marinus: NMR analysis of enzyme reactions. Eur. J. Biochem. 267:361–369. [DOI] [PubMed] [Google Scholar]
- 11.Kollár, R., B.B. Reinhold, E. Petrakova, H.J. Yeh, G. Ashwell, J. Drgonova, J.C. Kapteyn, F.M. Klis, and E. Cabib. 1997. Architecture of the yeast cell wall. Beta(1→6)-glucan interconnects mannoprotein, beta(1→)3-glucan, and chitin. J. Biol. Chem. 272:17762–17775. [DOI] [PubMed] [Google Scholar]
- 12.Fidel, P.L., Jr., and J.D. Sobel. 1998. Protective immunity in experimental Candida vaginitis. Res. Immunol. 149:361–373. [DOI] [PubMed] [Google Scholar]
- 13.Calderone, R.A., and W.A. Fonzi. 2002. Virulence factors in Candida albicans Curr. Opin. Microbiol. 9:327–335. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall, A., A. Cassone, F. Bistoni, J.E. Cutler, W. Magliani, J.W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell-mediated immune protective mechanisms in fungal diseases: an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36:95–105. [PubMed] [Google Scholar]
- 15.Keller, R.G., G.S. Pfrommer, and T.R. Kozel. 1994. Occurrence, specificities and functions of ubiquitous antibodies in human serum that are reactive with Cryptococcus neoformans cell wall. Infect. Immun. 62:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuoka, J. 2004. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 17:281–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, M. Gerloni, G. Morace, W. Magliani, C. Chezzi, and A. Cassone. 1994. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory yeast killer toxin-like anti-idiotypic antibodies. J. Immunol. 152:3175–3182. [PubMed] [Google Scholar]
- 18.Guyard, C., E. Dehecq, J.P. Tissier, L. Polonelli, E. Dei-Cas, J.C. Cailliez, and F.D. Menozzi. 2002. Involvement of beta-glucans in the wide-spectrum antimicrobial activity of Williopsis saturnus var. mrakii MUCL 41968 killer toxin. Mol. Med. 8:686–694. [PMC free article] [PubMed] [Google Scholar]
- 19.Girmenia, C., A.P. Iori, F. Boecklin, A. Torosantucci, P. Chiani, P. De Fabritiis, F. Taglietti, A. Cassone, and P. Martino. 1999. Fusarium infections in patients with severe aplastic anemia: review and implications for management. Haematologica. 84:114–118. [PubMed] [Google Scholar]
- 20.De Bernardis, F., A. Molinari, M. Boccanera, A. Stringaro, R. Robert, J.M. Senet, G. Arancia, and A. Cassone. 1994. Modulation of cell surface-associated mannoprotein antigen expression in experimental candidal vaginitis. Infect. Immun. 62:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casadevall, A., E. Dadachova, and L.A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, M.X., T.T. Brandhorst, T.R. Kozel, and B.S. Klein. 2001. Role of glucan and surface protein BAD 1 in complement activation by Blastomyces dermatitidis yeast. Infect. Immun. 69:7559–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi, K., M. Yoshida, I. Nakabayashi, H. Shinohara, N.N. Miura, Y. Adachi, and N. Ohno. 2005. Role of anti-β-glucan antibody in host defense against fungi. FEMS Immunol. Med. Microbiol. 44:99–109. [DOI] [PubMed] [Google Scholar]
- 24.Brown, G.D., and S. Gordon. 2005. Immune recognition of fungal beta-glucans. Cell. Microbiol. 7:471–479. [DOI] [PubMed] [Google Scholar]
- 25.Yuan, R.R., A. Casadevall, J. Oh, and M.D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA. 94:2483–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moragues, M.D., M.J. Omaetxebarria, N. Elguezabal, M.J. Sevilla, S. Conti, L. Polonelli, and J. Ponton. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71:5273–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1–23. [DOI] [PubMed] [Google Scholar]
- 28.Torosantucci, A., P. Chiani, and A. Cassone. 2000. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the beta-1,6 glucan of the fungal cell wall. J. Leukoc. Biol. 68:923–932. [PubMed] [Google Scholar]
- 29.Gantner, B.N., R.M. Simmons, and D.M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellberg, B.J., and J.E. Edwards Jr. 2003. The pathophysiology and treatment of Candida sepsis. Curr. Infect. Dis. Rep. 4:387–399. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, D.A. 2004. Vaccinate against aspergillosis! A call to arm of the immune system. Clin. Infect. Dis. 38:1131–1136. [DOI] [PubMed] [Google Scholar]
- 32.De Bernardis, F., M. Boccanera, D. Adriani, A. Girolamo, and A. Cassone. 2002. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect. Immun. 70:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traggiai, E., S. Becker, K. Subbarao, L. Kolesnikova, Y. Uematsu, M.R. Gismondo, B.R. Murphy, R. Rappuoli, and A. Lanzavecchia. 2004. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10:871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnie, J., and R. Mathews. 2003. The role of antibodies against HSP90 in the treatment of fungal infections. Drugs News Perspect. 16:205–210. [DOI] [PubMed] [Google Scholar]
- 36.Rooney, P.J., and B.S. Klein. 2002. Linking fungal morphogenesis with virulence. Cell. Microbiol. 4:127–137. [DOI] [PubMed] [Google Scholar]
- 37.d'Ostiani, C.F. G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implication for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torosantucci, A., G. Romagnoli, P. Chiani, A. Stringaro, P. Crateri, S. Mariotti, R. Teloni, G. Arancia, A. Cassone, and R. Nisini. 2004. Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendritic cells: a novel dimorphism-dependent mechanism to escape the host's immune response. Infect. Immun. 72:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bromuro, C., A. Torosantucci, M.J. Gomez, F. Urbani, and A. Cassone. 1994. Differential release of an immunodominant 65 kDa mannoprotein antigen from yeast and mycelial forms of Candida albicans. J. Med. Vet. Mycol. 32:447–459. [DOI] [PubMed] [Google Scholar]
- 40.Habeeb, A.F. 1966. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 14:328–336. [DOI] [PubMed] [Google Scholar]
- 41.Hill, M., J.J. Bechet, and A. d'Albis. 1979. Disuccinimidyl esters as bifunctional crosslinking reagents for proteins: assays with myosin. FEBS Lett. 102:282–286. [DOI] [PubMed] [Google Scholar]
- 42.Miron, T., and M. Wilchek. 1982. A spectrophotometric assay for soluble and immobilized N-hydroxysuccinimide esters. Anal. Biochem. 126:433–435. [DOI] [PubMed] [Google Scholar]
- 43.Dubois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugar and related substances. Anal. Chem. 28:350–356. [Google Scholar]
- 44.Cassone, A., A. Torosantucci, M. Boccanera, G. Pellegrini, C. Palma, and F. Malavasi. 1988. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J. Med. Microbiol. 27:233–238. [DOI] [PubMed] [Google Scholar]