Abstract
Lymphotoxin (LT)α knockout mice, as well as double LTα/tumor necrosis factor (TNF) knockout mice, show a severe splenic disorganization with nonsegregating T/B cell zones and complete absence of primary B cell follicles, follicular dendritic cell (FDC) networks, and germinal centers. In contrast, as shown previously and confirmed in this study, LTβ-deficient mice show much more conserved T/B cell areas and a reduced but preserved capacity to form germinal centers and FDC networks. We show here that similar to the splenic phenotype of LTβ-deficient mice, complementation of LTα knockout mice with TNF-expressing transgenes leads to a p55 TNF receptor–dependent restoration of B/T cell zone segregation and a partial preservation of primary B cell follicles, FDC networks, and germinal centers. Notably, upon lipopolysaccharide challenge, LTα knockout mice fail to produce physiological levels of TNF both in peritoneal macrophage supernatants and in their serum, indicating a coinciding deficiency in TNF expression. These findings suggest that defective TNF expression contributes to the complex phenotype of the LTα knockout mice, and uncover a predominant role for TNF and its p55 TNF receptor in supporting, even in the absence of LTα, the development and maintenance of splenic B cell follicles, FDC networks, and germinal centers.
Keywords: splenic architecture, gene targeting, complementation, follicular dendritic cells, germinal centers
Tumor necrosis factor (TNF), lymphotoxin α (LTα),1 and LTβ are structurally homologous cytokines, and their genes are closely clustered within the MHC (1, 2). TNF is expressed mainly by macrophages and T cells as either a transmembrane protein or a soluble homotrimeric molecule (3). LTα exists as a soluble homotrimer (LTα3) but also on the membrane of activated lymphocytes in heterotrimeric complexes with LTβ (1). TNF and LTα3 use the same cell surface receptors, the p55 and p75 TNFRs, which are expressed on a wide variety of cells (4), whereas the predominant surface LTα1β2 heterotrimer binds to the LTβR, which is expressed on cells of nonhematopoietic origin (5).
Recent studies in gene-targeted mice have revealed essential roles for TNF, LTα, and LTβ in secondary lymphoid organ structure and function. TNF was found to be essential for the formation of primary B cell follicles, follicular dendritic cell (FDC) networks, and germinal centers in all secondary lymphoid organs (6, 7). LTα knockout mice lack lymph nodes and Peyer's patches and show a severe disorganization of splenic architecture where B/T cell areas do not segregate, marginal zones are absent, and FDC networks and germinal centers do not form (8, 9). Interestingly, mice deficient in both the LTα and TNF genes show a phenotype identical to the LTα knockout phenotype (10). Remarkably, however, these phenotypes were not fully reproduced in LTβ knockout mice, in which mesenteric and cervical lymph nodes do develop, splenic white pulp lymphocytes segregate into B/T cell zones (11, 12), and some capacity to form FDC networks and germinal centers is preserved (12). These discrepancies led to the hypothesis that LTα should have additional functions independent of LTβ in the organogenesis of mesenteric and cervical lymph nodes and in the formation of distinct B/T cell areas, FDC networks, and germinal centers in the spleen.
The apparently similar phenotypes of LTα and double TNF/LTα (10) knockout mice and the differences these mice show when compared with LTβ knockout mice (11– 13) have led us to search for functional redundancies in the TNF/LT system by complementing LTα knockout mice with TNF-expressing transgenes. Surprisingly, although the lack of mesenteric and peripheral lymph nodes and Peyer's patches could not be rescued by transgenic expression of TNF, TNF-complemented LTα knockout mice displayed intact T/B cell segregation and retained a suboptimal capacity to develop primary B cell follicles that contained FDC networks and could support the formation of germinal centers. TNF-mediated restoration of LTα knockout splenic architecture was dependent on the presence of the p55TNFR. These observations suggested that altered TNF expression may contribute to the complex splenic phenotype of the LTα knockout mice. Indeed, defective TNF production in the LTα knockout mice could be documented by measuring TNF accumulation in LPS-stimulated sera and in the supernatants of macrophages from LTα knockout mice. Our studies support a model where LTs function to provide the optimal reticular/stromal splenic architecture for the efficient formation of B cell follicles and FDC networks, phenomena primarily dependent on TNF/p55TNFR interactions.
Materials and Methods
Mice.
LTα knockout mice (8) were obtained from The Jackson Laboratory (Bar Harbor, ME); LTβ knockout (12) mice were provided by Dr. K. Pfeffer (Technical University of Munich, Munich, Germany); and p55TNFR-deficient (14) mice were provided by Dr. H. Bluethmann (Hoffman-LaRoche Inc., Basel, Switzerland). Transgenic (Tg)1278 mice carrying ∼3 copies of a genomic 3.6-kb DNA fragment containing the entire wild-type human TNF gene together with 0.6 and 0.8 kb of 5′ and 3′ flanking sequences, respectively (15), and TgA86 mice carrying ∼50 copies of a 3.2-kb hybrid TNF/globin transgene construct containing the promoter of the murine TNF gene, the coding sequence of the murine TNFΔ1-12 gene (16), and the 3′-untranslated region (UTR) and polyadenylation site of the human β-globin gene (16) have been previously generated in our laboratory. Tg1278 and TgA86 mice were always heterozygous for the TNF transgenes. All mice used in this study were maintained under specific pathogen–free barriers in the animal facilities of the Hellenic Pasteur Institute and analyzed between 6 and 12 wk of age.
Immunocytochemical Analysis of Splenic Structure.
Spleens were harvested, embedded in O.C.T. compound (BDH Laboratory Supplies, Dorset, UK), and frozen in liquid nitrogen. Cryostat sections were cut at 7 μm, thaw-mounted on gelatinized slides, air dried, and stored desiccated at −20°C. Immediately before use, sections were fixed for 10 min in acetone containing 0.03% H2O2 to block endogenous peroxidase activity, followed by rehydration in PBS. All antibodies were diluted in PBS/0.1% BSA. For double immunostaining with anti-B220 and anti-CD3, sections were incubated with rat anti-CD3 clone KT3 (reference 17; provided by Dr. S. Gobbold, Sir William Dunn School of Pathology, Oxford, UK) followed by peroxidase-conjugated goat anti– rat IgG (Southern Biotechnology Associates, Inc., Birmingham, AL). After blocking with 20% normal rat serum in PBS, sections were incubated with biotin-conjugated rat anti-B220 (PharMingen, San Diego, CA), followed by avidin–alkaline phosphatase (ABC-AP kit; Vector Laboratories, Inc., Burlingame, CA). Double staining for IgM and IgD was performed as described elsewhere (6). For the analysis of FDC networks and germinal centers, mice were immunized with an intraperitoneal injection of 108 SRBC in PBS, and spleens were removed 10 d later. For double immunostaining of B cells and FDC networks or germinal centers, sections were incubated with rat anti-B220 (PharMingen), followed by peroxidase-conjugated goat anti–rat IgG. After blocking with 20% normal rat serum in PBS, sections were incubated with biotinylated anti–mouse complement receptor (CR)1 antibody (8C12; PharMingen [18]) or biotinylated peanut agglutinin (PNA; Sigma Chemical Co., St. Louis, MO), followed by avidin–alkaline phosphatase. In all of the above stainings, bound peroxidase activity was developed with diaminobenzidine (DAB; Sigma Chemical Co.), and alkaline phosphatase activity was visualized with naphthol AS-MX phosphate (Sigma Chemical Co.) and fast blue BB salt (Sigma Chemical Co.). For double staining with ER-TR9 and antisialoadhesin, sections were incubated with ER-TR9 (reference 19; provided by Dr. P. Leenen, Erasmus University, Rotterdam, The Netherlands) followed by alkaline phosphatase–conjugated goat anti–rat IgM/IgG (Southern Biotechnology Associates, Inc.). After blocking with 20% normal rat serum in PBS, sections were incubated with biotinylated rat antisialoadhesin (provided by Dr. P.R. Crocker, University of Oxford, Oxford, UK [20]) followed by peroxidase-conjugated streptavidin. Bound peroxidase activity was detected by staining with amino-ethylcarbazol (Sigma Chemical Co.), and alkaline phosphatase activity was visualized with naphthol AS-MX phosphate and fast blue BB salt. All incubations were carried out under humidified conditions, and slides were washed in PBS between steps.
Immunization Protocol.
Indicated groups of mice were immunized intraperitoneally with 108 SRBC in PBS on days 0 and 21. Mice were bled on day 28 for measurement of anti-SRBC serum antibodies.
ELISA for SRBC-specific Serum Antibodies.
Sera from immunized mice were assayed using SRBC-specific ELISA for IgG1 antibodies as described previously (6). In brief, 96-well Immuno-Maxisorp plates (Nunc, Roskilde, Denmark) were coated with a solubilized extract from SRBC (100 μl at 5 μg/ml [21]) suspended in carbonate buffer, pH 9.6. Plates were washed with 0.05% Tween 20 in PBS and blocked with 1% BSA in PBS. Serum samples diluted in PBS containing 0.05% Tween 20, 1% BSA, and 1 M NaCl were incubated overnight at 4°C. Horseradish peroxidase–conjugated goat anti–mouse IgG1 (Southern Biotechnology Associates, Inc.) was diluted 1:5,000 in PBS containing 1% BSA, and 100 μl/well was added and incubated for 1 h at room temperature. ELISAs were developed with 0.4 mg/ml o-phenyldiamine dihydrochloride (Sigma Chemical Co.) in 0.05 M phosphate-citrate buffer, pH 5.0, containing 0.03% H2O2, stopped with 2 M H2SO4, and OD490 from duplicate wells was measured using a microplate reader (MRX; Dynatech Laboratories, Inc., Chantilly, VA).
Measurement of TNF Production after LPS Stimulation.
Levels of TNF in macrophage supernatants and sera were determined as described previously (16). In brief, thioglycollate-elicited peritoneal macrophages were seeded at 5 × 105 cells/ml and incubated in the presence of 1 μg/ml LPS (Salmonella enteritidis, L6011; Sigma Chemical Co.) at 37°C, 5% CO2 for 24 h. Mice were injected intraperitoneally with 100 μg LPS in 0.5 ml PBS, and 90 min later blood samples were collected by cardiac puncture. The ELISA assay for murine TNF was provided by Dr. Wim Buurman (University of Limburg, Maastricht, The Netherlands) and performed as described previously (22).
Results
TNF-complemented LTα Knockout Mice.
LTα knockout mice (8, 9) as well as double LTα/TNF knockout mice (10) show a more severe disorganization of their splenic architecture compared with TNF (6) or LTβ knockout mice (11, 12). To determine whether the additional lymphoid abnormalities seen in the LTα knockout mice may be rescued by TNF-specific signals, we complemented LTα knockout mice with TNF-expressing transgenes (TgTNF/ LTα−/−). Two previously characterized TNF transgenic lines were used: Tg1278 mice expressing a human wild-type TNF transgene (15) and TgA86 mice expressing a mutant transmembrane form of murine TNF from a TNFΔ1-12/ globin hybrid gene construct (reference 16, and see Materials and Methods). Tg1278 mice are free of pathology and express a low level of wild-type human TNF mRNA in several tissues, including thioglycollate-elicited peritoneal macrophages, thymus, lung, spleen, kidney, brain, skin, and joints. Similar to endogenous murine TNF, low-level mRNA expression of the wild-type human TNF transgene does not result in a detectable level of TNF protein secretion in either sera or supernatants from thioglycollate- elicited peritoneal macrophages as assessed by ELISA or cytotoxicity assays (not shown). However, after LPS stimulation of ex vivo peritoneal macrophages, correct upregulation of transgene mRNA (15) and of protein production (not shown) could be demonstrated. On the other hand, TgA86 mice express a constitutively high level of the TNFΔ1-12/globin mRNA in several tissues, including thioglycollate-elicited peritoneal macrophages, thymus, lung, spleen, mesenteric lymph nodes, kidney, heart, brain, skin, and joints (16). Constitutive overproduction of a bioactive transmembrane TNF protein is suggested by the development of chronic inflammatory arthritis in these mice (16), indicating aberrant regulation probably resulting from the absence of the putatively suppressive TNF 3′-UTR from the mRNA of this specific transgene.
Macroscopic examination of TNF-complemented LTα knockout mice (n >7 per transgenic line) showed that they lack mesenteric and peripheral lymph nodes and Peyer's patches, confirming a TNF-independent role for LTs in the organogenesis of these lymphoid tissues. However, in both TgTNF/LTα−/− mouse lines, a substantial preservation of splenic structure could be observed (see below), indicating a composite nature of the LTα null mutation.
Rescued Splenic T/B Cell Segregation in TNF-complemented LTα Knockout Mice Is p55TNFR Dependent.
Double immunostaining with anti-B220 and anti-CD3 antibodies in spleen sections of Tg1278/LTα−/− and TgA86/LTα−/− mice revealed a conserved segregation of B and T cell zones, with T cells clustered around the central arterioles and B cells located peripheral to the T cell zone (Fig. 1). This structural feature is directly comparable to the appearance of B/T cell areas in the spleens of LTβ−/− mice (Fig. 1), but is lacking completely in the spleens of LTα knockout mice (reference 8, and Fig. 1). These results show that even in the absence of LTα, TNF is sufficient to maintain correct segregation of B/T cell areas in the spleen.
Figure 1.

Rescued T/B cell segregation and B cell follicle formation in TgTNF/LTα−/− mice is p55TNFR dependent. Immunocytochemical analysis was performed on cryostat sections of spleens from wild-type (WT), LTα−/−, LTβ−/−, 1278/LTα−/−, A86/LTα−/−, and A86/LTα−/−/p55TNFR−/− mice. Sections were stained with anti-B220 (blue) for B cells, anti-CD3 (brown) for T cells, anti-IgM (brown) for IgM+ B cells, and anti-IgD (blue) for IgD+ B cells. Original magnifications: ×40 for B220/CD3 staining, and ×100 for IgM/IgD staining.
To assess whether the observed involvement of TNF in rescuing B/T cell segregation in LTα knockout mice is p55TNFR dependent, double TgA86/LTα−/− mice were bred into a p55TNFR knockout background (14). Splenic architecture in triple TgA86/LTα−/−/p55TNFR−/− mice remained indistinguishable from control LTα−/− mice (Fig. 1) and LTα−/−/p55TNFR−/− mice (not shown), demonstrating that in the absence of the p55TNFR, transgenic TNF expression could not rescue the splenic phenotype in LTα knockout mice. Therefore, restoration of splenic structure in LTα knockout mice by TNF is mediated via the p55TNFR.
Rectified B Cell Follicles and FDC Networks but Absent Marginal Zones in Spleens from TgTNF/LTα−/− Mice.
To determine whether transgenic TNF expression could reconstitute deficient follicular organization in LTα knockout mice, we analyzed splenic sections from TgTNF/LTα−/− mice using double immunostaining with anti-IgM and anti-IgD antibodies. By this staining, B cell follicles containing IgM+/IgD+ B cells can be clearly discriminated from the IgMhigh/IgDlow marginal zone B cell population in splenic sections from wild-type mice (Fig. 1). Consistent with previous studies (23), double IgM/IgD staining revealed the absence of follicular organization in spleens from LTα knockout mice (Fig. 1). However, similar analyses in spleens from Tg1278/LTα−/−, TgA86/LTα−/−, and LTβ−/− mice (Fig. 1) revealed the presence of organized primary B cell follicles although at reduced size and numbers compared with wild-type controls. Double immunostaining with anti-B220 as a marker for B cells, and an antibody to CR1 (mAb 8C12) as a marker for FDCs (18), showed that the follicular structures observed in TgTNF/LTα−/− and LTβ−/− mice contain networks of CR1+ FDCs (Fig. 2). These FDC networks were greatly diminished in number and size; however, similar to wild-type FDCs, they showed a typical network organization and follicular localization. Thus, even in the absence of LTα, TNF has the capacity to support, albeit suboptimally, the development and maintenance of organized B cell follicles and FDC networks.
Figure 2.

Restoration of FDC networks and germinal centers but not marginal zones in TgTNF/LTα−/− mice. Immunocytochemical analysis was performed on cryostat sections of spleens from LTα−/−, LTβ−/−, 1278/LTα−/−, A86/LTα−/−, and wild-type control mice. For the detection of FDC networks and germinal centers, spleens were harvested from mice 10 d after immunization with 108 SRBC. Blue, CR1, PNA, and ER-TR9 stainings; brown, B220; red, sialoadhesin (Siaload.). Original magnifications: ×285 for CR1/B220 staining, ×155 for PNA/B220 staining, and ×100 for ER-TR9/ sialoadhesin staining.
To examine the structure of the splenic marginal zone in TgTNF/LTα−/− mice, we performed immunocytochemical analysis of splenic sections using markers specific for the specialized macrophage populations of the marginal zone. Double immunostaining with ER-TR9, an mAb recognizing marginal zone macrophages (19), and the 1C2 mAb against mouse sialoadhesin (20), which stains specifically the metallophilic macrophages in the spleen (24), revealed the characteristic concentric organization of these macrophage subsets peripheral to the white pulp in spleen sections from wild-type mice (Fig. 2). However, similar to spleen sections from LTα knockout mice (Fig. 2), double ER-TR9/antisialoadhesin staining of sections from TgTNF/LTα−/− and LTβ−/− spleens showed the absence of these macrophage populations of the marginal zone (Fig. 2). Absence of metallophilic macrophages was also shown using the MOMA-1 mAb (data not shown; provided by G. Kraal, Vrije Universiteit, Amsterdam, The Netherlands), and staining using the R3-3C12C7 anti–mucosal addressin cell adhesion molecule 1 mAb (provided by B. Holzmann, Technical University of Munich, Munich, Germany) demonstrated the complete absence of marginal zone mucosal addressin cell adhesion molecule 1 expression from the spleens of LTα−/−, Tg1278/LTα−/−, TgA86/LTα−/−, and LTβ−/− mice (not shown). Deficient marginal zone formation in these mice was also confirmed by the absence of the characteristic Ig-Mhigh/IgDlow marginal zone B cell population as assessed by double IgM/IgD staining (Fig. 1). Taken together, these data confirm the requirement for LT function in the development of the splenic marginal zone.
Germinal Center Formation and IgG Antibody Responses in TgTNF/LTα− /− Mice.
To investigate whether the rectified follicular organization in TgTNF/LTα−/− mice could support development of germinal centers, we analyzed germinal center formation in the spleens of wild-type, LTα−/−, LTβ−/−, and TgTNF/LTα−/− mice 10 d after immunization with the T cell–dependent (TD) antigen SRBC. Double immunocytochemical analysis using anti-B220 antibodies as a B cell marker and PNA as a marker for germinal center B cells demonstrated the presence of typical germinal centers forming within B cell follicles in wild-type mice (Fig. 2). Consistent with previous studies (9), control LTα−/− mice did not form typical germinal centers, although rare aggregates of PNA+ cells could be detected around central arterioles (reference 12, and Fig. 2). Similar analyses of spleen sections from immunized Tg1278/LTα−/−, TgA86/ LTα−/−, and LTβ−/− mice revealed the presence of PNA+ germinal centers forming within B cell follicles (Fig. 2). Although the number and size of these germinal centers are reduced compared with wild-type mice, their formation within B cell follicles, their typical appearance as B220+IgD− areas surrounded by IgD+ follicular mantle B cells (not shown), and the finding that they contain FDC networks distinguish them from the PNA+ patches often observed to form around central arterioles in LTα (12) and TNF knockout mice (6).
To investigate the ability of the TNF-complemented LTα knockout mice to respond to a TD immunization, we tested the secondary antibody responses of TgTNF/LTα−/− mice to the TD antigen SRBC. Wild-type, Tg1278/ LTα−/−, and LTα−/− mice were immunized intraperitoneally with SRBC on days 0 and 21, and anti-SRBC IgG1 antibody responses were measured on day 28 using an antigen-specific ELISA. Tg1278/LTα−/− mice showed increased levels of IgG1 anti-SRBC antibodies compared with LTα−/− mice, although they could not reach the levels produced by wild-type mice (Fig. 3). Similar results were observed using TgA86/LTα−/− mice (data not shown). Thus, the observed germinal centers in the TNF-complemented LTα−/− mice appear functional and contribute positively, albeit suboptimally, to the development of a TD humoral immune response.
Figure 3.
Immune response to the TD antigen SRBC. Wild-type (WT), LTα−/−, and 1278/LTα−/− mice were immunized intraperitoneally with 108 SRBC in PBS on days 0 and 21. Mice were bled on day 28, at which time serum titers to SRBC-specific IgG1 were determined by ELISA. Data are means ± SE of five mice per group.
A Reduced Level of TNF Production in LTα Knockout Mice.
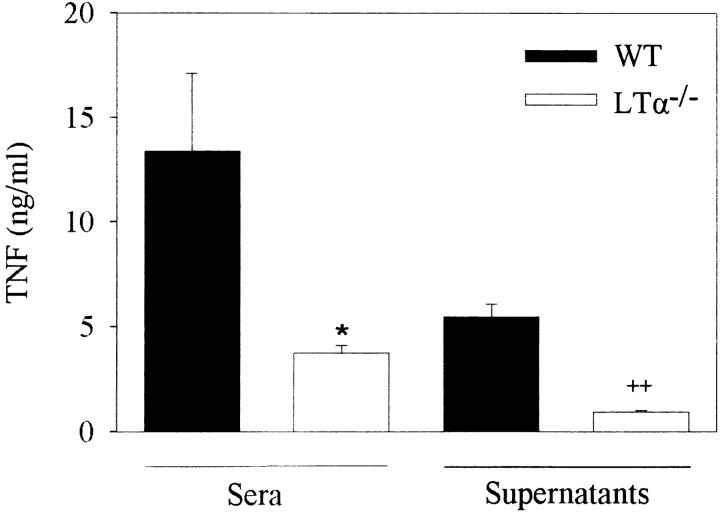
Given the surprising result of partial reconstitution of the splenic phenotype of the LTα knockout mice with TNF-expressing transgenes, and the striking similarities in the splenic architecture of TgTNF/LTα−/− and LTβ−/− mice, it would seem likely that the splenic phenotype of the LTα knockout mice could actually result from a coexistent deficiency in TNF production in these mice. To address this question, we measured levels of TNF protein production in LPS-stimulated LTα knockout mouse sera or ex vivo peritoneal macrophage supernatants. Compared with background-matched wild-type control mice, LTα knockout mice show severely reduced accumulation of TNF protein in both sera and macrophage exudates (Fig. 4), indicating that in addition to the LTα null mutation, this strain of mice may show further complications due to inherent defects in the neighboring TNF gene expression. Further analyses should address the underlying mechanism producing the additional defect on TNF expression and assess the impact of this phenomenon in the interpretation of different phenotypes occurring in the LTα knockout mouse strain.
Figure 4.
ELISA analysis of TNF levels after LPS induction. LTα−/− (n = 5) mice and wild-type (WT; n = 4) controls were injected intraperitoneally with 100 μg LPS, and serum levels of TNF were determined 90 min later. Thioglycollate-elicited peritoneal macrophages from LTα−/− (n = 4) mice and wild-type (n = 3) controls were stimulated with 1 μg/ml LPS for 24 h, and supernatants from individual cultures were assayed for the presence of TNF. Data are means ± SE per group. *P <0.03; ++ P <0.001 compared with wild-type mice.
Discussion
Targeted disruption of genes encoding ligands and receptors in the TNF/LT family have clearly established the important roles these molecules play in regulating the development and function of secondary lymphoid tissues (13, 25, 26). From these studies, a role has been proposed for LTαβ heterotrimers, presumably signaling through the LTβR, in the organogenesis of lymph nodes and Peyer's patches and in the regulation of splenic structural organization. However, apparent differences between the lymphoid phenotypes of LTα and LTβ knockout mice suggested that LTα should have additional biological activities independent of LTβ (11–13). For example, defects in splenic T/B cell organization and complete absence of FDC networks and typical germinal centers in LTα or double LTα/TNF knockout mice (8–10) could not be fully reproduced in LTβ-deficient mice (12), which show more conserved B/T cell organization and retain some capacity to form FDC networks and germinal centers (Figs. 1 and 2). Using complementation analysis, we show in this study that many of the phenotypic complications of LTα knockout mice in the spleen may be compensated by the reintroduction of functional TNF transgenes. Complementation of LTα knockout mice with TNF-encoding transgenes leads to a p55TNFR-dependent restoration of B/T cell zone segregation and to the partial preservation of B cell follicles, FDC networks, and germinal centers. Interestingly, the TNF-complemented LTα-deficient phenotype is strikingly similar to the splenic phenotype of LTβ-deficient mice, as demonstrated in this study (Figs. 1 and 2). These observations suggest that defective TNF expression may contribute to the complex phenotype of the LTα knockout mice. Alternatively, it may be suggested that complementation of the LTα knockout phenotype by TNF is due to a substitution for LT function rather than restoration of defective TNF expression, for example by transgenic overexpression of TNF. However, this seems unlikely, since phenotypic complementation by TNF occurs similarly with three independent transgenic lines carrying either correctly regulated or overexpressing TNF transgenes (i.e., Tg1278 mice [15] expressing correctly regulated levels of human wild-type TNF, TgA86 mice [16] overexpressing a bioactive murine transmembrane TNF, or Tg6079 mice [our unpublished data and not shown here] expressing wild-type murine TNF). In addition, transgenic expression of LTα in LTα knockout mice was reported recently as not sufficient to rescue defective splenic architecture, although it could restore lymph node organogenesis (27). Interestingly, the observed splenic phenotype of TgLTα/LTα−/− mice seems to be similar to the phenotype of TNF-deficient mice (6), with B cells organized in ring-like structures around the periarteriolar lymphoid sheath, and PNA+ patches forming around central arterioles (see Fig. 4 C in reference 27).
Most importantly, decreased expression of the endogenous TNF gene in LTα knockout mice could be documented in this study by TNF-specific quantitative assays (Fig. 4). It is not clear, however, whether this defect in TNF expression occurs at the level of gene transcription, mRNA translation, or protein processing. Although this certainly awaits further detailed characterization, it is tempting to speculate that defective expression of the TNF gene in the LTα knockout mouse strain is due to transcriptional interference caused by retention of a phosphoglycarate kinase (PGK)-neo selection cassette within the targeted LTα locus. This is now well documented in several other cases where retention of the PGK-neo cassette in targeted loci has yielded unexpected phenotypes due to the altered expression of neighboring genes (28, 29). In light of the evidence presented here, interpretation of the different phenotypes occurring in the LTα knockout mice should be carefully readdressed.
Interestingly, although expression of TNF is sufficient, even in the absence of LTα, to drive white pulp organization into distinct B and T cell areas and to partially support follicular structure and germinal center formation, the function of LTs is clearly required for the development of the marginal zone, as documented in this study but also as suggested previously by the complete absence of marginal zone structures in LTβ-deficient mice (11, 12). In this context, it is perhaps not very surprising that humoral responses to SRBC, as measured in the TNF-complemented LTα knockout system, although partially restored could not reach normal levels. This may be due to the presence of a suboptimal number of germinal centers in the spleen of these mice, but it may also be due to the documented complete absence of marginal zones in this system. Indeed, marginal zones are thought to play an important role in processing particulate antigens such as SRBC (30).
Taken together with the presence of mesenteric and cervical lymph nodes in LTβ-deficient mice (11, 12), our evidence that TNF-complemented LTα knockout mice still lack their mesenteric and peripheral lymphoid organs supports previous suggestions that LTα should have additional lymphoid organogenetic functions, independent of LTβ (11). It is perhaps interesting to note that correct B/T cell segregation, and also primary follicular organization and germinal center reactivity, appear in this study to be tightly regulated phenomena occurring in the absence of preserved marginal zone structures, and seemingly uninfluenced by a coinciding complete absence of secondary lymphoid organs. Therefore, it seems that they represent primary phenomena directly dependent on the local functioning of TNF and LTs.
Inhibition of LTαβ signaling either in transgenic mice expressing a soluble LTβR–IgG1 fusion protein (31), or by administration of soluble LTβR–Ig fusion proteins in normal adult mice (32), was shown to have profound effects on splenic organization. Similar effects were not observed when a p55TNFR–Ig fusion protein was administered to adult mice, suggesting that at least within the 3–4-wk time frame of inhibition, the TNF/p55TNFR system is not required for the maintenance of splenic architecture (32). We and others have previously suggested that a function for TNF/p55TNFR in splenic organization may be in the development and differentiation of FDC networks which would then support follicular organization and germinal center reactivity. If this is true, then given the long-living character of the FDCs, one would expect that only long-term inhibition of TNF signaling would be revealing for a role in the maintenance of splenic organization in the adult. The role of the TNF/p55TNFR system may also seem dispensable for correct B/T cell segregation, since this phenomenon is not affected in either TNF (6) or p55TNFR knockout mice (14). However, our present evidence suggests that even in the absence of LTs, TNF is sufficient to suboptimally support development and maintenance of follicular organization, indicating that the LT system shares a redundant role with TNF in regulating the conserved appearance of splenic white pulp areas. In addition to the splenic defects, administration of LTβR–Ig during gestation disrupted lymph node development (33), suggesting a basic organogenetic role for LTα/β in these processes. However, a differential role for TNF/p55TNFR in these phenomena is suggested by the presence of secondary lymphoid organs with clear B/T cell zone segregation in the TNF- (6) or p55TNFR-deficient strains of mice (14). As discussed above, a function for TNF/p55TNFR in splenic organization may be in the development and differentiation of FDC networks which would then support follicular organization and germinal center reactivity (6, 7, 34). In support of this hypothesis, recent bone marrow transplantation experiments in LTα- and p55TNFR-deficient mice showed that FDC clustering induced by wild-type bone marrow transfers is dependent on the presence of the p55TNFR on nonhematopoietic cells (35, 36). On the other hand, the LT/LTβR system is expected to function on hematopoietic lineage cell interactions with nonlymphocytic stroma elements (37, 38), and it may be that such interactions control the basic architecture, which appears essential for the organogenesis of the lymph nodes but also for the fine structural organization of the spleen. Formation of FDC networks should be a composite phenomenon requiring an optimal splenic infrastructure, perhaps provided by LTs, and a TNF/p55TNFR-specific signal which leads to the maturation/differentiation and/or follicular localization of FDCs. The capacity to form FDC networks and germinal centers even in the absence of LTs, as documented in both TNF-complemented LTα knockout mice and LTβ knockout mice, supports this hypothesis. Therefore, it is likely that the role of LTs is functionally distinct from that of TNF, and that both pathways need to cooperate for the optimal development and maintenance of splenic structure.
Acknowledgments
We wish to thank Klaus Pfeffer and Sergei Nedospasov for providing LTβ knockout mice and for useful discussions, Horst Bluethmann for providing p55TNFR knockout mice, Wim Buurman for the murine TNF-specific ELISA, Paul Crocker for antisialoadhesin antibody, Steve Cobbold for the KT3 anti-CD3 antibody, Peter Leenen for the ER-TR9 antibody, George Kraal for the MOMA-1 antibody, Dimitris Kontoyiannis for sharing unpublished data, and Anna Kefalaki for technical assistance with the histological analyses.
Abbreviations used in this paper
- FDC
follicular dendritic cell
- LT
lymphotoxin
- PNA
peanut agglutinin
- TD
T cell–dependent
- Tg
transgenic
- UTR
untranslated region
Footnotes
This project was supported in part by the Hellenic Secretariat for Research and Technology, and by European Commission grants BIO-CT96-0077 and BIO-CT96-0174.
References
- 1.Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 2.Pokholok DK, Maroulakou IG, Kuprash DV, Alimzhanov MB, Kozlov SV, Novobrantseva TI, Turetskaya RL, Green JE, Nedospasov SA. Cloning and expression analysis of the murine lymphotoxin beta gene. Proc Natl Acad Sci USA. 1995;92:674–678. doi: 10.1073/pnas.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 4.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 5.Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-beta-specific receptor. Science. 1994;264:707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 6.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha–deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc Natl Acad Sci USA. 1997;94:6319–6323. doi: 10.1073/pnas.94.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 10.Eugster HP, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 12.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Boehmer H. Lymphotoxins: from cytotoxicity to lymphoid organogenesis. Proc Natl Acad Sci USA. 1997;94:8926–8927. doi: 10.1073/pnas.94.17.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. . Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 15.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO (Eur Mol Biol Organ) J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexopoulou L, Pasparakis M, Kollias G. A murine transmembrane tumour necrosis factor transgene induces arthritis by cooperative p55/p75 tumour necrosis factor receptor signaling. Eur J Immunol. 1997;27:2588–2592. doi: 10.1002/eji.1830271018. [DOI] [PubMed] [Google Scholar]
- 17.Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140:3066–3072. [PubMed] [Google Scholar]
- 19.van Vliet E, Melis M, van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985;33:40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR, Freeman S, Gordon S, Kelm S. Sialoadhesin binds preferentially to cells of the granulocytic lineage. J Clin Invest. 1995;95:635–643. doi: 10.1172/JCI117708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly BS, Levy JG, Sikora L. The use of the enzyme-linked immunosorbent assay (ELISA) for the detection and quantification of specific antibody from cell cultures. Immunology. 1979;37:45–52. [PMC free article] [PubMed] [Google Scholar]
- 22.Bemelmans MHA, Gouma DJ, Buurman WA. LPS-induced sTNF-receptor release in vivo in a murine model. Investigation of the role of tumor necrosis factor, IL-1, leukemia inhibiting factor, and IFN-gamma. J Immunol. 1993;151:5554–5562. [PubMed] [Google Scholar]
- 23.Fu YX, Huang G, Matsumoto M, Molina H, Chaplin DD. Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc Natl Acad Sci USA. 1997;94:5739–5743. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crocker PR, Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989;169:1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasparakis M, Alexopoulou L, Douni E, Kollias G. Tumour necrosis factors in immune regulation: everything that's interesting is . . . new! . Cytokine Growth Factor Rev. 1996;7:223–229. doi: 10.1016/s1359-6101(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 26.Liu YJ, Banchereau J. Mutant mice without B lymphocyte follicles. J Exp Med. 1996;184:1207–1211. doi: 10.1084/jem.184.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacca R, Turley S, Soong L, Mellman I, Ruddle NH. Transgenic expression of lymphotoxin restores lymph nodes to lymphotoxin-α-deficient mice. J Immunol. 1997;159:4252–4260. [PubMed] [Google Scholar]
- 28.Olson EN, Arnold HH, Rigby PW, Wold BJ. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 29.Pham CT, MacIvor DM, Hug BA, Heusel JW, Ley TJ. Long-range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci USA. 1996;93:13090–13095. doi: 10.1073/pnas.93.23.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger R, Browning JL, Michie SA, van Ewijk W, McDevitt HO. Disrupted splenic architecture, but normal lymph node development in mice expressing a soluble lymphotoxin-beta receptor-IgG1 fusion protein. Proc Natl Acad Sci USA. 1996;93:13102–13107. doi: 10.1073/pnas.93.23.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay F, Majeau GR, Lawton P, Hochman PS, Browning JL. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur J Immunol. 1997;27:2033–2042. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- 33.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Hir M, Bluethmann H, Kosco-Vilbois MH, Muller M, di Padova F, Moore M, Ryffel B, Eugster HP. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto M, Fu YX, Molina H, Huang G, Kim J, Thomas DA, Nahm MH, Chaplin DD. Distinct roles of lymphotoxin alpha and the type I tumor necrosis factor (TNF) receptor in the establishment of follicular dendritic cells from non-bone marrow–derived cells. J Exp Med. 1997;186:1997–2004. doi: 10.1084/jem.186.12.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tkachuk M, Bolliger S, Ryffel B, Pluschke G, Banks TA, Herren S, Gisler RH, Kosco-Vilbois MH. Crucial role of tumor necrosis factor receptor 1 expression on nonhematopoietic cells for B cell localization within the splenic white pulp. J Exp Med. 1998;187:469–477. doi: 10.1084/jem.187.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Curr Top Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 38.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTβ+cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]