Abstract
Interleukin-1β–converting enzyme (ICE, caspase-1) regulates key steps in inflammation and immunity, by activating the proinflammatory cytokines interleukin (IL-)1β and IL-18, or mediating apoptotic processes. We recently provided evidence for the regulation of caspase-1 activity via an endogenous inhibitor expressed by human vascular smooth muscle cells (SMCs) (Schönbeck, U., M. Herzberg, A. Petersen, C. Wohlenberg, J. Gerdes, H.-D. Flad, and H. Loppnow. 1997. J. Exp. Med. 185:1287–1294). However, the molecular identity of this endogenous inhibitor remained undefined. We report here that the serine proteinase inhibitor (serpin) PI-9 accounts for the endogenous caspase-1 inhibitory activity in human SMCs and prevents processing of the enzyme's natural substrates, IL-1β and IL-18 precursor. Treatment of SMC lysates with anti–PI-9 antibody abrogated the caspase-1 inhibitory activity and coprecipitated the enzyme, demonstrating protein–protein interaction. Furthermore, PI-9 antisense oligonucleotides coordinately reduced PI-9 expression and promoted IL-1β release. Since SMCs comprise the majority of cells in the vascular wall, and because IL-1 is implicated in atherogenesis, we tested the biological validity of our in vitro findings within human atheroma in situ. The unaffected arterial wall contains abundant and homogeneously distributed PI-9. In human atherosclerotic lesions, however, PI-9 expression correlated inversely with immunoreactive IL-1β, supporting a potential role of the endogenous caspase-1 inhibitor in this chronic inflammatory disease. Thus, our results provide new insights into the regulation of this enzyme involved in immune and inflammatory processes of chronic inflammatory diseases, and point to an endogenous antiinflammatory action of PI-9, dysregulated in a prevalent human disease.
Keywords: caspase-1, interleukin 1β, PI-9, smooth muscle cells, atherosclerosis
Introduction
IL-1β–converting enzyme (ICE, caspase-1) 1 2 is the prototype of a family of cysteine proteases termed caspases, sharing the active site cysteine and aspartate binding clefts 3. The biological function of caspase-1 was originally thought to be restricted to the maturation process of IL-1β, a central mediator in the cytokine network 4 5 6 7. Proteolytic maturation of the inactive, 33-kD IL-1β precursor (proIL-1β) into the 17-kD, biologically functional form 8 9 10 11 results from cleavage at the Asp116–Ala117 site 1 2 12 13 14. However, recent studies revealed that caspase-1 mediates processes relevant to immune and inflammatory reactions, in addition to IL-1β activation. The enzyme has been implicated in the activation of another inflammatory cytokine, IL-18, originally termed IFN-γ inducing factor (IGIF) 15 16 17 18 19 20. Similar to IL-1β, IL-18 is expressed as an inactive precursor (24 kD), requiring proteolytic conversion via caspase-1 into the active, 18-kD form 21. The biological function of IL-18 was originally thought to be restricted to the induction of another inflammatory cytokine, IFN-γ, which has well-known roles in inflammation and Th1 immune responses 18 20 22. However, recent work indicated a much broader role for IL-18 in immunity and inflammation, mediating the expression of other proinflammatory cytokines, certain chemokines, and Fas ligand 16. In addition to the activation of proinflammatory mediators, caspase-1 is the prototype of a family of enzymes implicated in the control of apoptosis. Although the exact function of caspase-1 is controversial, this enzyme may participate in programmed cell death 23 24 25 26 27.
Since caspase-1–mediated processing of IL-1β and IL-18 as well as the promotion of apoptosis are considered crucial processes in chronic inflammatory diseases, the (physiologic and therapeutic) regulation of caspase-1 activity has garnered considerable interest in recent years. The enzyme was originally isolated and cloned from cells of the monocytic lineage 1 2 12 13 14. However, not surprisingly given the widespread expression of its substrate, IL-1β precursor 4, other cell types also express caspase-1, including endothelial cells (ECs) and smooth muscle cells (SMCs), fibroblasts, and epithelial and epidermal cells 28 29 30. Typically, caspase-1 is synthesized as an inactive 45-kD precursor and autocatalytically processed to form an active homodimeric complex consisting of the 10- and 20-kD subunit, (p20/p10)2 31 32. However, detection of mature immunoreactive caspase-1 protein does not always correspond to its biological activity 29 33. Indeed, regulation of caspase-1 activity remains obscure. Early studies on modulators of caspase-1 activity revealed that besides synthetic peptides 1 34, certain viral proteins can block the enzyme's activity 35 36 37. Cowpox virus effectively diminished inflammatory responses against viral infection in the chick via a specific inhibitor of caspase-1, encoded by the cytokine response modifier A (CrmA) gene 35. Previously, we and others have provided evidence for the presence of a human-derived endogenous inhibitor 29 33. We described a constitutively expressed, heat-labile, cell-associated caspase-1 inhibitory activity in human vascular SMCs 29, the most abundant cell type in arteries and a central participant in the pathogenesis of vascular diseases 38 39.
The viral caspase-1 inhibitor CrmA belongs to the family of serine proteinase inhibitors, termed serpins 40. Recently, we cloned the human serpin PI-9. This protein shares 54% identity with residues found in the reactive center loop of the cowpox virus CrmA serpin 41 42 and, as recently described, inhibits granzyme B–mediated apoptosis as well as caspase-1–mediated cleavage of synthetic substrates 43 44. PI-9 shares several biochemical characteristics with the endogenous caspase-1 inhibitory activity previously identified in vascular SMCs, including the constitutive and cell-associated expression, as well as a molecular mass of 42 kD 41. Thus, we tested the hypothesis that PI-9 accounts for the endogenous caspase-1 inhibitory activity in human vascular SMCs.
We report here that human vascular SMCs constitutively express PI-9, and that this serpin accounts for the endogenous caspase-1 inhibitory activity in these cells, preventing processing of the native caspase-1 substrates IL-1β and IL-18 precursor. The inhibitory activity of PI-9 requires protein–protein interaction with the enzyme. Since vascular SMCs comprise the most abundant cell type in arteries, and IL-1 likely participates in the pathogenesis of vascular diseases 38 39, we further analyzed the expression of PI-9 within undiseased and atherosclerotic human arteries. The homogenous, abundant expression of PI-9 in normal tissue contrasts with its pattern of expression in atherosclerotic lesions. Within human atheroma, the inhibitor localized inversely with caspase-1 and IL-1β expression. Our results suggest a novel endogenous antiinflammatory function of PI-9, and indicate dysregulation of this control mechanism in an important human disease.
Materials and Methods
Materials.
Human recombinant mature IL-1β was obtained from Endogen. Human recombinant precursor IL-1β (proIL-1β) was purchased from Cistron. Recombinant human PI-8 and PI-9, as well as the respective polyclonal antibodies (raised against the respective full-length protein; affinity-purified), were generated as described previously 41. The anti–IL-1β antibody was obtained from Upstate Biotechnology. The anti-ICEP20 antibody was provided by Santa Cruz biotechnology. Control mouse and rabbit Ig were obtained from Sigma Chemical Co. Recombinant human caspase-1 was provided by Dr. Nancy Thornberry (Merck Laboratories, Rahway, NJ). Recombinant human caspase-3 was purchased from Transduction Laboratories. Gelsolin was provided by Dr. T. Azuma (Brigham and Women's Hospital). The synthetic tetrapeptide fluorogenic caspase-3 substrate (Ac-DEVD-AMC) and the synthetic caspase inhibitor DEVD-CHO were obtained from PharMingen.
Cell Isolation and Culture.
Human vascular SMCs were isolated from human saphenous veins by explant outgrowth 45, and cultured in DMEM (BioWhittaker) supplemented with 1% l-glutamine (BioWhittaker), 1% penicillin/streptomycin (BioWhittaker), and 10% fetal bovine serum (FBS; Atlanta Biologicals). Cells were subcultured after trypsinization (0.5% trypsin [Worthington Biochemicals], 0.2% EDTA [EM Science]), in 75-cm2 culture flasks (Becton Dickinson) and used throughout passages two to four. SMCs were cultured 24 h before the experiment in IT (insulin/transferrin) medium lacking FBS, as described previously 46. SMCs were characterized by immunostaining with anti-SMC α-actin antibody (Enzo Diagnostics Inc.).
Mononuclear phagocytes were isolated by density gradient centrifugation 47, using Lymphocyte Separation Medium (Organon-Teknika), and subsequent counterflow elutriation from freshly prepared human PBMCs obtained from leukopacs of healthy donors (provided by Dr. B. Rollins, Dana-Farber Cancer Institute, Boston, MA). The purity of monocytes/macrophages was ≥96%, as determined by FACS® analysis (anti–human CD68 mAb FITC; PharMingen).
As the source of IL-18, THP-1 cells were obtained from American Type Culture Collection (TIB-202) and were maintained in RPMI 1640 with 2.5 g/liter glucose, 1 mM Hepes, 1 mM sodium pyruvate, 50 μM 2-ME, containing 10% human serum. To obtain lysates, cells were pelleted (500 g, 10 min), resuspended in 106 cells/ml sterile water, and exposed to three freeze–thaw cycles.
Culture media and FBS contained <40 pg endotoxin/ml as determined by chromogenic Limulus amebocyte assay (QLC-1000; BioWhittaker).
Processing Assay.
For processing, recombinant human proIL-1β (20 nM), proIL-18 (equivalent to 50,000/ml cells), or gelsolin was incubated for 30 min with recombinant human caspase-1 (15 nM), native caspase-1 obtained from lysates of monocyte cultures (obtained by three freeze–thaw cycles), or recombinant human caspase-3 (25 nM) at 37°C in a final volume of 50 μl processing buffer (10 mM Hepes, 2 mM dithiothreitol, 5% glycerol; final concentrations). To analyze inhibition of processing, the indicated concentrations of PI-8 or PI-9 were preincubated with either the enzyme (recombinant or monocyte-derived caspase-1 or caspase-3) or the substrate (proIL-1β, proIL-18, or gelsolin) for the respective duration. For control purposes, a synthetic caspase inhibitor (DEVD-CHO) was used instead of the serpin. Processing was stopped by heating the samples (10 min, 95°C) in 10 μl SDS-PAGE (5×) sample buffer (0.2 M Tris, 5% glycerol, 0.1% SDS, 3% β-ME, 0.1 mg/ml bromophenol blue; final concentrations). Finally, samples were applied to SDS-PAGE and developed by either Coomassie stain (gelsolin) or Western blot analysis using the anti–human IL-1β or IL-18 antibody (1:1,000). To analyze the caspase-1 inhibitory activity in SMCs, culture lysates were harvested in processing buffer. After three freeze–thaw cycles, the lysates were preincubated with the recombinant enzyme (15 nM, 30 min, 37°C) before the IL-1β or IL-18 precursor (20 nM) was added for an additional 30 min.
For immunoprecipitation experiments, lysates of SMC cultures were harvested and incubated with nonimmune rabbit serum (24 h, 4°C; Vector Laboratories) to preclear the samples. The cell extracts were immunoprecipitated with the specific anti–PI-9 antibody (6 h, 4°C) and pelleted by subsequent addition of goat anti–rabbit IgG (18 h, 4°C; Jackson ImmunoResearch Laboratories) as well as protein A–Sepharose beads (2 h, 4°C; Amersham Pharmacia Biotech). Supernatants (corresponding to PI-9–depleted lysates of SMC cultures) and precipitates were either resuspended in SDS-PAGE loading buffer (200 mmol/liter Tris, 5% glycerol, 0.1% SDS, 3% β-ME, 0.1 mg/ml bromophenol blue), separated by SDS-PAGE, and analyzed by Western blotting, or were resuspended in processing buffer and applied to the processing assay.
Antisense Experiments.
Antisense PI-9 (5′-GAAAGAGTTTCCATGATGCAG-3′) and control (5′-TTACCGCGCCGTAGACGGGCA-3′) phosphorothioate oligodeoxynucleotides were synthesized and purified via reverse-phase HPLC by Integrated DNA Technologies, Inc. Subconfluent cultures of human vascular SMCs were washed twice with DMEM and subsequently incubated with DMEM containing Lipofectin (1 μg/ml; Life Technologies, Inc.) and the respective oligodeoxynucleotide (5 μM). Cells were maintained in DMEM for 72 h, before the 24-h stimulation (TNF-α, 50 ng/ml). Finally, lysates and supernatants of these SMC cultures were harvested and applied to Western blot analysis for PI-9 or caspase-1, and IL-1β ELISA, respectively.
Western Blot Analysis.
Cell extracts, equilibrated by total protein (25 μg total protein/lane), culture supernatants, or processing assay preparations were separated by standard SDS-PAGE under reducing conditions and blotted to polyvinylidene difluoride membranes (Bio-Rad) using a semidry blotting apparatus (0.8 mA/cm2, 30 min; Bio-Rad). Blots were blocked and first and second mAbs were diluted in 5% defatted dry milk/PBS/0.1% Tween 20. After 1 h of incubation with the respective primary antibody, blots were washed three times (PBS/0.1% Tween) and the secondary, peroxidase-conjugated, goat anti–mouse or goat anti–rabbit antibody (Jackson ImmunoResearch Laboratories) was added for an additional 1 h. Finally, the blots were washed (20 min, PBS/0.1% Tween 20) and immunoreactive proteins were visualized using the Western blot chemiluminescence system (NEN). Densitometric analysis of immunoreactive bands employed Image-Pro® software (Media Cybernetics) applied to digital images of the respective Western blots.
ELISA.
Release of IL-1β from human vascular SMCs was measured by ELISA, following the recommendations of the manufacturer (Endogen). In brief, SMC supernatants (200 μl) obtained from the antisense experiments were added for 1 h to 96-well modules (Nunc) coated with the capturing antibody (1 μg/ml, 4°C overnight). Subsequently the plates were washed (PBS/0.1% Tween) three times, and the respective biotin-labeled detecting mouse anti–human IL-1β mAb (0.5 μg/ml) was added (1 h). Finally, wells were incubated for 30 min with alkaline phosphatase (Vectastain ABC kit, AK-500; Vector Laboratories) and were washed four times. Antibody binding was detected by adding p-nitrophenyl phosphate (1.39 mg/ml; Sigma Chemical Co.), and absorbance was measured at 405 nm in a Dynatech plate reader. The amount of cytokine detected was calculated from a standard curve prepared from recombinant mature IL-1β. Samples were assayed in triplicate. Error bars represent SD.
Immunohistochemistry.
Surgical specimens of human carotid atheroma and aorta were obtained by protocols approved by the Human Investigation Review Committee at the Brigham and Women's Hospital. Nonatherosclerotic tissue was obtained from both carotid arteries and aortae, whereas atherosclerotic tissue employed only carotid specimen. Serial cryostat sections (5 μm) were cut, air dried onto microscope slides (Fisher Scientific), and fixed in acetone at −20°C for 5 min. Sections were preincubated with PBS containing 0.3% hydrogen peroxidase activity. The sections were then incubated (30 min) with primary or control (mouse myeloma protein MOPC-21; Sigma Chemical Co.) antibody, diluted in PBS supplemented with 5% appropriate serum. The subsequent processing was performed according to the manufacturer's recommendations (Universal Dako LSAB kit; Dako). Antibody binding was visualized with 3-amino-9-ethyl carbazole (Vector) according to the recommendations provided by the supplier. For colocalization of caspase-1 with PI-9, or either molecule with the respective cell type, double-immunofluorescence staining was performed. The goat anti–human ICEP20 antibody (1:100) was applied for 90 min followed by biotinylated anti–mouse secondary antibody for 45 min and Texas red–conjugated streptavidin (Amersham Pharmacia Biotech). Subsequent to application of the avidin/biotin blocking kit (Vector Laboratories), rabbit anti–human PI-9 antibody (1:50), anti–muscle actin mAb for SMCs (Enzo Diagnostics), anti-CD31 mAb for ECs (1:400; Dako), or anti-CD68 mAb for macrophages (Mφ, 1:600; Dako) was added, and sections were incubated overnight at 4°C. Subsequently, the appropriate secondary antibodies were applied for 30 min followed by streptavidin-FITC (Amersham Pharmacia Biotech).
Biochemical Analysis of Human Atherosclerotic Lesions.
Frozen tissue from nonatherosclerotic and atheromatous specimens, dichotomized by morphological criteria as stable or vulnerable plaques as described previously 48, were homogenized (Ultra-turrax T 25; IKA-Labortechnik) and lysed (0.3 g tissue/ml lysis buffer: 10 mM sodium phosphate, 150 mM sodium chloride, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 0.2% sodium azide). The lysates were clarified (13,000 g, 15 min), and the protein concentration for each tissue extract as well as for the cell culture samples was determined using a bicinchoninic acid (BCA) protein assay according to the instructions of the manufacturer (Pierce Chemical Co.). 50 μg total protein was applied to Western blot analysis.
Results
The Serpin PI-9 Inhibits Processing of the IL-1β and IL-18 Precursor by Native or Recombinant Caspase-1.
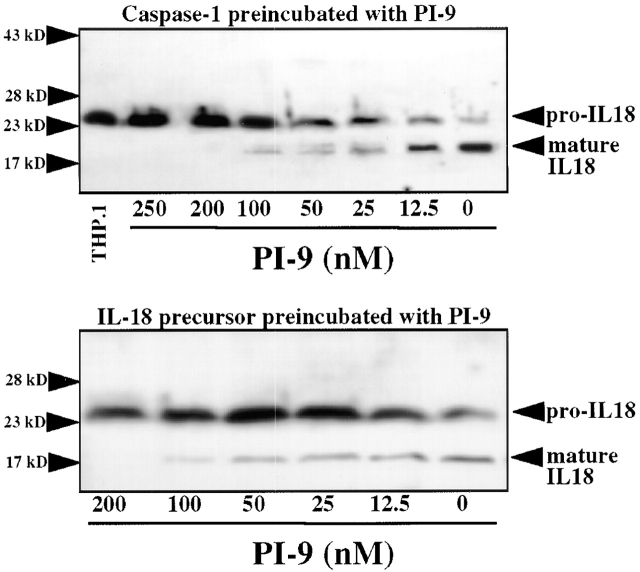
To determine whether PI-9 indeed inhibits processing of the native substrate by caspase-1, a processing assay was established in which the enzyme cleaves the 33-kD recombinant human IL-1β precursor (proIL-1β) into the expected 1 29 49 predominant 28- and 17-kD (as well as an occasional 25-kD) forms, as detected by Western blot analysis (Fig. 1 A). The inhibitory capacity of PI-9 was explored by addition of the serpin to the assay at various time points, i.e., allowing preincubation with either the enzyme or the substrate. When added after coincubation of proIL-1β with native (monocyte-derived) or recombinant caspase-1, PI-9 did not affect processing (Fig. 1 A). However, simultaneous incubation of PI-9, enzyme, and substrate in this assay yielded partial inhibition of proIL-1β processing, and when preincubated with the enzyme, PI-9 completely inhibited processing of the IL-1β precursor by native or recombinant caspase-1 (Fig. 1A and Fig. B). In addition to the time of preincubation, inhibition of caspase-1 processing activity by PI-9 depended on the temperature during preincubation, requiring preincubation at 37°C (using ≥100 nM PI-9) for maximal inhibition and showing diminished inhibitory activity at room temperature or 4°C. Partial inhibition occurred after times of preincubation as short as 5 min. In contrast to PI-9, a related serpin family member, PI-8 (50; as well as the more distant relative ovalbumin), did not affect caspase-1 activity, even when added at high concentrations (up to 200 μg/ml, ≈5 μM) or preincubated for extended periods of time (up to 24 h). Interestingly, PI-9 differentially inhibited the processing into the two predominant products, the 28- and 17-kD forms (Fig. 2). Complete inhibition of processing into the mature 17-kD form of IL-1β required 4–6 μg/ml (≈100–150 nM) PI-9 (n = 5; with an IC50 ≈ 57 ± 16 nM), whereas inhibition of processing into the 28-kD form required higher concentrations (>20 μg/ml; >500 nM) of PI-9 (n = 5; with an IC50 ≈ 272 ± 69 nM). Partial inhibition occurred when using as little as 0.5–1 nM PI-9. Similar concentration- and time-dependencies of the inhibition of caspase-1 processing activity were obtained when a second native substrate for the enzyme, IL-18, was used (Fig. 3). Interestingly, inhibition of the IL-18 processing activity also required the preincubation of the enzyme rather than the substrate for optimal effectiveness.
Figure 1.
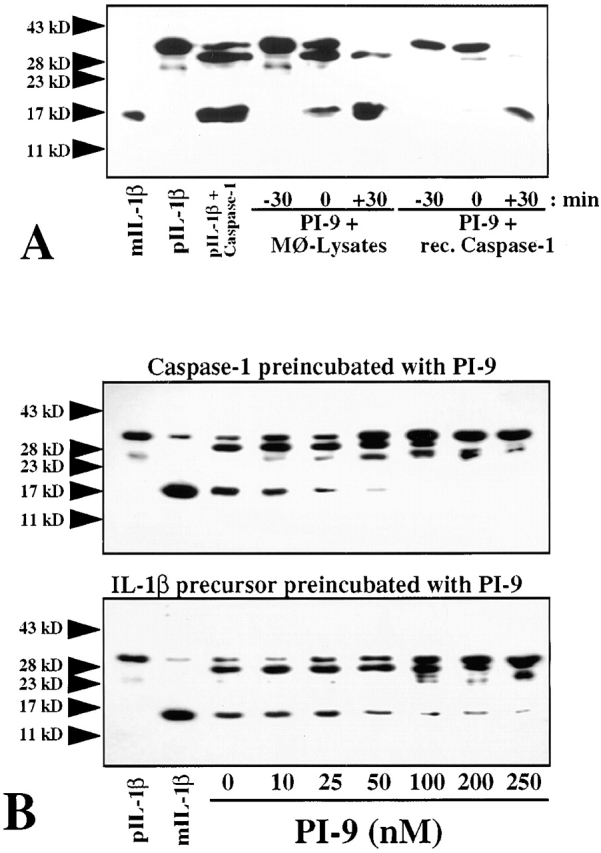
The human serpin PI-9 inhibits native and recombinant caspase-1 processing activity. (A) Human recombinant PI-9 (100 nM) was either preincubated for 30 min (−30) with native, monocyte-derived (Mφ-Lysates; equivalent to 106 Mφ/ml) or recombinant (rec.) caspase-1 (15 nM), applied simultaneously (0), or added 30 min after incubation (+30) of proIL-1β (20 nM) with the enzyme for 30 min at 37°C in a final volume of 50 μl processing buffer. (B) Human recombinant caspase-1 (15 nM, top) or human recombinant IL-1β precursor (20 nM, bottom) was preincubated for 30 min with the indicated concentrations of PI-9, before application to the processing assay. Processing was stopped by heating the samples in 10 μl SDS-PAGE sample buffer. The preparations were analyzed by 15% SDS-PAGE and subsequent Western blot analysis using anti–human IL-1β. For control purposes, recombinant mature (mIL-1β, 20 nM) and precursor (pIL-1β, 20 nM) IL-1β were applied. The positions of the molecular weight markers are indicated on the left (in kD). Similar data were obtained in three (A) or five (B) independent experiments.
Figure 2.
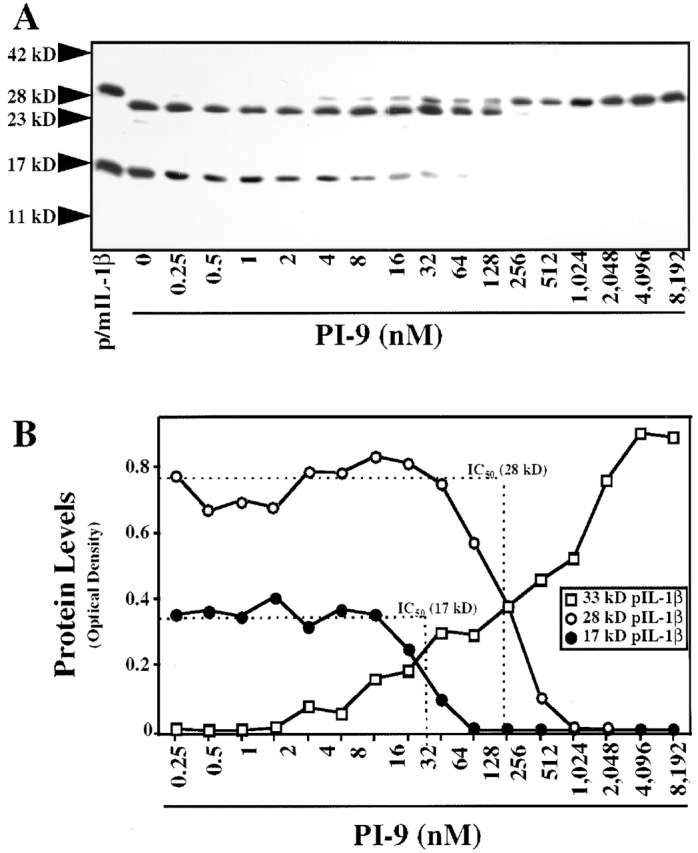
PI-9 concentration-dependently inhibits recombinant caspase-1 processing activity. (A) Human recombinant caspase-1 (15 nM) was preincubated with the indicated concentrations of PI-9 for 30 min at 37°C, before being added for 30 min (37°C) to proIL-1β (20 nM) in 50 μl processing buffer. Reactions were stopped by heating the samples in 10 μl SDS-PAGE sample buffer, and the preparations were applied to 15% SDS-PAGE and subsequent Western blot analysis using anti–human IL-1β. For control purposes, recombinant mature and precursor IL-1β were applied in combination (m/pIL-1β, both at 20 nM). The positions of the molecular weight markers are indicated on the left (in kD). (B) Densitometric analysis of immunoreactive cleavage products obtained in the processing assays described in A. The IC50 was determined in reference to untreated recombinant proIL-1β (20 nM, 33-kD form) or mature IL-1β (20 nM, 17-kD form). Similar data were obtained in eight independent experiments.
Figure 3.
The human serpin PI-9 inhibits processing of the IL-18 precursor by caspase-1. Human recombinant caspase-1 (15 nM, top) or human recombinant IL-18 precursor (equivalent to 50,000/ml THP.1 cells) was preincubated for 30 min (37°C) with the indicated concentrations of PI-9, before either the substrate (IL-18, top) or the enzyme (bottom) was added (30 min, 37°C). Processing was stopped by heating the samples in 10 μl SDS-PAGE sample buffer. Samples were analyzed by 15% SDS-PAGE and subsequent Western blot analysis using anti–human IL-18. For control purposes, THP.1 lysate (THP.1; equivalent to 106 cells/ml) was applied. The positions of the molecular weight markers are indicated on the left (in kD). Similar data were obtained in three independent experiments.
We further tested the specificity of the PI-9–mediated inhibition of caspase-1 processing activity by analyzing whether the serpin inhibits other caspases. Neither PI-9 nor PI-8 inhibited caspase-3 activity, using either a native substrate, gelsolin 51, in a processing assay similar to that used for analysis of caspase-1 activity, or a fluorogenic synthetic peptide (data not shown).
The Serpin PI-9 Accounts for the Endogenous Caspase-1 Inhibitory Activity of Human Vascular SMCs.
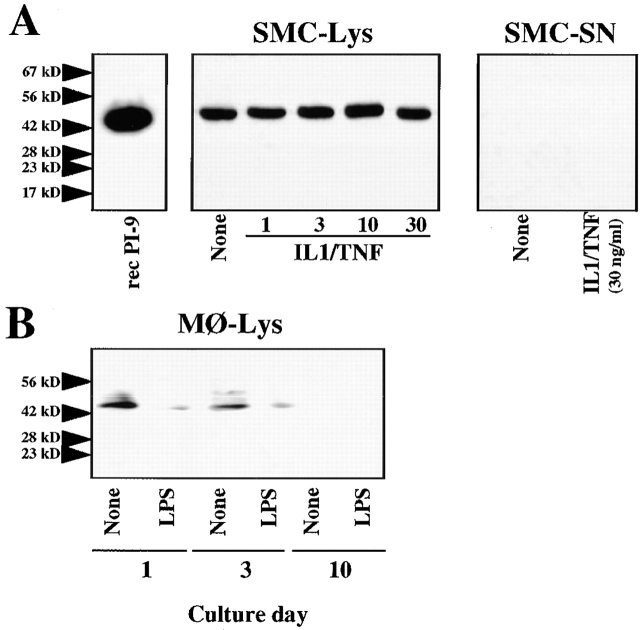
To extend the above observations, obtained with recombinant material, to extracts of human vascular SMCs, previously found to express a heretofore unidentified caspase-1 inhibitory activity (29; see also Fig. 5 A), we analyzed PI-9 expression in these cells. SMCs indeed expressed immunoreactive PI-9 constitutively as a cell-associated immunoreactive band with an apparent molecular mass of 42 kD (Fig. 4), resembling the biochemical characteristics described for the endogenous caspase-1 inhibitory activity. Stimulation with either IL-1β, TNF-α, or IFN-γ, or combinations thereof, affected neither the cell-associated, constitutive expression of PI-9 nor the release of the serpin. In contrast, constitutive expression of PI-9 in freshly isolated peripheral blood monocytes was diminished after either stimulation with LPS or culture-mediated differentiation (Fig. 4 B).
Figure 5.
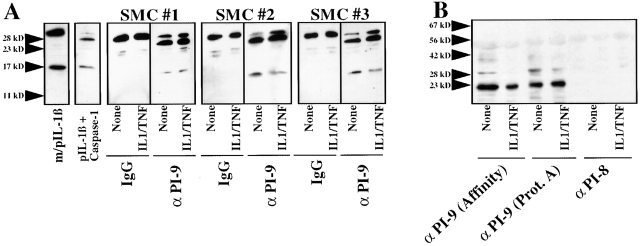
Human vascular SMCs depleted of endogenous PI-9 lack caspase-1 inhibitory activity. Human vascular SMCs were cultured for 24 h serum-free in IT medium, before being incubated for 24 h with fresh medium in the absence (None) or presence of human recombinant IL-1β/TNF-α (10/50 ng/ml). (A) Culture lysates of SMCs (equivalent to 107 cells/ml) were incubated with the anti–human PI-9 antibody (24 h, 4°C) and subsequently precipitated with protein A–Sepharose (500 g, 10 min). The remaining supernatants were incubated with human recombinant caspase-1 (15 nM; 1 h, 37°C) before being added to recombinant IL-1β precursor (20 nM) for 30 min at 37°C, and application to Western blot analysis using anti-human IL-1β. Combined mature (20 nM) and precursor (20 nM) IL-1β were applied as controls (m/pIL-1β). (B) Culture lysates of SMCs (equivalent to 107 cells/ml) were incubated with either affinity- or protein A–purified anti–human PI-9 or affinity-purified PI-8 antibody (24 h, 4°C), and subsequently precipitated with protein A–Sepharose (500 g, 10 min). The precipitates were applied to Western blot analysis using anti–human ICEP20. The positions of the molecular weight markers are indicated on the left (in kD). Similar data were obtained in three independent experiments, using SMC isolates of five different donors.
Figure 4.
Human vascular SMCs express PI-9 constitutively and in a cell-associated manner. (A) Lysates of SMCs, cultured for 24 h in IT medium in the absence (None) or presence of the respective concentrations of human recombinant mature IL-1β/TNF-α, were obtained by three freeze–thaw cycles. Lysates (Lys), equilibrated for total protein (50 μg/lane), as well as supernatants (SN, 50 μl; obtained from cultures stimulated with 30 ng/ml IL-1β/TNF-α) were analyzed by Western blotting with anti–human PI-9 antibody. (B) Similarly, lysates of peripheral blood mononuclear cells cultured for 1, 3, or 10 d (50 μg total protein/lane) were applied to Western blot analysis using anti–human PI-9 antibody. Recombinant PI-9 (recPI-9, 20 nM) was applied for control purposes. The positions of the molecular weight markers are indicated on the left (in kD). Similar data were obtained in three independent experiments.
Immunoprecipitation of SMC lysates with the anti–human PI-9 antibody depleted coordinately the serpin (data not shown) and the caspase-1 inhibitory activity, as demonstrated by the finding that anti–PI-9–treated SMC lysates no longer prevented cleavage of the IL-1β precursor (Fig. 5 A). Interestingly, the anti–PI-9 immunoprecipitate contained both PI-9 (data not shown) and caspase-1 (Fig. 5 B). Control experiments revealed that the anti–PI-9 antibody does not cross-react with recombinant caspase-1. The precipitate did not contain proIL-1β processing activity (data not shown), indicating complete inhibition of endogenously expressed caspase-1 via endogenous PI-9. Neither anti–PI-8 (Fig. 5 B) nor nonimmune rabbit control IgG (data not shown) immunoprecipitated caspase-1.
Inhibition of PI-9 Expression Induces the Release of IL-1β from Human Vascular SMCs.
To explore further the potential importance of PI-9–mediated regulation of caspase-1 processing activity, we treated cultures of unstimulated or TNF-α–stimulated vascular SMCs with PI-9 antisense oligonucleotides. This treatment coordinately reduced PI-9 expression (Fig. 6, top) and augmented the release of active IL-1β from TNF-α–stimulated cultures (Fig. 6, middle). As SMCs retain IL-1β intracellularly under usual conditions 52 53, this finding demonstrates that diminished expression of endogenous PI-9 indeed allows maturation of the IL-1β precursor in intact cells. PI-9 antisense treatment did not affect endogenous caspase-1 expression (Fig. 6, bottom), demonstrating specificity of the observed alterations in IL-1β release.
Figure 6.
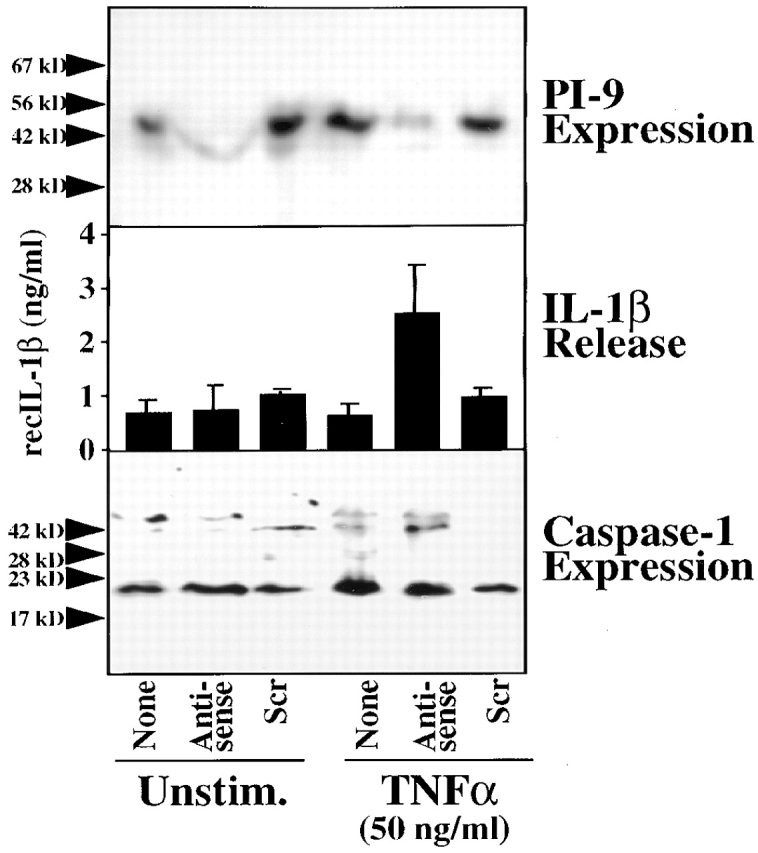
PI-9 antisense treatment induces the release of IL-1β into the supernatant of vascular SMC cultures. Cultures of SMCs were treated with lipofectin (1 μg/ml; GIBCO BRL) and PI-9 antisense or scrambled (Scr) phosphorothioate oligodeoxynucleotides for 72 h in the absence (None) or presence of recombinant TNF-α (50 ng/ml) during the last 24 h. Lysates of these cultures were analyzed by Western blotting using anti–PI-9 (top) or anti–caspase-1 (bottom), whereas supernatants were assayed for IL-1β by ELISA (middle), using recombinant IL-1β (recIL-1β) as standard. The positions of the molecular weight markers are indicated on the left (in kD). Similar data were obtained in three independent experiments.
Differential Expression of PI-9, Caspase-1, and IL-1β in Nonaffected and Atherosclerotic Arteries.
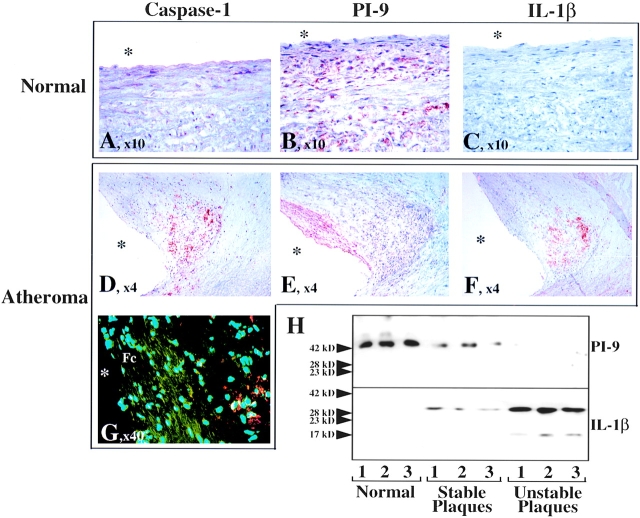
Since SMCs comprise the most abundant cell type in arteries and participate in vascular diseases, e.g., via expression of proinflammatory cytokines such as IL-1, we examined the expression of PI-9 in normal (n = 5) tissue and colocalized its expression with that from caspase-1 and IL-1β within atherosclerotic (n = 5) arteries. SMCs in normal arteries had low, but detectable, levels of caspase-1 (Fig. 7 A), which colocalized with abundant and homogeneously distributed PI-9 (Fig. 7 B). Normal vessels did not stain for IL-1β (Fig. 7 C). Expression of PI-9 was less intense and homogenous within atherosclerotic lesions compared with unaffected arterial tissue. Furthermore, PI-9 and caspase-1 localize inversely in atheroma. Caspase-1 (Fig. 7 D) as well as IL-1β (Fig. 7 F) localized most prominently in the macrophage-enriched shoulder region, whereas PI-9 localized most prominently in the SMC-enriched fibrous cap of the atherosclerotic plaque (Fig. 7 E), further demonstrated by immunofluorescence double labeling for PI-9 (green) and caspase-1 (red) (Fig. 7 G). The caspase-1 signal predominated over PI-9 in the more macrophage-rich shoulder area, whereas in the SMC-enriched fibrous cap PI-9 staining exceeded immunodetectable caspase-1. These findings corroborate with the reduced constitutive or absent expression of PI-9 in activated and/or differentiated mononuclear phagocytes (Fig. 4 H). In accordance with the immunohistochemical findings, Western blot analysis further demonstrated diminished total PI-9 concentrations in protein extracts of atherosclerotic lesions compared with unaffected tissue (Fig. 7 H). Moreover, Western blot analysis for PI-9 correlated inversely with enhanced immunoreactive IL-1β levels (Fig. 7 H). Notably, mature IL-1β was found primarily in extracts of atherosclerotic lesions, with morphologic characteristics of those prone to rupture and thus likely to cause acute clinical complications (defined by a large lipid core and thin fibrous cap, as described previously [48, 54]).
Figure 7.
Differential expression of caspase-1 and PI-9 in human atherosclerotic lesions. Serial cryostat sections of frozen specimens of human nonatherosclerotic aorta (A–C) and carotid atheroma (D–G) were stained with (A and D) goat anti–human ICEP20 (1:100), (B and E) rabbit anti–human PI-9 (1:50), or (C and F) mouse anti–human IL-1β (1:100) antibody. (G) For colocalization of PI-9 (green) with caspase-1 (red), double-immunofluorescence staining was performed. The asterisk indicates the lumen of the vessels. (H) Frozen tissue from nonatherosclerotic (Normal) as well as stable or unstable atheromatous carotid plaques was analyzed by Western blotting using the anti–PI-9 (top) or anti–IL-1β (bottom) antibody. The positions of the molecular weight markers are indicated on the left (in kD). Analysis of tissue obtained from five nonatherosclerotic as well as five stable and seven vulnerable atherosclerotic surgical specimens of different donors showed similar results.
Discussion
Increasing evidence suggests a critical role of caspase-1 in inflammation, linking enhanced caspase-1 activity with the progression of various diseases, e.g., via the activation of the proinflammatory cytokine IL-1β and furthermore by promoting apoptosis. Despite the scientific interest in and potential importance of caspase-1 in inflammatory and immune processes, little is known regarding regulation of the enzyme's activity. This study identifies the serpin PI-9 as an endogenous caspase-1 inhibitor in human vascular SMCs in vitro, and provides evidence that an imbalance between PI-9 and caspase-1 expression prevails at sites of chronic inflammatory diseases, such as atherosclerosis, in vivo.
Early studies of caspase-1 expression revealed mechanisms of autocatalytic activation of the inactive 45-kD precursor form, via intermediate forms, into the enzymatically active (p20/p10)2 homodimer 1 2 31 32. Further studies demonstrated that caspase-1 protein expression, although in immunoreactive mature forms, does not necessarily correspond to biological function. We previously reported the presence of the precursor and intermediate, and furthermore of the 20-kD mature, forms of caspase-1 in human vascular SMCs 29. However, these cells lack caspase-1 processing activity, due to a constitutively expressed caspase-1 inhibitory activity. The demonstration of such inhibitory activity furthermore explained the previous observation from our group and others, that human vascular SMCs express IL-1β upon stimulation but do not release this mediator 29.
Characteristics of the inhibitory activity resembled those described here for the serpin PI-9 expressed by vascular SMCs in vitro. Both are expressed constitutively and in a cell-associated manner, and the reported molecular mass of 42 kD for PI-9 agreed with the expected molecular mass of the caspase-1 inhibitory activity in SMCs 29 41. PI-9 reportedly localizes in the cytosol 41, the compartment where most caspase-1 resides 55, facilitating potential interactions between these two mediators. Although the molecular mechanism of the PI-9–mediated inhibition of caspase-1 exceeds the scope of this study, coprecipitation of the enzyme using anti–PI-9 antibody established protein–protein interactions, as also supported by the findings of Annand et al. 44 using synthetic substrates. Furthermore, the demonstration that optimal inhibition of IL-1β and IL-18 processing activity required the preincubation of the serpin with the enzyme, rather than the substrate, suggested that PI-9 interacts with caspase-1 rather than the cytokine. The interaction with the serpin seems selective, since PI-9 did not affect the enzymatic activities of another member of the caspase family, caspase-3 (CPP-32), as also suggested by Annand et al. 44. Further hints regarding the molecular mechanisms underlying the inhibition of caspase-1 emerge from previous studies analyzing the inhibition of caspase-1 by a viral serpin, CrmA 35. CrmA rapidly inhibits caspase-1, forming a tight complex with a low equilibrium constant for inhibition. Thus, the viral serine proteinase inhibitor CrmA, like a number of serpins, inhibits a nonserine proteinase. Ray and colleagues further suggested that caspase-1, a cysteine proteinase, shares substrate binding geometries resembling those found in serine proteinases, and that it is the substrate binding geometry, not the catalytic mechanism of a proteinase, that dictates its reactivity with protein inhibitors 37. The observations reported here agree with the applicability of this model to PI-9–caspase-1 interactions. The finding that PI-9 affects caspase-1 activity at equimolar stoichiometry and that complete inhibition of caspase-1 activity requires a molar ratio of 1:5–1:8 of enzyme to inhibitor resembles those obtained in experiments with CrmA 37. In vivo in undiseased tissue, PI-9 levels clearly exceed those of caspase-1, which lacks mature IL-1β. However, atherosclerotic lesions, characterized by chronic inflammation and by the presence of mature IL-1β, exhibit decreased PI-9 expression. Our immunoprecipitation and antisense experiments support the causal relationship between these observations.
The characterization of PI-9 as a human-derived, endogenous modulator of caspase-1, together with the differential expression of the enzyme and its inhibitor at sites of chronic inflammation in vivo, indicates the likely relevance of our observations in physiological as well as pathological processes. Our findings suggest that constitutive levels of PI-9 expression might limit caspase-1 activity, and represent a novel endogenous antiinflammatory mechanism. However, in diseased tissues (e.g., atheroma), augmentation of caspase-1 expression as well as imbalance between the enzyme and its inhibitor favor caspase-1 activity and hence inflammation. Because of the pivotal proximal role of IL-1β in the cytokine cascade and the involvement of caspase-1 in apoptosis, this imbalance between the enzyme and its inhibitor PI-9 could lower the threshold for activation of these two major pathways 4 56. The present observations should stimulate further exploration of the role of this new antiinflammatory pathway in atherosclerosis and other diseases.
Acknowledgments
The authors thank E. Shvartz, M. Muszynski, I. Chulsky, K. Williams, and E. Simon-Morrissey (Brigham and Women's Hospital) for their skillful technical assistance.
This work was performed during the tenure of the Paul Dudley White Fellowship of the American Heart Association by U. Schönbeck and supported by National Heart, Lung, and Blood Institute grant HL34636 to P. Libby.
Footnotes
Abbreviations used in this paper: CrmA, cytokine response modifier A; EC, endothelial cell; FBS, fetal bovine serum; ICE, IL-1β–converting enzyme; IT, insulin/transferrin; Mφ, macrophage(s); PI, proteinase inhibitor; SMC, smooth muscle cell.
References
- Thornberry N.A., Bull H.G., Calaycay J.R., Chapman K.T., Howard A.D., Kostura M.J., Miller D.K., Molineaux S.M., Weidner J.R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Cerretti D.P., Kozlosky C.J., Mosley B., Nelson N., Van Ness K., Greenstreet T.A., March C.J., Kronheim S.R., Druck T., Cannizzaro L.A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Lomedico P.T., Gubler U., Hellmann C.P., Dukovich M., Giri J.G., Pan Y.-C.E., Collier K., Semionow R., Chua A.O., Mizel S.B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli . Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Auron P.E., Webb A.C., Rosenwasser L.J., Mucci S.F., Rich A., Wolff S.M., Dinarello C.A. Nucleotide sequence of human monocyte interleukin-1 precursor cDNA. Proc. Natl. Acad. Sci. USA. 1984;81:7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C.J., Mosley B., Larsen A., Cerretti D.P., Braedt G., Price V., Gillis S., Henney C.S., Kronheim S.R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–643. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Lonnemann G., Endres S., van der Meer J.W., Cannon J.G., Koch K.M., Dinarello C.A. Differences in the synthesis and kinetics of release of interleukin 1 alpha, interleukin 1 beta and tumor necrosis factor from human mononuclear cells. Eur. J. Immunol. 1989;19:1531–1537. doi: 10.1002/eji.1830190903. [DOI] [PubMed] [Google Scholar]
- Mosley B., Dower S.K., Gillis S., Cosman D. Determination of the minimum polypeptide lengths of the functionally active sites of human interleukins 1α and 1β. Proc. Natl. Acad. Sci. USA. 1987;84:4572–4576. doi: 10.1073/pnas.84.13.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D.J., Strickler J., Simon P., Young P.R. Structure-function mapping of interleukin 1 precursors. Cleavage leads to a conformational change in the mature protein. J. Biol. Chem. 1991;266:7081–7086. [PubMed] [Google Scholar]
- Black R.A., Kronheim S.R., Cantrell M., Deeley M.C., March C.J., Prickett K.S., Wignall J., Conlon P.J., Cosman D., Hopp T.P., Mochizuki D.Y. Generation of biologically active interleukin-1β by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- Kostura M.J., Tocci M.J., Limjuco G., Chin J., Cameron P., Hillman A.G., Chartrain N.A., Schmidt J.A. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc. Natl. Acad. Sci. USA. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.A., Kronheim S.R., Sleath P.R. Activation of interleukin 1β by a co-induced protease. FEBS Lett. 1989;247:386–391. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Howard A.D., Kostura M.J., Thornberry N.A., Ding G.J.F., Limjuco G., Weidner J., Salley J.P., Hogquist K.A., Chaplin D.D., Mumford R.A. IL-1β-converting enzyme requires aspartic acid residues for processing of the IL-1β precursor at two distinct sites and does not cleave 31 kDa IL-1α. J. Immunol. 1991;147:2964–2969. [PubMed] [Google Scholar]
- Gillespie M.T., Horwood N.J. Interleukin-18perspectives on the newest interleukin. Cytokine Growth Factor Rev. 1998;9:109–116. doi: 10.1016/s1359-6101(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A., Novick D., Puren A.J., Fantuzzi G., Shapiro L., Muhl H., Yoon D.Y., Reznikov L.L., Kim S.H., Rubinstein M. Overview of interleukin-18more than an interferon-gamma inducing factor. J. Leukoc. Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- Dinarello C.A. Role of pro- and anti-inflammatory cytokines during inflammationexperimental and clinical findings. J. Biol. Regul. Homeost. Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- Dinarello C.A. IL-18a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. NY Acad. Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G., Dinarello C.A. Interleukin-18 and interleukin-1 betatwo cytokine substrates for ICE (caspase-1) J. Clin. Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- Munder M., Mallo M., Eichmann K., Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18a novel pathway of autocrine macrophage activation. J. Exp. Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander R.M., Gagliardini V., Rotello R.J., Yuan J. Functional role of interleukin 1β (IL-1β) in IL-1β–converting enzyme–mediated apoptosis. J. Exp. Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander R.M., Gagliardini V., Hara H., Fink K.B., Li W., MacDonald G., Fishman M.C., Greenberg A.H., Moskowitz M.A., Yuan J. Expression of a dominant negative mutant of interleukin-1β converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J. Exp. Med. 1997;185:933–940. doi: 10.1084/jem.185.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura M., Okuma E., Yuo A., Sasaki T., Mukai C., Takaku F., Ishizaka Y. Room temperature-induced apoptosis of Jurkat cells sensitive to both caspase-1 and caspase-3 inhibitors. Cancer Lett. 1998;132:7–16. doi: 10.1016/s0304-3835(98)00116-5. [DOI] [PubMed] [Google Scholar]
- Pasinelli P., Borchelt D.R., Houseweart M.K., Cleveland D.W., Brown R.H., Jr. Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper-zinc superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1998;95:15763–15768. doi: 10.1073/pnas.95.26.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.S., Kochanek P.M., Chen M., Watkins S.C., Marion D.W., Chen J., Hamilton R.L., Loeffert J.E., Graham S.H. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 1999;13:813–821. doi: 10.1096/fasebj.13.8.813. [DOI] [PubMed] [Google Scholar]
- Wong M.L., Bongiorno P.B., Gold P.W., Licinio J. Localization of interleukin-1 beta converting enzyme mRNA in rat brain vasculatureevidence that the genes encoding the interleukin-1 system are constitutively expressed in brain blood vessels. Pathophysiological implications. Neuroimmunomodulation. 1995;2:141–148. doi: 10.1159/000096884. [DOI] [PubMed] [Google Scholar]
- Schönbeck U., Herzberg M., Petersen A., Wohlenberg C., Gerdes J., Flad H.D., Loppnow H. Human vascular smooth muscle cells express interleukin-1β–converting enzyme (ICE), but inhibit processing of the interleukin-1β precursor by ICE. J. Exp. Med. 1997;185:1287–1294. doi: 10.1084/jem.185.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K., Kitajima T., Bergstresser O.R., Takashima A. Interleukin-1 beta converting enzyme in murine Langerhans cells and epidermal-derived dendritic cell lines. Eur. J. Immunol. 1995;25:2137–2141. doi: 10.1002/eji.1830250803. [DOI] [PubMed] [Google Scholar]
- Walker N.P.C., Talanian R.V., Brady K.D., Dang L.C., Bump N.J., Ferenz C.R., Franklin S., Ghayur T., Hackett M.C., Hammhill L.D. Crystal structure of the cysteine protease interleukin-1β-converting enzymea (p20/p10)2 homodimer. Cell. 1994;78:343–352. doi: 10.1016/0092-8674(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Wilson K.P., Black J.A., Thomson J.A., Kim E.E., Griffith J.P., Navia M.A., Murcko M.A., Chambers S.P., Aldape R.A., Raybuck S.A., Livingston D.J. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Sympson C.J., Werb Z., Bissell M.J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D.J. In vitro and in vivo studies of ICE inhibitors. J. Cell. Biochem. 1997;64:19–26. [PubMed] [Google Scholar]
- Ray C.A., Black R.A., Kronheim S.R., Greenstreet T.A., Sleath P.R., Salvesen G.S., Pickup D.J. Viral inhibition of inflammationcowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Bump N.J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- Komiyama T., Ray C.A., Pickup D.J., Howard A.D., Thornberry N.A., Peterson E.P., Salvesen G. Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosisa perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Potempa J., Korzus E., Travis J. The serpin superfamily of proteinase inhibitorsstructure, function, and regulation. J. Biol. Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- Sprecher C.A., Morgenstern K.A., Mathewes S., Dahlen J.R., Schrader S.K., Foster D.C., Kisiel W. Molecular cloning, expression, and partial characterization of two novel members of the ovalbumin family of serine proteinase inhibitors. J. Biol. Chem. 1995;270:29854–29861. doi: 10.1074/jbc.270.50.29854. [DOI] [PubMed] [Google Scholar]
- Sun J., Bird C.H., Sutton V., McDonald L., Coughlin P.B., De Jong T.A., Trapani J.A., Bird P.I. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J. Biol. Chem. 1996;271:27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- Bird C.H., Sutton V.R., Sun J., Hirst C.E., Novak A., Kumar S., Trapani J.A., Bird P.I. Selective regulation of apoptosisthe cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol. Cell. Biol. 1998;18:6387–6398. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annand R.R., Dahlen J.R., Sprecher C.A., De Dreu P., Foster D.C., Mankovich J.A., Talanian R.V., Kisiel W., Giegel D.A. Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem. J. 1999;342:655–665. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Kariya B. Morphogenesis of vascular smooth muscle in atherosclerosis and cell structure. In: Bohr D.F., Somlyo A.P., Sparks H.Y., editors. Handbook of Physiology, The Cardiovascular System. American Physiological Society; Bethesda, MD: 1980. pp. 66–91. [Google Scholar]
- Libby P., O'Brien K.V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J. Cell Physiol. 1983;115:217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- Sukhova G.K., Schönbeck U., Rabkin E., Schoen F.J., Poole A.R., Billinghurst R.C., Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- Howard A.D., Kostura M.J., Thornberry N., Ding G.J., Limjuco G., Weidner J., Salley J.P., Hogquist K.A., Chaplin D.D., Mumford R.A. IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J. Immunol. 1991;147:2964–2969. [PubMed] [Google Scholar]
- Dahlen J.R., Foster D.C., Kisiel W. Expression, purification, and inhibitory properties of human proteinase inhibitor. Biochemistry. 1997;36:14874–14882. doi: 10.1021/bi970977p. [DOI] [PubMed] [Google Scholar]
- Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T.J., Kirschner M.W., Koths K., Kwiatkowski D.J., Williams L.T. Caspase-3-generated fragment of gelsolineffector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J.M., Birinyi L.K., Auger K.S., Dinarello C.A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J. Clin. Invest. 1986;78:1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S.J.C., Auger K.R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J. Exp. Med. 1987;165:1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton C.V., Crook D., Davies M.J., Oliver M.F. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler. Thromb. Vasc. Biol. 1997;17:1337–1345. doi: 10.1161/01.atv.17.7.1337. [DOI] [PubMed] [Google Scholar]
- Singer I.I., Scott S., Chin J., Bayne E.K., Limjuco G., Weidner J., Miller D.K., Chapman K., Kostura M.J. The interleukin-1β–converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J. Exp. Med. 1995;182:1447–1459. doi: 10.1084/jem.182.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]