Abstract
Splenic B lineage cells expressing recombination activation genes (RAG+) in mice immunized with 4-hydroxy-3-nitrophenyl-acetyl coupled to chicken γ-globulin (NP-CGG) and the adjuvant aluminum-hydroxide (alum) have been proposed to be mature B cells that reexpress RAG after an antigen encounter in the germinal center (GC), a notion supported by findings of RAG expression in peripheral B lymphocyte populations activated in vitro. However, recent studies indicate that these cells might be immature B cells that have not yet extinguished RAG expression. Here, we employ RAG2–green fluorescent protein (GFP) fusion gene knock-in mice to show that RAG+ B lineage cells do appear in the spleen after the administration of alum alone, and that their appearance is independent of T cell interactions via the CD40 pathway. Moreover, splenic RAG+ B lineage cells were detectable in immunized RAG2-deficient mice adoptively transferred with bone marrow (BM) cells, but not with spleen cells from RAG+ mice. Although splenic RAG+ B cells express surface markers associated with GC B cells, we also find the same basic markers on progenitor/precursor BM B cells. Finally, we did not detect RAG gene expression after the in vitro stimulation of splenic RAG− mature B cells with mitogens (lipopolysaccharide and anti-CD40) and cytokines (interleukin [IL]-4 and IL-7). Together, our studies indicate that RAG+ B lineage cells from BM accumulate in the spleen after immunization, and that this accumulation is not the result of an antigen-specific response.
Keywords: alum, germinal center, receptor editing, immunoglobulin re-rearrangement
Introduction
During B cell development in the bone marrow (BM), Ig heavy (IgH) and Ig light (IgL) chain variable region genes are assembled from germline variable (V), diversity (D), and joining (J) gene segments by a process referred to as V(D)J recombination (for a review, see reference 1). V(D)J recombination is absolutely dependent on the expression of the products of recombination activating gene (RAG)1 and RAG2, which together initiate V(D)J recombination by recognizing and cleaving the recombination signal sequences that flank different V, D, and J coding segments (for a review, see reference 1).
RAG1 and RAG2 are coordinately expressed during B cell development, as sequential IgH D to JH and VH to DJH occurs in B220loCD43+IgM− progenitor B cells, and IgL VL to JL rearrangements take place in B220loCD43loIgM− precursor B cells 2 3 4 5 6. The production of IgH and IgL chains from functionally rearranged genes leads to the expression of surface IgM and developmental progression to the immature B cell stage. Immature BM B cells are B220loIgMloIgD−heat stable antigen (HSA)hipB130-140+ and many have continued RAG expression 7 8 9 10 11 12. As immature B cells develop into the transitional stage, they downregulate RAG and migrate to the spleen 6 11 12. During development, only a small fraction of the newly generated, immature B cells join the pool of long-lived mature, B220hiIgMloIgDhiHSAlopB130–140− splenic B cells; at the mature B cell stage, RAG expression is virtually undetectable (9 10 11 12; for a review, see reference 13). Thus, most B cells in a normal spleen of an unimmunized adult mouse do not express RAG.
Very young mice (e.g., <4 wk) have a substantial population of splenic, RAG+ B lineage cells that presumably represent pre-B/immature B cells present from remnants of splenic lymphopoiesis that occurs before the shift to adult BM 11 12. Older mice (>9 wk) immunized with 4-hydroxy-3-nitrophenyl-acetyl coupled to chicken γ-globulin (NP-CGG), and an adjuvant such as aluminum-hydroxide (alum), develop RAG+, IgM− or IgMlo, B220lo, GL-7+ or GL-7−, pB130-140+, peanut agglutinin (PNA)+, HSA+ splenic B lineage cells that phenotypically resemble pre-B/immature B cells 11 12 14 15. Some of these RAG+ spleen cells have been reported to localize to the germinal center (GC) 14 16 17. At least three, not necessarily mutually exclusive, hypotheses have been proposed concerning the origin of the B220lo RAG+ splenic B cells. One proposes reexpression of RAG and other markers of early B cell developmental stages in mature, splenic GC B cells after activation in response to antigens 14 15 16 17 18 19 20 21. The second involves generation of pre-B/immature B cells from residual hematopoietic islands in the spleen 11 22 23 24. The third involves the emigration of RAG+ pre-B/immature B cells from the BM to the spleen after immunization 11 12 22 23.
Secondary IgH and IgL V region gene rearrangements have been detected in various transgenic B cells (for reviews, see references 22, 25), such as IgH and IgL site-directed mutant B cells (for reviews, see references 22, 25) and primary normal B cells 14 15 16 17 19 20 26 27 28. These findings provided evidence that secondary VL to JL rearrangements or IgH V gene replacements (receptor editing) in newly generated, BM B cells may contribute substantially to the generation of the Ab repertoires and to the establishment of tolerance 22. The occurrence of a related mechanism of secondary, antigen-dependent V gene rearrangements in peripheral B cells, referred to as “receptor revision,” was hypothesized based on the finding of RAG+ B lineage cells in the spleens of immunized mice 11 12 14 15 16 and supported by findings of V(D)J recombination in splenic B cells after immunization 15 21 or in vitro activation 17 18 19 20. However, recent studies using a RAG reporter transgenic mouse line (see below) demonstrated that RAG− splenic B cells could not be induced to express RAG in vivo or in vitro, questioning the notion that RAG genes can be reexpressed in mature B cells 12. In addition, work with RAG2–green fluorescent protein (GFP) fusion gene knock-in mice indicated that the splenic RAG+ cells were similar in phenotype to BM pro/pre-B cells, supporting the possibility that the RAG+ cells that appear after immunization may derive from immature BM B lineage cells 11.
V(D)J recombination absolutely depends on RAG expression 29 30. Therefore, to easily identify live cells that express RAG in various populations, we generated reporter mice in which a RAG2–GFP fusion gene replaced the endogenous RAG2 coding exon 11. The advantages of this approach are that expression of the RAG2–GFP fusion protein appears to closely mimic that of the endogenous RAG protein during normal development and that the fusion protein functions to support V(D)J recombination sufficient to provide normal lymphocyte development 11. However, a limitation of the RAG2–GFP fusion gene mouse model is a relatively low GFP signal 11. In parallel experiments, others have generated either transgenic mice in which a GFP protein was driven by RAG gene-regulatory regions (NG-BAC [12, 23]) or a mouse in which GFP was targeted in place of the RAG1 coding sequence (RAG1/GFP replacement [31]). Whereas the NG-BAC transgenic or RAG1/GFP replacement mice provide a much stronger signal, GFP alone appears to be more stable than endogenous RAG and thus the reporter gene expression may extend somewhat beyond normal RAG protein expression 12 23 31. Analyses of the different strains of RAG reporter mice have yielded largely similar conclusions and, when viewed together, may provide information beyond that available with individual approaches (23; see Discussion).
To further elucidate the nature of peripheral RAG+ B lineage cells in immunized mice, we employed the RAG2–GFP fusion mice and other approaches to identify the source of the splenic RAG+ lymphocytes after immunization and to further characterize the conditions that lead to their appearance.
Materials and Methods
Mice.
CD40L−/− (C57BL/6 [B6], 129 S-Tnfsf5, or B6 S-Tnfsf5; The Jackson Laboratory) 32 were bred with RAG2-GFP mice 11. 129 mice and C3H mice were obtained from Taconic Farms.
Flow Cytometry.
Single-cell suspensions were stained with FITC-, PE-, CyChrome (CyC)-, and biotin (bi)-conjugated Abs and analyzed by a FACSCalibur™ (Becton Dickinson) as described 11. The following Abs were used (BD PharMingen and Southern Biotechnology Associates, Inc.): FITC–anti-GL7; PE–anti-CD43 (S7), -IgMa (Igh-6a), −c-kit (2B8), -IgD 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26, -IgM (1B4B1), -CD24 (M1/69), -CD25 (PC61), -CD4 (RM4-5), −GR-1 (RB6-8C5), -F480 (provided by L.A. Herzenberg, Stanford School of Medicine, Stanford, CA), -κ (187.1), -λ (JC5-1), -CD23 (B3B4), and −c-kit (2B8); CyC–anti-B220 (RA3-6B2), -CD8 (53-6.7), and −syndecan-1 (CD138, 281-2); bi–anti-MAC-1 (M1/70), -F480 (provided by L.A. Herzenberg), -PNA, −pB130-140 (493) (provided by M. Carroll, Harvard Medical School, Boston, MA and prepared by the authors), and bi–GL-7 (provided by G. Kelsoe, Duke University Medical School, Durham, NC). Streptavidin-PE, -CyC, and -allophycocyanin were used to reveal bi-conjugated Abs. For most FACS® plots, ≥100,000 events were collected; dead cells were excluded by forward scatter gating. Data were analyzed with either CELLQuest™ (Becton Dickinson) or FlowJo (Tree Star, Inc.) software. Cell sorting was performed on a MoFlo machine (Cytomation) or an Epics Altra (Beckman Coulter).
Immunizations.
RAG2+/+, RAG2+/GFP, and RAG2GFP/GFP mice (10–13 wk old) were immunized intraperitoneally with a single dose of 100 μg of NP-CGG, provided by G. Kelsoe or prepared from individual components (Biosearch Technologies, Inc.) as described previously 33, that had been precipitated in alum as described previously 15, except that 1 ml (as opposed to 300 μl) of the final suspension was used for intraperitoneal immunizations. Spleens were taken at times indicated after immunization and dissociated into single-cell suspensions for FACS® analysis. Immunizations had no effect on thymus cellularity or T cell subsets on days 8 and 16 after immunization (data not shown).
Splenic B Cell Cultures.
Splenic B cells were purified from single-cell suspensions of either 10–15-wk-old male C3H mice, CD40L−/− mice, or RAG2–GFP mice 11 by sorting (MoFlo; Cytomation) based on B220 and IgD surface expression or by cytotoxic T cell depletion as described 19. Culture conditions were as published 19. In brief, splenic B cell preparations were cultured at 1–3 × 106 cells/ml for 2–3 d in 1 ml RPMI 1640 medium (10% FCS, 10−5 M 2-mercaptoethanol, 100 U/ml penicillin G, and 100 μg/ml streptomycin) with 20 μg/ml LPS from Escherichia coli 055:B5 (Sigma-Aldrich), 1 μg/ml anti-CD40 mAbs (BD PharMingen), 10 ng/ml recombinant murine IL-4 (PeproTech), or 10 ng/ml recombinant murine IL-7 (PeproTech) as indicated.
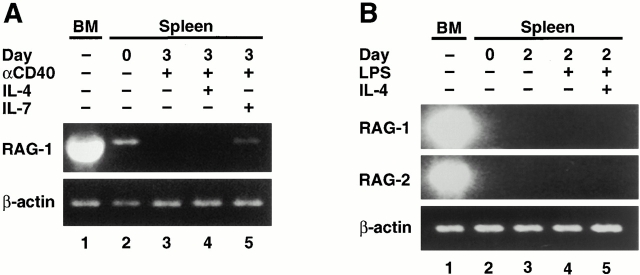
Reverse Transcription PCR Analysis.
Total RNA from splenic cultures was isolated using TRIzol (Life Technologies) or TriPure (Roche Molecular Biochemicals) reagent, reverse transcribed with SuperScript II (Life Technologies) or Omniscript (QIAGEN), and primed with random hexamers according to the directions of the manufacturer (Life Technologies). PCR reactions (25 μl) contained 5 μl of the cDNA preparation, 3 pmol of both sense and antisense oligonucleotide primer, 0.2 mM of each dNTP, 2 mM MgCl2, and 1 U Taq DNA polymerase in 1× PCR buffer (QIAGEN). Intron spanning primers for RAG1, RAG2, TdT, λ-5, Vpre-B, and β-actin were as described by Li et al. 4, except that the λ-5 sense primer was 5′-CTTGAGGGTCAATGAAGCTCAGA-3′. Primers for detection of ε and γ-2b IgH germline transcripts were as published previously 34. Primers for the amplification of glyceraldehyde 3-phosphate dehydrogeanse (GAPDH) were GAPDH-S (5′-TCCACCACCCTGTTGCTGTA-3′) and GAPDH-A (5′-ACCACAGTCCATGCCATCAC-3′). PCR reactions were performed on a GeneAmp thermocycler (model 9600; PerkinElmer) with the following conditions: 3 min at 94°C; 18–45 cycles for 30 s at 94°C, 15 s at 60°C (or 58°C for ε and γ-2b), and 45 s at 72°C; and finally, 10 min at 72°C. In every experiment, the cycle number was titrated for every primer pair to be in the exponential phase of amplification (with the exception of BM samples, where the signal intensity relative to spleen samples was determined by serial dilution). PCR products were resolved on 2% agarose gels and visualized by ethidium bromide staining.
ELISA Analysis.
Detection of anti–NP-specific Abs was done as described previously 35. An anti-NP IgG1 standard mAb (PE-VHCγ1 [33]) was provided by G. Kelsoe.
Adoptive Transfer.
RAG2GFP/GFP spleen, RAG2GFP/GFP BM, 129 spleen, or 129 BM cells from pooled 9–16-wk-old mice were adoptively transferred intravenously into 5-wk-old RAG2−/− recipients. Donor spleen cells from old RAG2–GFP mice did not express GFP 11. Ly9.1 was used to distinguish recipient (Ly9.1−) B220lo cells from those of the donor (Ly9.1+). Mice adoptively transferred with BM received 1.8 × 107 cells per mouse and mice adoptively transferred with spleen received 9.2 × 107 cells per mouse. There were four to five mice in each group, three of which were immunized the day after transfer with 100 μg NP-CGG/alum, while the remaining mice served as naive controls. In addition, three 10-wk-old RAG2GFP/GFP and three 10-wk-old 129 mice were immunized as positive controls. Unimmunized 10-wk-old RAG2GFP/GFP and 129 mice served as naive controls. RAG2−/− mice served as a negative control. Spleen and BM cells were analyzed by FACS® for the presence of RAG2–GFP+ cells in the case of RAG2GFP/GFP transferred mice, and B220lo493+ or B220loGL-7+ cells in the case of both RAG2GFP/GFP and 129 transferred mice after gating on Ly9.1+ cells.
Results
Appearance of RAG+ Splenic B Cells after Immunization Is Independent of Antigen and the CD40 Pathway.
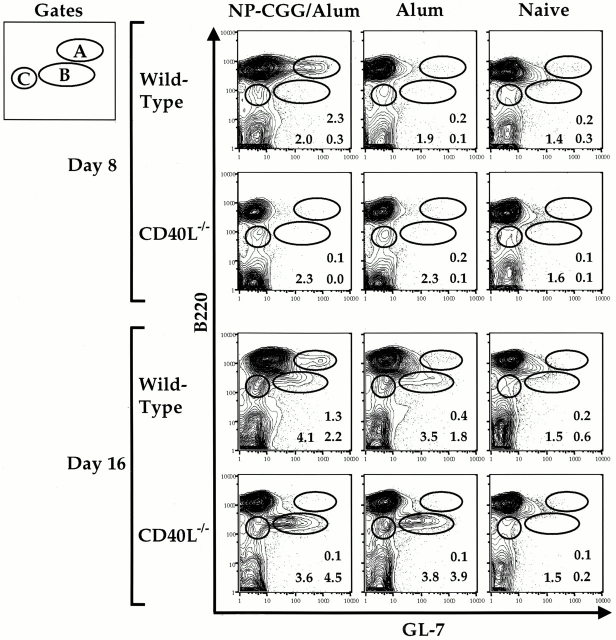
RAG+ splenic B lineage cells appear in the spleen of mature (≥9 wk) mice after immunization with the antigen NP-CGG 11 12 23. To characterize the requirement for antigen and T cell help with respect to the appearance of this cell population, we immunized wild-type (wt) and CD40L−/− mice with alum in the presence or absence of NP-CGG. As expected, on day 8 after immunization we detected a population of B220hiGL-7++ splenic, B lineage cells not seen in naive animals (11 14 15; Fig. 1, gate A). Appearance of this “GC B cell population” 14 15 was dependent on immunization with antigen and was absent in CD40L−/− mice, consistent with a requirement for helper T cells and an intact CD40 pathway for the initiation of the GC reaction 24 32 36. We also found considerable numbers of splenic B220lo cells on day 16, but not on day 8, after immunization 11 12 15; these cells can be subdivided into B220loGL-7+ and B220loGL-7− subpopulations (Fig. 1, gates B and C, respectively [11]). Significantly, B220lo cells appeared on day 16 both in wt mice and in CD40L−/− mice injected with alum alone (Fig. 1). Alum injection of both wt and CD40L−/− mice also resulted in spleens containing high numbers of granulocytes (data not shown). We concluded that the B220lo B lineage cells that appear in the spleen on day 16 after immunization are part of a generalized response to the alum adjuvant in the absence of antigen or CD40L-mediated T cell interactions.
Figure 1.
The appearance of RAG-expressing B220lo cells in the spleen after immunization is independent of antigen and the CD40 pathway. Splenic cells from 10-wk-old CD40L−/− or wt mice immunized intraperitoneally with alum alone, NP-CGG with alum, or PBS were analyzed at days 8 and 16 after injection and analyzed for GL-7 and B220 expression. The percentages of cells out of total live lymphocytes for the gated populations are shown. FACS® plots are shown from representative mice of five experiments where the indicated number of mice was analyzed at days 8 and 16 for each treatment: wt NP-CGG/alum day 8 n = 10, day 16 n = 10; CD40L−/− NP-CGG/alum day 8 n = 2, day 16 n = 2; wt alum day 8 n = 4, day 16 n = 12; CD40L−/− alum day 8 n = 5, day 16 n = 5; naive n = 12, where wt mice were either 129 or RAG2–GFP. The mean and standard deviation for the percentages of subsets out of total live lymphocytes observed on day 8 and day 16 are as follows: at day 8, wt NP-CGG/alum mice exhibited 1.8 ± 0.8% B220hiGL-7++ cells (gate A) compared with percentage values of 0.12 and 0.16 for the CD40L−/− mice immunized with NP-CGG/alum, 0.54 ± 0.2 for wt alum, 0.14 ± 0.05 for CD40L−/− alum, and 0.43 ± 0.3 for naive mice; at day 16, the B220loGL-7+ subset (gate B) was observed at percentage values of 6.5 ± 6.1 for wt NP-CGG/alum, percentage values of 0.77 and 12 for the CD40L−/− mice immunized with NP-CGG/alum, 3.0 ± 2.8 for wt alum, 6.3 ± 2.3 for CD40L−/− alum, and 0.5 ± 0.5 for naive mice; at day 16, the B220loGL-7− subset (gate C) was observed at percentage values of 8.0 ± 5 for wt NP-CGG/alum, percentage values of 5.9 and 9.6 for the CD40L−/− mice immunized with NP-CGG/alum, 4.8 ± 1.6 for wt alum, 6.4 ± 2.4 for CD40L−/− alum, and 2.9 ± 1.3 for naive mice.
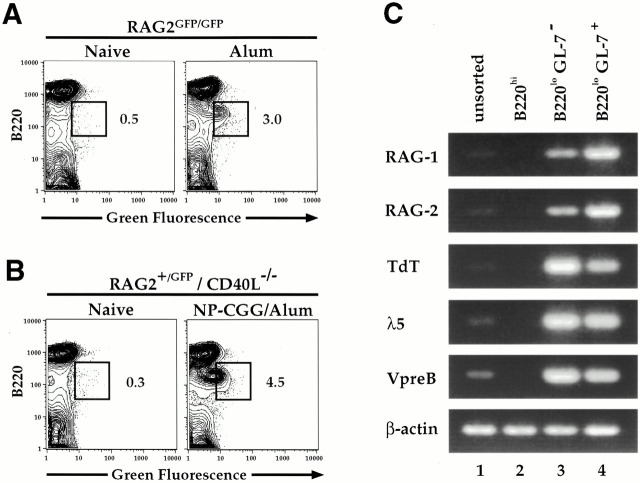
To assess whether the splenic B220lo B cell population induced 16 d after the injection of alum was RAG+, we assayed for the expression of the RAG2–GFP fusion protein in RAG2–GFP mice injected with alum, with or without NP-CGG (Fig. 2). The B220lo B cells appearing in the spleen after the application of alum alone on day 16 clearly were RAG2–GFP+(Fig. 2 A). To test whether B220lo B cells induced by alum plus NP-CGG in CD40L−/− mice were RAG+, we immunized RAG2–GFP mice bred into the CD40L−/− background (Fig. 2 B). Again, we observed that the B220lo B cells induced in the CD40L−/− background 16 d after immunization were RAG+ and that the size of this population was similar to that induced in a wt background (compare Fig. 2B and A). The latter finding clearly shows that generation of the RAG+ splenic B lineage cells occurs in a fashion that is independent of the pathways required for GC formation. Neither mature B220hiGL-7−/lo splenic B cells nor the antigen-dependent B220hiGL-7++ cells appearing on day 8 expressed RAG even after immunization with the antigen NP-CGG (data not shown, and reference 11).
Figure 2.
RAG2–GFP+ cells are present in the spleen on day 16 after alum immunization alone and in the absence of the CD40L pathway. (A) RAG2GFP/GFP mice (13 wk old) were immunized intraperitoneally with alum or PBS; their splenic cells were analyzed on day 16 for RAG2–GFP expression by FACS®. Percentages of B220loRAG2–GFP+ cells out of total live lymphocytes are shown on FACS® plots of representative mice of two experiments representing four RAG2–GFP mice which received alum and two naive mice analyzed at day 16. (B) Splenic cells from 10-wk-old CD40L−/−RAG2+/GFP mice immunized with NP-CGG and alum were analyzed on day 16 for RAG2–GFP expression by FACS®. Percentages of B220lo RAG2–GFP+ cells out of total live lymphocytes are shown for representative mice of two experiments representing three immunized mice of the CD40L−/− background and three naive mice of the 129 background. (C) RNA was purified from unsorted, sorted B220hi (reanalysis 95% purity), sorted B220loGL-7− (reanalysis 80% purity), and sorted B220loGL-7+ cells (reanalysis 90% purity) from three 12-wk-old, day 16 CD40L−/− alum-immunized mice and used for RT-PCR analysis of RAG1, RAG2, TdT, λ-5, and Vpre-B. β-Actin was used as a control to ensure equivalent amounts of RNA. One out of five representative experiments is shown.
To further characterize the RAG+ splenic, B lineage subsets that appear in the late stages of the immune response, we sorted splenic B cell populations from CD40L−/− mice on day 16 after alum injection (Fig. 2 C). Reverse transcription (RT)-PCR analysis of RNA isolated from unsorted, B220hi, B220loGL-7−, and B220loGL-7+ populations was performed to examine the expression of several progenitor/precursor B cell markers. As expected, RAG1 and RAG2 expression was extremely low to undetectable in the unsorted cells (Fig. 2 C, lane 1) and the B220hi mature B cell populations (Fig. 2 C, lane 2). In contrast, RAG expression was strongly enriched in the B220loGL-7− and B220loGL-7+ subsets (Fig. 2 C, compare lanes 3 and 4 with lane 1). Of note, transcripts from several other pro/pre-B–specific genes (including TdT, λ-5, and Vpre-B) were also enriched in the B220lo population (Fig. 2 C). Similar results were obtained with B220loGL-7− and B220loGL-7+ populations sorted from CD40L+/+ mice immunized with NP-CGG and alum (data not shown).
We concluded that the splenic B220lo subsets appearing during the late stages of the immune response express the RAG genes together with markers characteristic of developing pro-B and pre-B cells in the bone marrow. Furthermore, RAG expression does not require specific antigens or an intact CD40 pathway.
Common Surface Marker Expression on Developing BM B Cells and Postimmunization RAG2–GFP+ Splenic B-Lineage Cells.
To determine whether the RAG+ splenic B cells expressed any additional developmental B cell markers, we compared surface marker expression on B220lo cells in the spleen and on the BM of RAG2–GFP mice. The general expression patterns of the B220loGFP+ and B220loGFP− subsets in the spleen after NP-CGG and alum administration closely correlated with those of the corresponding pro- and/or pre-B populations in the BM (Fig. 3). Thus, B220loGFP+ and B220loGFP− cells in the spleen uniformly expressed the immature B cell marker pB130-140 8, the BM B cell, and the Ab-secreting cell markers PNA 37 and syndecan-1 38. Similarly, GL-7 expression levels were essentially the same on both subsets, with GL-7− cells being relatively more abundant in the splenic B220loGFP− compartment. Of note, most of the B220loGFP+ cells expressed little or no IgM, whereas the relative contribution of IgM+ cells was greater in the B220loGFP− compartment, consistent with this subset representing a later developmental stage (see legend to Fig. 3). Notably, splenic B220loGFP+ and B220loGFP− cells both expressed low levels of CD43, with only a small subset being CD43hi, consistent with a predominantly pre-B cell phenotype of the splenic B220lo populations. Together, the RT-PCR and FACS® analysis of the B220lo subsets show that the RAG-expressing cells detectable in the spleen in the late stages of an immune response predominantly resemble BM pre-B cells, with a smaller number potentially representing pro-B and immature B cells.
Figure 3.
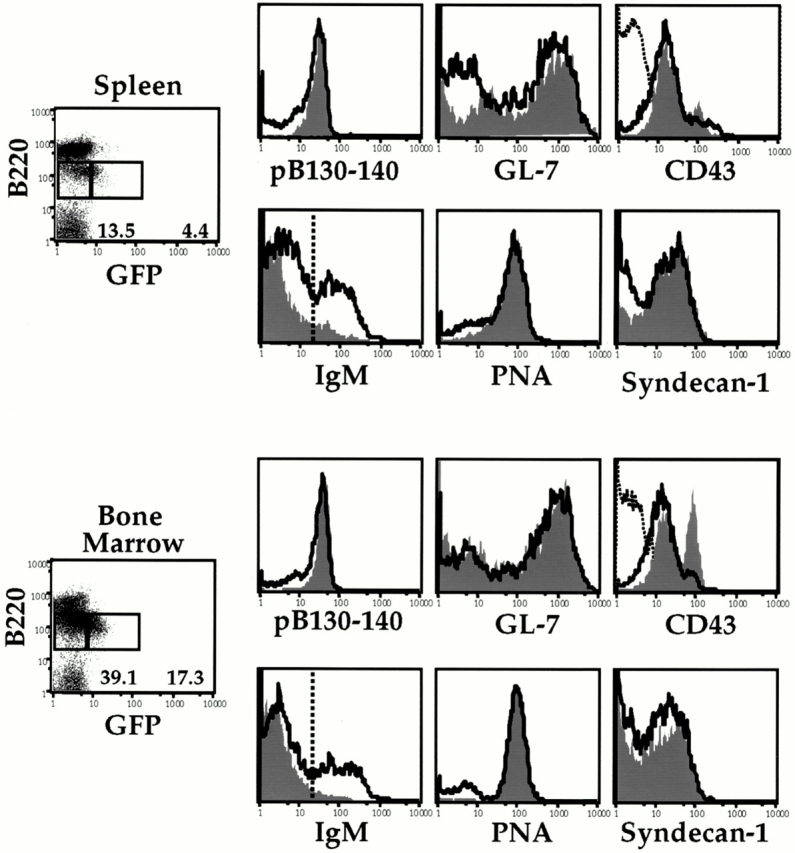
B220lo cells present in immunized spleen resemble pro/pre/immature B cells. Splenic and BM RAG2–GFP+ and RAG2–GFP− cells from NP-CGG/alum–immunized (intraperitoneally) mice at day 16 were analyzed for cell surface expression by staining for pB130-140, GL-7, CD43, IgM, PNA, syndecan-1, and B220. Histograms depicting the expression of the various cell surface markers generated from the RAG2–GFP+ gated cells (shaded) are overlayed with histograms generated from RAG2–GFP− gated cells (line). In the CD43 histogram panels, B220hi gated cells were used to generate CD43− histograms as a reference (dotted line). Percentages of RAG2–GFP+ and RAG2–GFP− cells out of total live B lymphocytes are shown. In the spleen, 10% of GFP+ cells were IgM+ and 32% of GFP− cells were IgM+. In the BM, 5% of GFP+ cells were IgM+ and 30% of GFP− cells were IgM+. Gates are as indicated on the IgM histogram panels. FACS® plots are shown from representative mice of two experiments representing eight RAG2–GFP immunized mice and four 129 naive mice. BM RAG2–GFP+ and RAG2–GFP populations exhibit the same respective cell surface phenotype regardless of whether cells were analyzed from immunized or naive mice. BM or splenic GFP+ and GFP− cells from mice immunized with alum alone exhibited a similar phenotype as the data shown.
Adoptive Transfer of RAG2–GFP Cells Confirm That RAG+ Developing BM B Cells Populate the Spleen after Immunization.
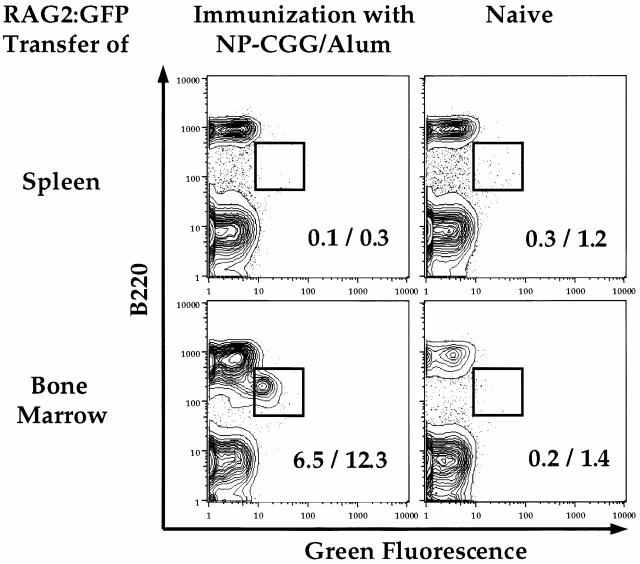
The characteristics of splenic RAG+ B cells are consistent with the notion that they arise from B lineage BM cells. To directly test whether BM cells can give rise to splenic RAG+ cells after immunization, we performed adoptive transfer experiments. BM and spleen cells from either RAG2–GFP or 129 wt donor mice were transferred intravenously into RAG2−/− mice; the following day, the recipients were either immunized with NP-CGG and alum or left unimmunized. By day 16, splenic RAG+ cells were observed only in immunized recipient mice that received RAG2–GFP BM (Fig. 4). Similarly, immunized mice that received BM cells from 129 wt mice contained the splenic B220lopB130-140+ population enriched in RAG+ cells (data not shown). No splenic B220lopB130-140+cells or GFP+ cells were detectable in mice that received 129 wt or RAG2–GFP spleen cells. Immunized control mice and immunized recipients of spleen cells produced antigen-specific Abs after immunization, whereas the BM recipients did not, probably due to insufficient numbers of mature T and/or B cells at the time of immunization (data not shown).
Figure 4.
RAG2–GFP+ BM cells populate the spleen after immunization. RAG2GFP/GFP spleen, RAG2GFP/GFP BM, 129 spleen, or 129 BM cells from pooled 9–16-wk-old mice were adoptively transferred (intravenously) into 5-wk-old RAG2−/− recipients which were immunized with NP-CGG/alum (intraperitoneally) the following day or left unimmunized. Splenic cells from the recipient mice were analyzed by FACS® at day 16 for the presence of RAG2–GFP-expressing cells. Cells are gated for the marker Ly9.1 and displayed as B220 versus RAG2–GFP. Percentages of cells gated for green fluorescence out of B220+ cells (%, right) or total live lymphocytes (%, left) are shown. After immunization, zero out of six mice receiving spleen cells and four out of five mice receiving BM cells showed evidence of B220lo or RAG2-GFP+ cells in the spleen. All spleens from immunized positive control mice contained this population.
These results separate the antigen-specific immune response, which leads to the production of Abs, from the antigen-independent response that leads to the appearance of RAG+ splenic B cells. While we cannot exclude the possibility that transfer conditions did not allow expansion of potential RAG+ precursors potentially present in hematopoietic islands of the donor spleen after immunization, these data show that RAG+ BM cells populate the spleen after immunization. From our combined data, we concluded that immunization leads to the appearance of splenic RAG+ B lineage cells which originate from the BM.
Lack of RAG Reexpression in Activated Mature Splenic B Cells In Vitro.
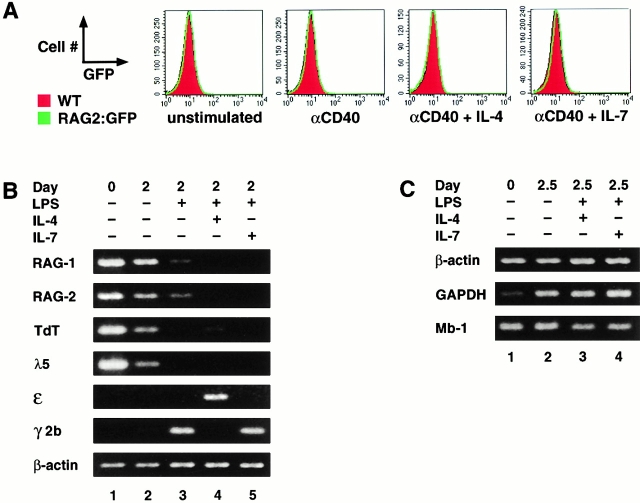
Some studies have detected induction of RAG expression in mature splenic B cells stimulated in vitro 17 18 19 20, whereas others have not 12 26. To further elucidate this phenomenon, we assayed for RAG expression in B220hi splenic B lymphocytes from RAG2–GFP mice cultured with anti-CD40 or anti-CD40 plus IL-4 or IL-7. No RAG expression, as measured by green fluorescence, was detected either in total lymphocytes or in subsets defined by forward scatter or IgD under any of these culture conditions (Fig. 5 A, and data not shown). To further elucidate this issue, we employed RT-PCR to assay for RAG transcripts in purified splenic B cells of normal mice after culture for 2 d in RPMI medium alone, in the presence of LPS, LPS plus IL-4, or in the presence of LPS plus IL-7 17 19. Levels of RAG1 and 2 transcripts were normalized to β-actin transcripts (Fig. 5 B). Whereas uncultured, splenic B cells showed significant RAG RNA signals (Fig. 5 B, lane 1), this level decreased in cultured, unstimulated B cells (Fig. 5 B, lane 2) and declined further in samples stimulated with LPS alone (Fig. 5 B, lane 3). Surprisingly, no induction of RAG1 or RAG2 transcripts was detected in cultures stimulated with LPS plus IL-4 (Fig. 5 B, lane 4) or LPS plus IL-7 (Fig. 5 B, lane 5). Levels of TdT and λ-5 transcripts paralleled those of RAG transcripts. As a positive control, we demonstrated that the expression of germline Cε or Cγ2b transcripts was appropriately induced by LPS or LPS and IL-4 (Fig. 5 B).
Figure 5.
Lack of RAG upregulation in splenic B cells after in vitro stimulation. (A) Sorted B220hi splenic B cells from RAG2GFP/GFP and 129 wt mice were cultured in vitro in RPMI medium alone (unstimulated) or in the presence of anti-CD40, anti-CD40 plus IL-4, or anti-CD40 plus IL-7 for 3 d and analyzed for RAG2–GFP expression by FACS®. Histograms of cultured wt cells (shaded) are overlayed with histograms of cultured RAG2GFP/GFP cells (line). (B) Spleen cells from 10–12-wk-old C3H mice were depleted of T cells by treatment with anti-Thy1.2 mAbs and complement. 80% of the remaining cells were B cells and 2.3% were T cells as determined by FACS® analysis. A second cytotoxic depletion did not significantly improve the removal of T cells (data not shown). The cells were cultured as indicated. Subsequently, RNA was isolated and used for RT-PCR analysis of RAG1, RAG2, TdT, and λ-5. Germline ε and γ2b IgH transcripts were measured to control for LPS (induction of γ2b germline transcript) and of IL-4 (induction of ε germline transcript and suppression of LPS-induced γ2b germline transcript) activity. Activity of IL-7 was determined by its ability to promote proliferation in fetal liver cultures (data not shown). β-Actin was used as a control to ensure equivalent amounts of RNA. One of three similar experiments is shown. In several similar experiments using slightly different conditions (total spleen cultures, two times the cytotoxic T cell depletion, anti-CD40 instead of LPS), RAG transcript levels in activated samples never surpassed those already present in the uncultured cells (data not shown). (C) RT-PCR analysis of the transcript levels of GAPDH and Mb-1 in β-actin–adjusted splenic B cell samples obtained as described in B and treated as indicated.
To exclude the possibility that the β-actin transcript signal used for normalization biased against the detection of RAG upregulation in various culture conditions, we compared the transcript levels of GAPDH and Mb-1, two other markers commonly used for normalization, in β-actin-adjusted samples (Fig. 5 C). Whereas β-actin and Mb-1 levels were consistent under the various culture conditions, GAPDH levels were quite low in uncultured B cells and increased after activation, making this an inappropriate marker for standardization (Fig. 5 C, lane 1). Overall, the decrease in RAG expression after activation, coupled with the correlation between the RAG, TdT, and λ-5 transcript levels, suggests that the preexisting RAG levels in the uncultured samples result from a small number of developing B cells already present in the spleens of unimmunized mice.
To avoid a potentially inhibitory effect of non-B cells on RAG reexpression in the cultures and to reduce the RAG background levels of uncultured samples, we also assessed RAG transcript levels in sorted B220hiIgD+ and B220hi mature B cells after in vitro culture. The combination of anti-CD40 and IL-7 for 3 d prolonged the very low levels of RAG transcripts already present in the uncultured, sorted B220hiIgD+ B cell samples (Fig. 6 A, lanes 2 and 5). However, as observed with the unsorted cultures (Fig. 5), the level of RAG transcripts after stimulation with anti-CD40, IL-4, and IL-7 never exceeded the levels already present in the uncultured samples (Fig. 6 A, and data not shown). To eliminate contaminating RAG-expressing B220lo cells from the sorted population, B220hi cells were analyzed. Notably, when no preexisting RAG RNA was detectable in uncultured B cells, such as for the sorted B220hi B cells (Fig. 6 B, lane 2), no detectable signal appeared upon mitogen/cytokine stimulation (Fig. 6 B, lanes 4 and 5). In conclusion, our data suggest that cultivation in the presence of certain stimuli (in particular IL-7) may prolong preexisting RAG RNA levels in uncultured splenic B cell preparations, presumably by promoting the growth or survival of small numbers of developing RAG+ B lymphocytes. However, the in vitro culture conditions tested do not lead to reexpression of RAG transcripts in mature RAG− B cells to levels detectable by our assays.
Figure 6.
No RAG reexpression in RAG− splenic B cells after in vitro stimulation. (A) Sorted B220hiIgD+ splenic B cells (99% purity by FACS® reanalysis with 0.1% contaminating B220lo cells) from a pool of five 15-wk-old C3H mice were left uncultured or were cultured in vitro for 3 d in RPMI medium as indicated. RT-PCR analysis of the RAG1 transcript levels was performed using β-actin for normalization. Uncultured BM cell RNA served as a positive control. Absolute levels of RAG transcripts in the sorted B cell samples were ∼1:2,000 of those in total BM as determined by serial dilutions (data not shown). The functional integrity of these cultures was confirmed by their ability to perform isotype class switching 4–5 d after appropriate stimulation (data not shown). (B) Splenic B cells sorted based on very high B220 expression were cultured in vitro. RNA was purified from uncultured cells or from samples cultured for 2 d, and RAG1 and RAG2 transcripts were assayed by RT-PCR with high sensitivity (up to 45 cycles) using β-actin for normalization. Uncultured BM cells were analyzed as a positive control.
Discussion
RAG+ Splenic B Cells in Immunized Mice Largely Represent Recent BM Immigrants That Appear via an Antigen- and CD40L-independent Process.
RAG+ splenic B lineage cells in mice immunized with NP-CGG and alum have been proposed to be mature B cells that reexpress RAG after an antigen encounter in the GC 14 15 16 17 18 19 20 21, allowing additional rearrangement of endogenous Ig genes and receptor revision (15 21; for a review, see reference 22). However, it was recently shown that these postimmunization RAG+ cells appeared after the administration of alum alone 23, suggesting that these cells are not antigen specific. In this regard, we now extend these results by showing that the appearance of these cells does not appear to require T cell interactions or a GC reaction, as we observed them in the spleens of “immunized” CD40L−/− mice. In addition, our adoptive transfer studies indicate that the RAG+ splenic B lineage cells in immunized older mice largely represent recent immigrants from the BM. Our findings argue that the majority of splenic RAG+ cells that appear after immunization derive from the migration of differentiating BM RAG+ B lineage cells to the spleen, rather than from the reinduction of RAG in mature B cells.
Previous studies observed RAG+ cells in the GC after immunization 11 12 14 15 16 17 19 20, suggesting that receptor revision occurs in response to the antigen. In support of this notion, studies coupling somatic mutation and Ig re-rearrangement in human tonsil B cells and transgenic mouse B cells also supported the existence of peripheral B lineage cells that revise their receptors (39 40 41; for a review, see reference 22). However, it was also noted that RAG+ GC cells predominantly lost Ig expression and expressed several pro/pre-B markers such as λ-5, Vpre-B, and TdT 14 15 26. One potential explanation for this common gene expression was that the mature B cells underwent a process of “neoteny” in which the cells reverted to a pre/pro-B–like phenotype along with the reactivation of RAG genes 14. However, pre-B cells in the BM also express several standard GC B cell markers including PNA and GL-7 (this study; 14, 37, 42, 43; for reviews, see references 44, 45), raising the possibility that BM pre-B cell immigrants to the spleen could be readily confused with traditional GC B cells. Correspondingly, recent studies using NG-BAC RAG reporter mice showed no evidence of RAG reexpression in either adoptively transferred RAG− spleen cells or whole spleen cells after immunization 12 23. These studies also showed that RAG+ cells present in the spleen on day 16 after immunization did not represent proliferating GC cells 23. Overall, our current results support the general conclusion of the latter studies, that the majority of RAG+ cells in the spleen after immunization do not derive from mature B cells.
Persistence of Preexisting RAG Expression Rather Than RAG Reexpression in Mitogen/Cytokine–stimulated Splenic B Cell Cultures.
The initial notion that mature B cells could be induced to reexpress RAG genes derived substantially from studies that reported the induction of RAG transcripts in mature peripheral B cells after in vitro culture in the presence of various activators and cytokines 16 17 18 19 21. However, other studies have found that RAG is not induced in LPS plus IL-4–treated splenic B cells 12 26. In this regard, we found that preexisting RAG transcript levels did in fact decline when splenic B lineage cell populations were cultured without stimulation or with mitogen treatment alone, although the addition of cytokines, notably IL-7, could prolong baseline RAG expression. In this context, IL-7 is known to promote survival and/or proliferation of RAG+ developing B cells 46 47 48. While the population of RAG+ splenic B lineage cells with a pro/pre-B phenotype decreased to levels undetectable by the RAG2–GFP reporter assay in 9-wk-old mice, such cells still may be the source of the low preexisting RAG levels detected in uncultured splenic B cell preparations from older mice via the more sensitive RT-PCR assay. Outgrowth of splenic pre/pro-B cells and/or differentiation into RAG+ immature B cells could account for the apparent RAG induction observed in previous studies. Though we find no evidence for RAG induction in splenic B cells at the population level, it is conceivable that there could be induction of RAG in a minor subset of B cells. Finally, preliminary studies also suggest that our finding of RAG transcripts in cultured IgD+ BM B cells after treatment with anti-IgM 11 may result from the selection of preexisting RAG+ cells (Seidl, K., unpublished data).
Why Does Alum Administration Cause BM RAG-expressing Lymphocytes to Immigrate to the Spleen?
It is thought that adjuvants such as alum augment the immune response to antigens by inducing the innate immune system through similar mechanisms as natural infections 49 50. Recent studies of NG-BAC transgenic mice demonstrated that both alum injection and malaria infection suppress lymphopoiesis in the BM, but that lymphopoiesis recovers by day 16 accompanied by the accumulation of RAG+ splenic cells 23. This increased production of B lineage cells, similar to that described following sublethal irradiation 9 10 23, may directly contribute to the accumulation of RAG+ spleen cells. In our study, alum administration caused local inflammation in the spleen, as evidenced by the recruitment of granulocytes. The infiltrating granulocytes may secrete as yet undefined cytokines that, in turn, induce the migration, proliferation, or differentiation of BM RAG+ cells. A similar function has been described previously for macrophages (for a review, see reference 51). This influx of developing B cells into the spleen during an immune response raises the question of whether such cells could play an direct or indirect role in the generation of antigen-specific cells and, if so, what mechanisms would enforce tolerance with respect to antigen-specific cells generated in the periphery 11.
Immunization May Lead to Differentiation of BM B-Lineage Cells in the Spleen.
Studies of both unimmunized and immunized NG-BAC mice revealed a much greater level of GFP+IgM+ splenic B lineage cells than we found in the corresponding RAG2–GFP mice. Although these findings might seem contradictory, they are in fact likely to be complementary when viewed with respect to the differences in the two reporter systems. Thus, the longer half-life of the GFP protein versus the endogenous RAG proteins in the NG-BAC mice may allow the detection of cells that recently expressed RAG for a short period after the expression of the endogenous RAG genes was extinguished 12 23. On the other hand, the RAG2–GFP fusion protein appears to closely resemble endogenous RAG2 with respect to expression 11. Therefore, when taken together, the results of these two separate approaches would be quite consistent with the notion that, in response to alum, RAG+ pre-B and immature B cells migrate from the BM to the spleen where they can undergo further differentiation to become mature B cells.
Acknowledgments
We thank Garnett Kelsoe, Michel Nussenzweig, Mark Schlissel, Hitoshi Ohmori, and David Nemazee for sharing relevant unpublished data and for their helpful discussions. We also thank Juanita Campos at the Howard Hughes Cell Sorter Facility, Department of Genetics, Harvard Medical School, and the Dana-Farber Cancer Institute Core Flow Cytometry Facility for their excellent technical assistance. F.W. Alt is an Investigator, and F. Gärtner was an Associate of The Howard Hughes Medical Institute.
K.J. Seidl was supported by postdoctoral fellowship PF-99-125-01-CIM from the American Cancer Society. This work was supported by National Institutes of Health grant AI20047 (to F.W. Alt).
Footnotes
Abbreviations used in this paper: alum, aluminum-hydroxide; B6, C57BL/6; bi, biotin; BM, bone marrow; CyC, CyChrome; GC, germinal center; GAPDH, glyceraldehyde 3-phosphate dehydrogeanse; GFP, green fluorescent protein; HSA, heat stable antigen; NP-CGG, 4-hydroxy-3-nitrophenyl-acetyl coupled to chicken γ-globulin; PNA, peanut agglutinin; RAG, recombination activating gene; RT, reverse transcription; wt, wild-type.
References
- Lansford R., Okada A., Chen J., Oltz E.M., Blackwell T.K., Alt F.W., Rathbun G. Mechanism and control of immunoglobulin gene rearrangement. 2nd ed. In: Hames B., Glover D., editors. Molecular Immunology. Oxford University Press; Oxford: 1996. pp. 248–282. [Google Scholar]
- Wilson A., Held W., MacDonald H.R. Two waves of recombinase gene expression in developing thymocytes. J. Exp. Med. 1994;179:1355–1360. doi: 10.1084/jem.179.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U., Leu T.M., Schatz D.G., Werner A., Rolink A.G., Melchers F., Winkler T.H. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Li Y.S., Hayakawa K., Hardy R.R. The regulated expression of B lineage–associated genes during B cell differentiation in bone marrow and fetal liver. J. Exp. Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Misulovin Z., Suh H., Hardy R.R., Jankovic M., Yannoutsos N., Nussenzweig M.C. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science. 1999;285:1080–1084. doi: 10.1126/science.285.5430.1080. [DOI] [PubMed] [Google Scholar]
- Rolink A.G., ten Boekel E., Yamagami T., Ceredig R., Andersson J., Melchers F. B cell development in the mouse from early progenitors to mature B cells. Immunol. Lett. 1999;68:89–93. doi: 10.1016/s0165-2478(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Carsetti R., Kohler G., Lamers M.C. Transitional B cells are the target of negative selection in the B cell compartment. J. Exp. Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A.G., Andersson J., Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur. J. Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Allman D.M., Ferguson S.E., Cancro M.P. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J. Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- Allman D.M., Ferguson S.E., Lentz V.M., Cancro M.P. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- Monroe R.J., Seidl K.J., Gaertner F., Han S., Chen F., Sekiguchi J., Wang J., Ferrini R., Davidson L., Kelsoe G., Alt F.W. RAG2:GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity. 1999;11:201–212. doi: 10.1016/s1074-7613(00)80095-3. [DOI] [PubMed] [Google Scholar]
- Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., Nussenzweig M.C. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- Osmond D.G., Rolink A., Melchers F. Murine B lymphopoiesistowards a unified model. Immunol. Today. 1998;19:65–68. doi: 10.1016/s0167-5699(97)01203-6. [DOI] [PubMed] [Google Scholar]
- Han S., Zheng B., Schatz D.G., Spanopoulou E., Kelsoe G. Neoteny in lymphocytesRag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- Han S., Dillon S.R., Zheng B., Shimoda M., Schlissel M.S., Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- Hikida M., Mori M., Kawabata T., Takai T., Ohmori H. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J. Immunol. 1997;158:2509–2512. [PubMed] [Google Scholar]
- Hikida M., Nakayama Y., Yamashita Y., Kumazawa Y., Nishikawa S.I., Ohmori H. Expression of recombination activating genes in germinal center B cellsinvolvement of interleukin 7 (IL-7) and the IL-7 receptor. J. Exp. Med. 1998;188:365–372. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz M., Kouskoff V., Nakamura T., Nemazee D. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 1998;394:292–295. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida M., Mori M., Takai T., Tomochika K., Hamatani K., Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- Hikida M., Ohmori H. Rearrangement of lambda light chain genes in mature B cells in vitro and in vivo. Function of reexpressed recombination-activating gene (RAG) products. J. Exp. Med. 1998;187:795–799. doi: 10.1084/jem.187.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou F., Casellas R., Suh H., Qin X.F., Besmer E., Pelanda R., Nemazee D., Rajewsky K., Nussenzweig M.C. V(D)J recombination in mature B cellsa mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Weigert M. Revising B cell receptors. J. Exp. Med. 2000;191:1813–1818. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka H., Gonzalez-Aseguinolaza G., Tsuji M., Nussenzweig M.C. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J. Exp. Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Zheng B., Takahashi Y., Kelsoe G. Distinctive characteristics of germinal center B cells. Semin. Immunol. 1997;9:255–260. doi: 10.1006/smim.1997.0081. [DOI] [PubMed] [Google Scholar]
- Radic M.Z., Zouali M. Receptor editing, immune diversification, and self-tolerance. Immunity. 1996;5:505–511. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- Meffre E., Papavasiliou F., Cohen P., de Bouteiller O., Bell D., Karasuyama H., Schiff C., Banchereau J., Liu Y.J., Nussenzweig M.C. Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells. J. Exp. Med. 1998;188:765–772. doi: 10.1084/jem.188.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 1997;94:9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz M., Nemazee D. BCR ligation induces receptor editing in IgM+IgD− bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Kuwata N., Igarashi H., Ohmura T., Aizawa S., Sakaguchi N. Cutting edgeabsence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J. Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- Renshaw B.R., Fanslow W.C., III, Armitage R.J., Campbell K.A., Liggitt D., Wright B., Davison B.L., Maliszewski C.R. Humoral immune responses in CD40 ligand–deficient mice. J. Exp. Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J. Immunol. 1995;155:3377–3384. [PubMed] [Google Scholar]
- Zelazowski P., Carrasco D., Rosas F.R., Moorman M.A., Bravo R., Snapper C.M. B cells genetically deficient in the c-Rel transactivation domain have selective defects in germline CH transcription and Ig class switching. J. Immunol. 1997;159:3133–3139. [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987;48:757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Oxenius A., Campbell K.A., Maliszewski C.R., Kishimoto T., Kikutani H., Hengartner H., Zinkernagel R.M., Bachmann M.F. CD40–CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+ T cell functions. J. Exp. Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.L., Birbeck M.S., Wallis V.J., Forrester J.A., Davies A.J. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Sanderson R.D., Lalor P., Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.C., Wilson K., Liu Y.J., Banchereau J., Pascual V., Capra J.D. Receptor revision of immunoglobulin heavy chain variable region genes in normal human B lymphocytes. J. Exp. Med. 2000;191:1881–1894. doi: 10.1084/jem.191.11.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brard F., Shannon M., Prak E.L., Litwin S., Weigert M. Somatic mutation and light chain rearrangement generate autoimmunity in anti–single-stranded DNA transgenic MRL/lpr mice. J. Exp. Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wildt R.M., Hoet R.M.A., van Venrooij W.J., Tomlinson I.M., Winter G. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J. Mol. Biol. 1999;285:895–901. doi: 10.1006/jmbi.1998.2396. [DOI] [PubMed] [Google Scholar]
- Osmond D.G., Batten S.J. Genesis of B lymphocytes in the bone marrowextravascular and intravascular localization of surface IgM-bearing cells in mouse bone marrow detected by electron-microscope radioautography after in vivo perfusion of 125I anti-IgM antibody. Am. J. Anat. 1984;170:349–365. doi: 10.1002/aja.1001700310. [DOI] [PubMed] [Google Scholar]
- Laszlo G., Hathcock K.S., Dickler H.B., Hodes R.J. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J. Immunol. 1993;150:5252–5262. [PubMed] [Google Scholar]
- Kelsoe G. The germinal centera crucible for lymphocyte selection. Semin. Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa K. B lineage differentiation stages resolved by multiparameter flow cytometry. Ann. NY Acad. Sci. 1995;764:19–24. doi: 10.1111/j.1749-6632.1995.tb55800.x. [DOI] [PubMed] [Google Scholar]
- Era T., Nishikawa S., Sudo T., Wang F.H., Ogawa M., Kunisada T., Hayashi S. How B-precursor cells are driven to cycle. Immunol. Rev. 1994;137:35–51. doi: 10.1111/j.1600-065x.1994.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Rolink A.G., Winkler T., Melchers F., Andersson J. Precursor B cell receptor–dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J. Exp. Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. An ancient system of host defense. Curr. Opin. Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- Fearon D.T. Seeking wisdom in innate immunity. Nature. 1997;388:323–324. doi: 10.1038/40967. [DOI] [PubMed] [Google Scholar]
- Osmond D.G. Population dynamics of bone marrow B lymphocytes. Immunol. Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]