Abstract
A diverse and complex array of lipids plays a vital role in structuring and organizing cell membranes. However, the details of lipid requirements for global membrane organization are poorly understood. One obstacle to this understanding is the difficulty of accurately manipulating the lipid composition of commonly studied mammalian cells. In contrast, the lipid composition of cells of ectotherms changes with changes in environmental temperatures. Thus, comparison of lipid probe diffusion in cells from animals living at different temperatures, together with biochemical analysis, can be used toward understanding membrane organization. We used two dialkyindocarbocyanine iodide (DiI) probes, of differing chain length, to probe lipid organization in terms of their lateral diffusion in eggs of the sea urchin Strongylocentrotus purpuratus. The lateral diffusion of our probes changed in urchins developing in the year of an “El Niño” weather event, which raised the ocean temperature by several degrees, suggesting alterations in membrane domain composition and structure. Indeed the changes in lateral diffusion were correlated with lower levels of unsaturated fatty acids and cholesterol in animals of the “El Niño” year than in animals of the preceding or following years. We found similar trends comparing DiI diffusion in membranes of eggs from 15 °C waters with those from 10°C. Our findings establish a new approach for manipulating and studying membrane organization.
It is now widely recognized that biological membrane organization is extremely complex (Edidin, 1997; Edidin 2007; Shaikh and Edidin, 2006). Experimental and theoretical studies have led to notion of membrane lipid and protein domains, picket fences, and protein-protein clustering as mechanisms by which cells propagate signals form the plasma membrane (Edidin, 1997; Edidin 2003). A major limitation toward understanding membrane organization has been developing tools that can measure changes in membrane organization, especially lipid organization. For example, while work with model membranes predicts that lipid probes can differentiate between liquid-ordered, lo, domains (so-called lipid rafts) and liquid-disordered, ld, domains, experiments on cells to change the proportion of lo and ld domains of mammalian cells are often confounded by metabolic effects of feeding or removing particular lipids, for example cholesterol (Kwik et al., 2006; Shaikh and Edidin 2007).
In contrast to the difficulties of manipulating lipid composition of homeothermic, mammalian, cells, lipid composition of cells of ectothermic animals can be changed in as response to the temperature of the environment (Edidin and Sessions, 1984; Hazel, 1995). Thus, a good starting point for evaluating the effects of lipids on membrane organization is to work with cells of ectothermic animals living at different temperatures. Temperature shifts can then be used to work out the relationship between the plasma membrane heterogeneity reported by lateral diffusion of lipid probes, temperature and lipid composition of cells.
In the present study, we measure the temperature dependence of lateral diffusion of 2 different DiI lipid probes in the plasma membranes of sea urchin, Strongylocentrotus purpuratus, eggs obtained from the same population, nominally living at 15°C, in three different years, including an “El Niño” event (1983) which raised ocean water temperature by several degrees during the months in which the eggs developed. We also compare DiI diffusion in the membrane of these eggs to that in egg membranes from animals growing at 10°C. We find that in eggs developing in the year before the El Niño the lateral diffusion of the short-chain lipid probe, DiI C12, is only weakly dependent upon temperature, while the diffusion of the longer-chain DiI C16 increases linearly with temperature over the range 15–30°C. In contrast, diffusion of all probes is insensitive to temperature in eggs developing in an El Niño year of higher ocean temperature, which resulted in a reduction in unsaturated membrane lipids and membrane cholesterol. Diffusion of the probes in membranes from eggs developing at 10°C was consistent with the lipid composition of these membranes relative to that of the 15 °C population.
Materials and Methods
Two dialkylcarbocyanine iodide (DiI) fluorophores (Invitrogen, Carlsbad, CA) varying in chain length (C12 or C16) were used to probe the organization of sea urchin eggs using FRAP microscopy (Kwik et al., 2003); the eggs did not label with the longer chain DiI, C18. A second extreme probe, DiI C10, proved to be significantly water soluble; hence it was not a reliable probe of membrane lipid organization. Sea urchin eggs were isolated as previously described in each of three different years. Year 2 was the “El Niño” year (Kinsey et al., 1980; Wolf et al,, 1981). In this year the ocean temperature was raised by 1–3 °C, as measured at the Scripps Institute of Oceanography (La Jolla, CA). In year 3, eggs were obtained from two different populations, one living in 10°C waters and the other at 15 °C. FRAP measurements provided a diffusion coefficient D and mobile fraction R using a bleach time of 4ms. All values for R and D are reported as Winsorized means; in this approach, the error of a mean is reduced by dropping high and low outliers from the data set (Tukey and McLaughlin, 1963). Lipids were extracted from sea urchin eggs using the method of Bligh and Dyer (Bligh and Dyer, 1959).
Total cholesterol content of isolated plasma membranes (Kinsey et al., 1980) was determined using a cholesterol oxidase kit (Sigma Chemical Co., St. Louis, MO) and acyl chain composition was determined with gas chromatography (Weaver, 1985) and verified with mass spectroscopy. The reported cholesterol values are mole percent of total phospholipids and normalized to total protein per cell.
Results and Discussion
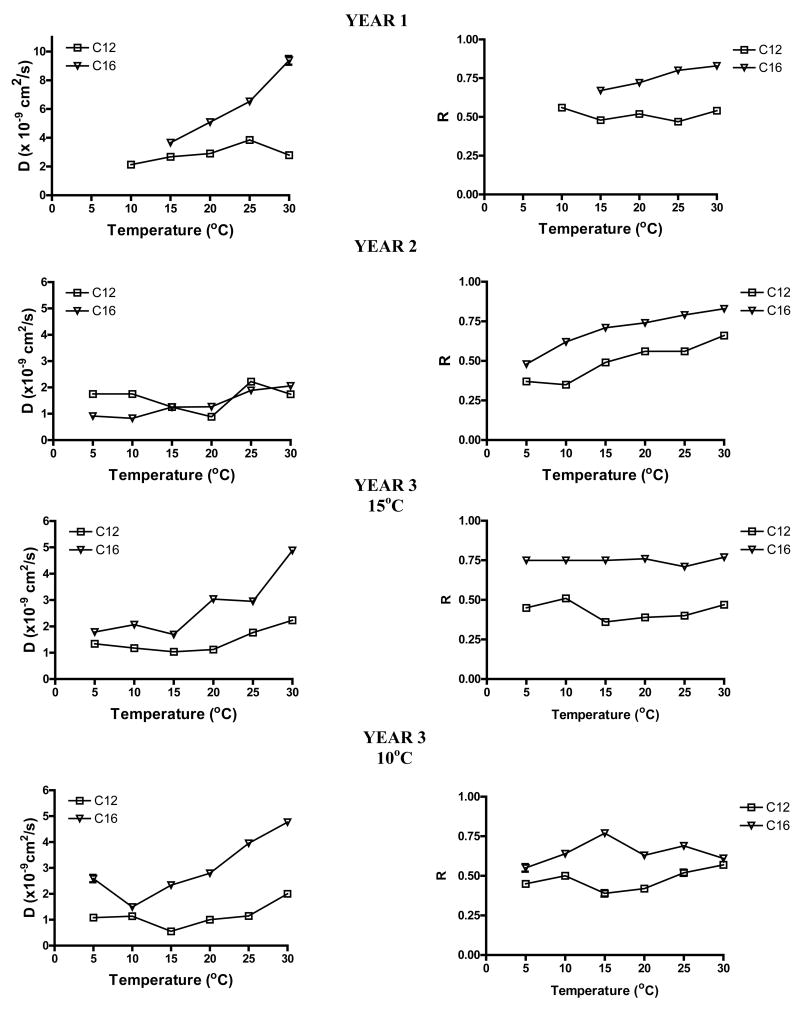
In eggs developing the year before El Niño, the diffusion coefficients for DiI C12, and C16 fell within the range measured previously for these probes in S. purpuratus eggs (Wolf et al., 1981), 2-9.5 × 10-9 cm2/s, over the temperature range of 10–30 °C (Fig. 1, left panel). However, the diffusion coefficients for the two probes were not identical functions of temperature. Diffusion of DiI C16 was most sensitive to temperature increasing about 3-fold over the range 15–30 °C. D for C12 increased at most 2-fold over a larger temperature range, 10–30 °C. D of both probes was significantly lower in egg membranes from animals in the next year, an El Niño year. D is still nearly linear with temperature, but the range of values is reduced by 2-4 fold to 0.9-2.1 × 10-9 cm2/s. The diffusion coefficients for C12 now fall within the same range as those for C16. The trends in D for the year after the El Niño, year 3, are similar to those of year 1. The effects of T on D of DiI C12 are larger than in year 1. D of both probes was most similar to D of the probes in eggs of another population of S. purpuratus living at 10 °C. The mobile fractions of the probes in all years were close to those published earlier for S. pupuratus eggs from this population (Fig. 1, right panel) (Wolf et al, 1981). However, the temperature dependence of mobile fraction was stronger in eggs from the El Niño year. The mobile fraction of probes in year 3 eggs from animals living at 15 °C was unaffected by changes in temperature similar to the mobile fraction of probes in eggs from a population of animals at 10 °C.
Figure 1.
Diffusion coefficient D (left) and mobile fraction R (right) values as a function of temperature from three different years. Samples were collected from the Pacific ocean for all three years. An additional population in year 3 was collected from a 10°C Pacific ocean. D and R values are plotted as Winsorized means ± S.D. and were calculated from FRAP recovery curves. Error bars are generally smaller than the data points and ranged from 0.01–0.3. Note that the ordinate scale is different for the diffusion coefficient data for Year 1. N = 9–57 animals at each temperature.
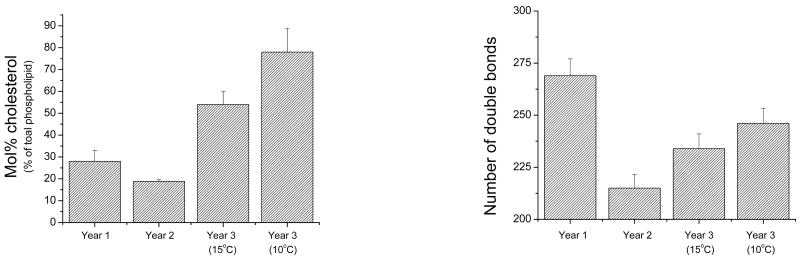
The changes in probe diffusion were paralleled by changes in the lipid composition of crude membrane fractions isolated from the eggs. It can be seen in Figure 2 that the ocean warming, and destruction of kelp beds associated with the El Niño was associated with decreases in membrane cholesterol (mol% of total phospholipid) and in unsaturated lipid acyl chains. These changes were only partly reversed in the year after the El Niño. Indeed, consistent with the diffusion data, the lipid composition of eggs shed at 15 °C in the year after the El Niño most resembled the composition of eggs from animals living at 10 °C.
Figure 2.
Lipid analysis of sea urchin eggs from three different years. (Left) Mol % cholesterol determined via cholesterol oxidase. Cholesterol values are relative to total phospholipid content and normalized to total protein. (Right) Double bonds per hundred non-hydroxy fatty acids determined with gas chromatography and verified with mass spectroscopy.
The unequal temperature dependence of D and R for lipid probes of different chain lengths is consistent with changes in the organization of sea urchin egg plasma membrane lipids. One possible interpretation is that a change in probe diffusion reflects a change in membrane domains, local concentrations of particular composition deviating from the average for the membrane. Changes in the diffusibility of the probes correlate with changes in membrane lipid composition and could change with domain composition. The high temperature sensitivity and high absolute D of the longest chain probe, DiI C16 at first sight seems paradoxical. Measurements of the partition of DiI’s into gel and fluid lipid phases suggest that at least a fraction of DiI C16 is “gel preferring” (Klausner and Wolf, 1980). However, the behavior of the probe is consistent with a membrane in which sub-microscopic islands of disordered (short-chain or highly unsaturated) lipids are distributed in a continuum of more ordered lipids. D and R then would reflect continuity of domains and probe partition preferences, rather than depending only on lipid viscosity. Other possibilities include changes in smaller-scale lipid organization, or in lipid-protein interactions. It may also be that the diffusion of the shorter-chain probes is more influenced by membrane proteins than is the lateral diffusion of DiI C16.
The high levels of cholesterol content in year 3, albeit surprising, are not inconsistent with reported literature values. The range of cholesterol values reported for Strongylocentrotus species is 36 to 86 mole percent of phospholipids; our values fall within this range (Campisi and Scandella, 1980; Kozhina et al., 1978). Since we used a crude membrane preparation, we cannot completely rule out the possibility of cholesterol from cortical granules, although one would then expect that the year 1 membranes would have the highest cholesterol to phospholipid ratio. In spite of the drawbacks of the crude membrane preparation, the lipid extracts examined in this study, while they may not strictly be composed of material from the plasma membrane, certainly reflect the composition of those membranes.
Our results point to the possibility of studying membrane organization in the cells of ectothermic animals, invertebrates and vertebrates, whose lipid composition can be readily changed by changes in their growth temperature. We suggest that the approach used here can be applied to a variety of ectotherms, notably fish, and used to work out the organization of membrane lipids and the genetics of this organization.
Acknowledgments
Funding from the National Institutes of Health to M.E. (AI14584).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Campisis J, Scandella CJ. Bulk membrane fluidity increases after fertilization or partial activation of sea urchin eggs. J Biol Chem. 1980;255:5411–5419. [PubMed] [Google Scholar]
- Edidin ME. Lipid microdomains in cell surface membranes. Curr Opin Struct Biol. 1997;7:528–532. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- Edidin ME. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Edidin M, Sessions AVV. Heterogeneity in the plasma membrane lipids of eukaryotic cells. Ann NY Acad Sci. 1984;414:8–18. doi: 10.1111/j.1749-6632.1983.tb31670.x. [DOI] [PubMed] [Google Scholar]
- Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Decker GL, Lennarz WJ. Isolation and partial characterization of the plasma membrane of sea urchin egg. J Cell Biol. 1980;87:248–254. doi: 10.1083/jcb.87.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheets MP, Edidin M. Membrane cholesterol, Lateral mobility & the PI(4,5)P2-dependent organization of cell actin. Proc Natl Acad Sci USA. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhina VP, Terekhova TA, Svetashev VI. Lipid composition of gametes and embryos of the sea urchin strongylocentrotus intermedius at early stages of development. Develop Biol. 1978;62:412–517. doi: 10.1016/0012-1606(78)90232-4. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Wolf DE. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry. 1980;19:6199–6203. doi: 10.1021/bi00567a039. [DOI] [PubMed] [Google Scholar]
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Membranes are not just rafts. Chem Phys Lipids. 2006;144:1–3. doi: 10.1016/j.chemphyslip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- Tukey JW, McLaughlin DH. Less vulnerable confidence and significance procedure for location based on a single sample: Trimming/Winsorization. Indian Journal of Statistics (A) 1963;25:331–352. [Google Scholar]
- Weaver FE. Studies on the effects of temperature and membrane composition on the organization of the eukaryotic cell membranes. Johns Hopkins University; Baltimore, MD: 1985. Ph.D. Thesis. [Google Scholar]
- Wolf DE, Kinsey E, Lennarz W, Edidin M. Changes in the organization of the sea urchin egg plasma membrane upon fertilization: indications from the lateral diffusion rates of lipid-soluble fluorescent dyes. Dev Biol. 1981;81:133–138. doi: 10.1016/0012-1606(81)90355-9. [DOI] [PubMed] [Google Scholar]