Abstract
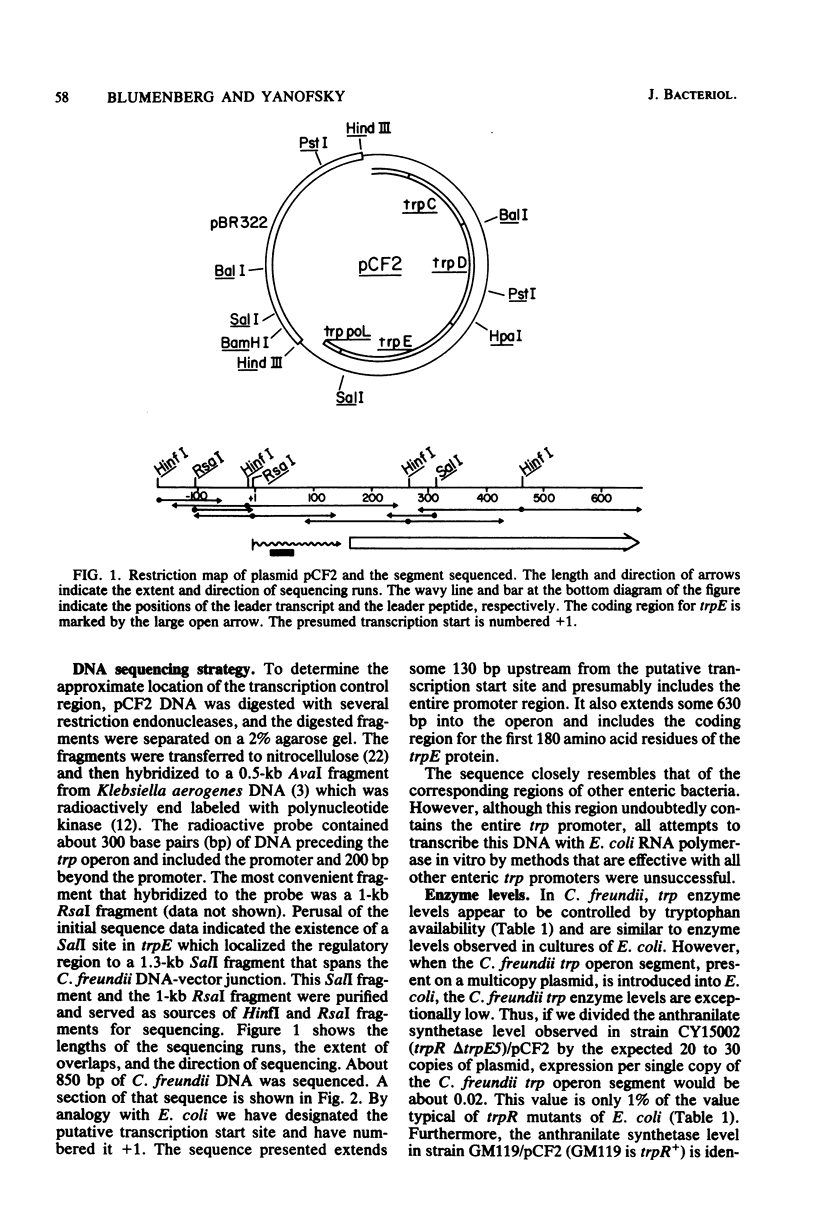
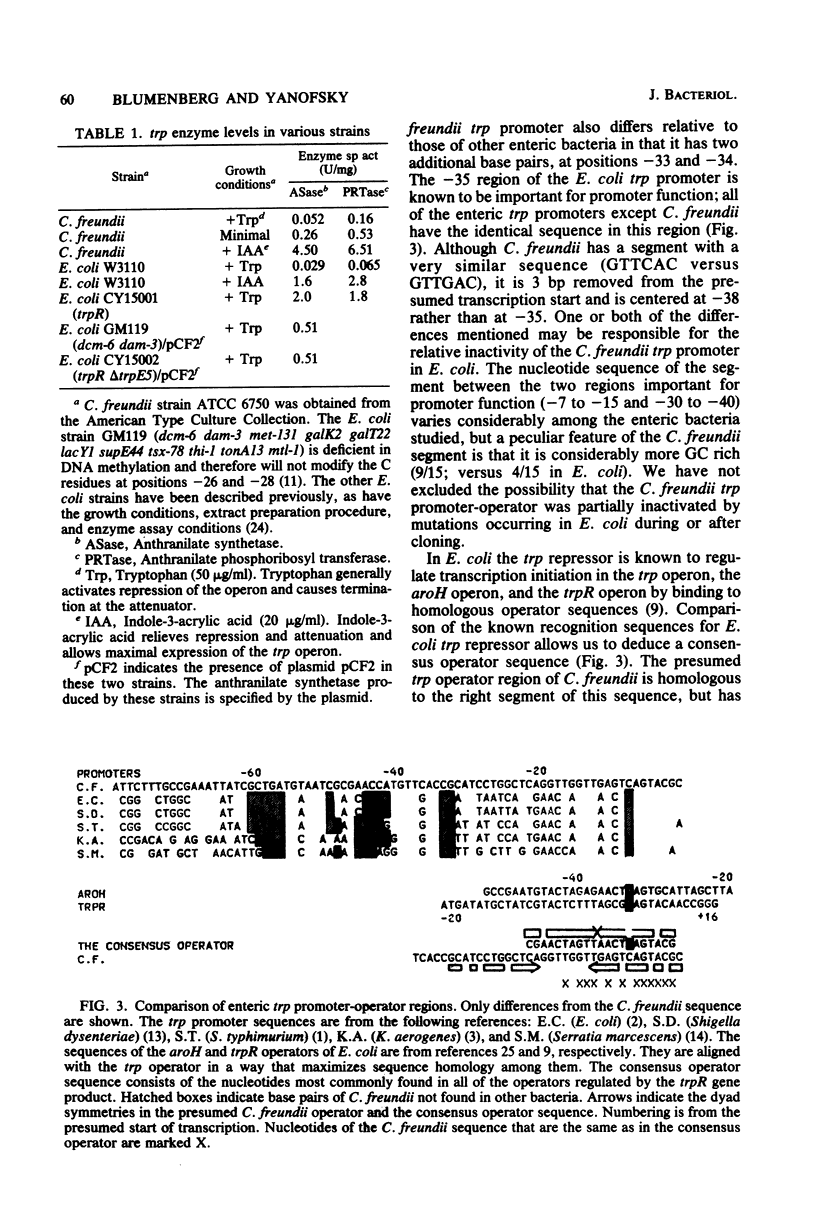
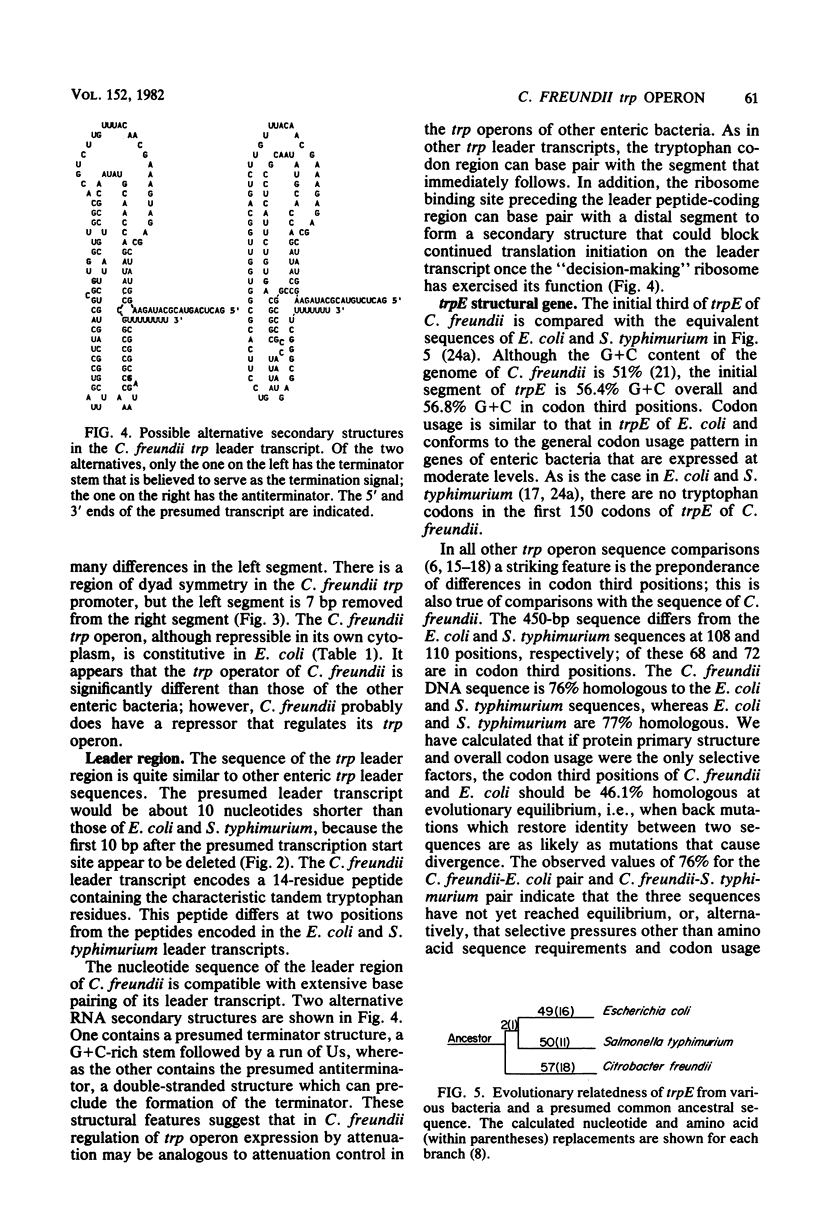
The regulatory region of the trp operon of Citrobacter freundii was sequenced and compared with the corresponding regions of other enteric bacteria. Significant differences were noted in the promoter region. These differences are presumably responsible for the weak expression of the cloned trp operon in Escherichia coli. The presumed operator region, although nonfunctional in E. coli, has dyad symmetry, but the sequence of the symmetrical region differs appreciably from those of operators that can be regulated by the E. coli trp repressor. The sequence of the trp leader region of C. freundii resembles that of other enteric bacteria, suggesting that the C. freundii operon is also regulated by attenuation. Comparison of the sequence of the initial portion of trpE with the homologous regions of E. coli and Salmonella typhimurium indicates that the three organisms probably are evolutionary equidistant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. N., Brown K. D., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):139–152. doi: 10.1016/s0022-2836(78)80002-3. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):113–137. doi: 10.1016/s0022-2836(78)80001-1. [DOI] [PubMed] [Google Scholar]
- Blumenberg M., Yanofsky C. Regulatory region of the Klebsiella aerogenes tryptophan operon. J Bacteriol. 1982 Oct;152(1):49–56. doi: 10.1128/jb.152.1.49-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Nichols B. P., Yanofsky C. Nucleotide sequence of the trpB gene in Escherichia coli and Salmonella typhimurium. J Mol Biol. 1980 Oct 5;142(4):489–502. doi: 10.1016/0022-2836(80)90259-4. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largen M., Belser W. L. Tryptophan biosynthetic pathway in the Enterobacteriaceae: some physical properties of the enzymes. J Bacteriol. 1975 Jan;121(1):239–249. doi: 10.1128/jb.121.1.239-249.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Konrad E. B. Hyper-recombination in dam mutants of Escherichia coli K-12. Mol Gen Genet. 1976 Dec 22;149(3):273–277. doi: 10.1007/BF00268528. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Yanofsky C. The regulatory region of the trp operon of Serratia marcescens. Nature. 1978 Dec 14;276(5689):684–689. doi: 10.1038/276684a0. [DOI] [PubMed] [Google Scholar]
- Miozzari G., Yanofsky C. Naturally occurring promoter down mutation: nucleotide sequence of the trp promoter/operator/leader region of Shigella dysenteriae 16. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5580–5584. doi: 10.1073/pnas.75.11.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Blumenberg M., Yanofsky C. Comparison of the nucleoside sequence of trpA and sequences immediately beyond the trp operon of Klebsiella aerogenes. Salmonella typhimurium and Escherichia coli. Nucleic Acids Res. 1981 Apr 10;9(7):1743–1755. doi: 10.1093/nar/9.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Miozzari G. F., van Cleemput M., Bennett G. N., Yanofsky C. Nucleotide sequences of the trpG regions of Escherichia coli, Shigella dysenteriae, Salmonella typhimurium and Serratia marcescens. J Mol Biol. 1980 Oct 5;142(4):503–517. doi: 10.1016/0022-2836(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Yanofsky C. Nucleotide sequences of trpA of Salmonella typhimurium and Escherichia coli: an evolutionary comparison. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5244–5248. doi: 10.1073/pnas.76.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., van Cleemput M., Yanofsky C. Nucleotide sequence of Escherichia coli trpE. Anthranilate synthetase component I contains no tryptophan residues. J Mol Biol. 1981 Feb 15;146(1):45–54. doi: 10.1016/0022-2836(81)90365-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Functional analysis of wild=type and altered tryptophan operon promoters of Salmonella typhimurium in Escherichia coli. J Mol Biol. 1980 Dec 5;144(2):143–161. doi: 10.1016/0022-2836(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Rifampin resistance mutations that alter the efficiency of transcription termination at the tryptophan operon attenuator. J Bacteriol. 1981 Mar;145(3):1334–1341. doi: 10.1128/jb.145.3.1334-1341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., vanCleemput M. Nucleotide sequence of trpE of Salmonella typhimurium and its homology with the corresponding sequence of Escherichia coli. J Mol Biol. 1982 Mar 5;155(3):235–246. doi: 10.1016/0022-2836(82)90003-1. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]


