Abstract
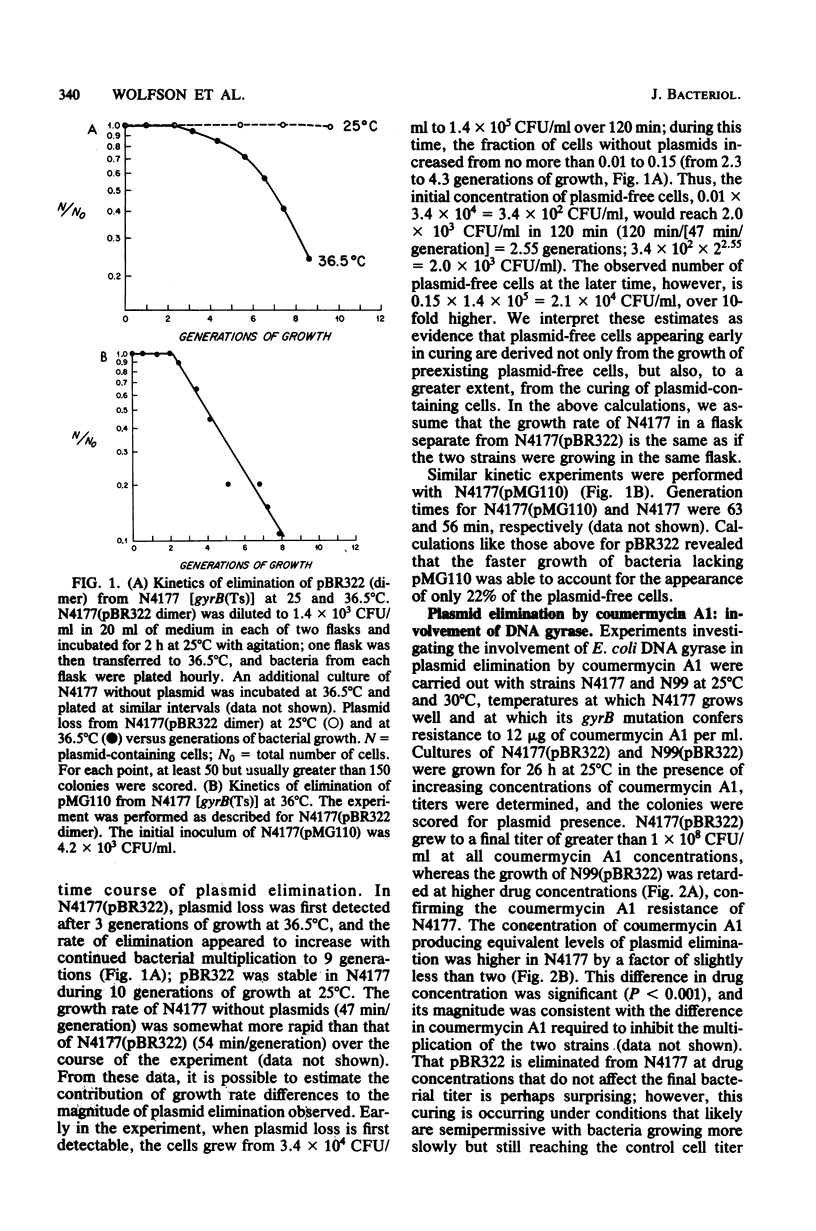
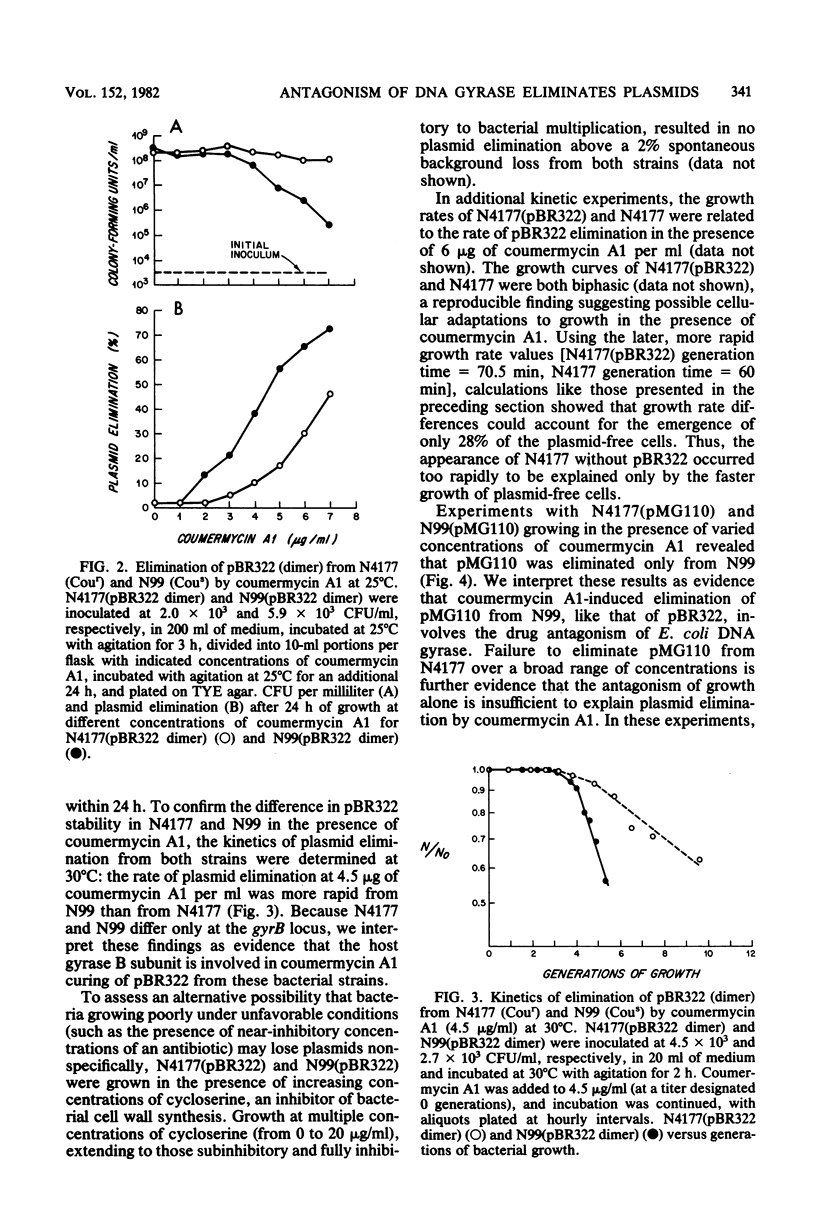
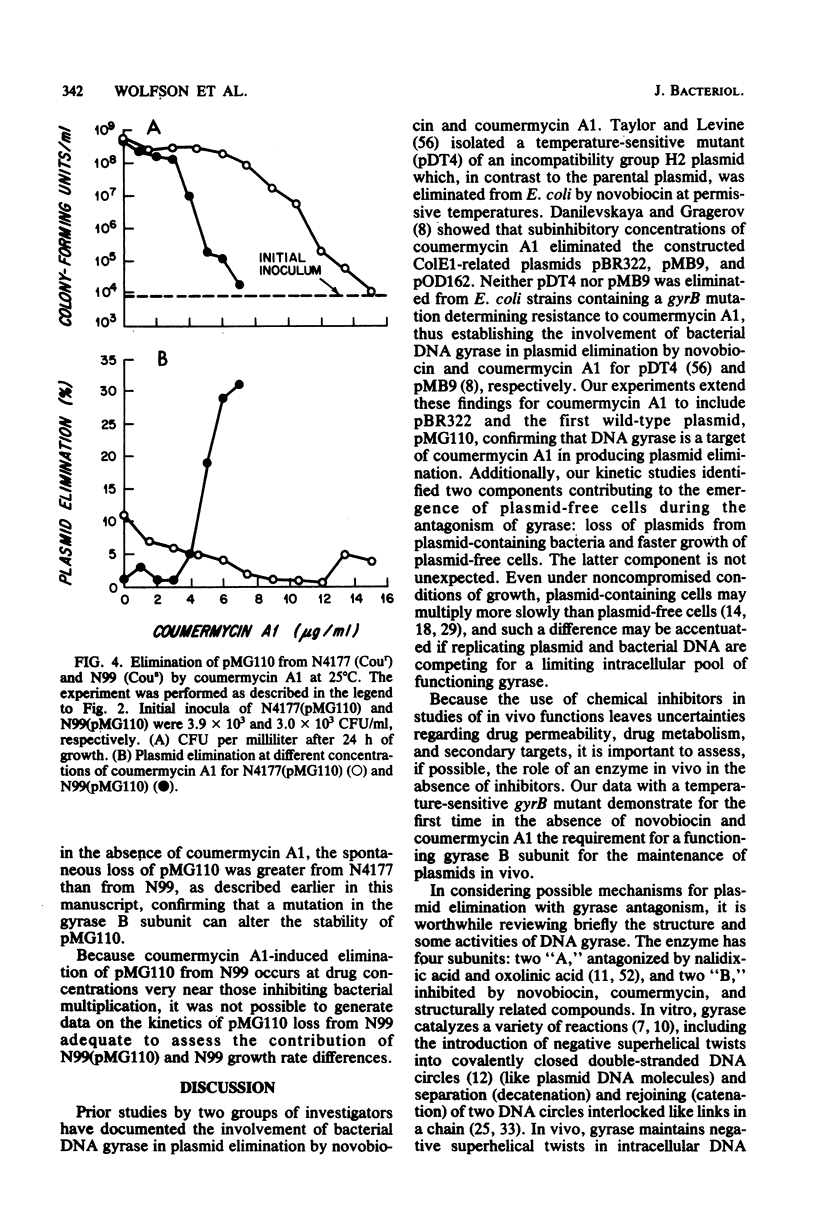
The constructed plasmid pBR322 and the native plasmid pMG110 were eliminated (cured) from growing Escherichia coli cells by the antagonism of the B subunit of the bacterial enzyme DNA gyrase. The antagonism may be by the growth of cells (i) at semipermissive temperatures in a bacterial mutant containing a thermolabile gyrase B subunit or (ii) at semipermissive concentrations of coumermycin A1, an antibiotic that specifically inhibits the B subunit of DNA gyrase. The kinetics of plasmid elimination indicate that plasmid loss occurs too rapidly to be explained solely by the faster growth of that plasmid-free bacteria and, therefore, represents interference with plasmid maintenance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastarrachea F., Willetts N. S. The elimination by acridine orange of F30 from recombination-deficient strains of Escherichia coli K12. Genetics. 1968 Jun;59(2):153–166. doi: 10.1093/genetics/59.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzicalupo P., Tocchini-Valentini G. P. Curing of an Escherichia coli episome by rifampicin (acridine orange-F + -F - -Hfr-lac). Proc Natl Acad Sci U S A. 1972 Feb;69(2):298–300. doi: 10.1073/pnas.69.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Ausubel F. M. Recombination between bacterial plasmids leading to the formation of plasmid multimers. Cell. 1976 Dec;9(4 Pt 2):707–716. doi: 10.1016/0092-8674(76)90134-3. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Danilevskaya O. N., Gragerov A. I. Curing of Escherichia coli K12 plasmids by coumermycin. Mol Gen Genet. 1980 Apr;178(1):233–235. doi: 10.1007/BF00267235. [DOI] [PubMed] [Google Scholar]
- Fuke M., Inselburg J. Electron microscopic studies of replicating and catenated colicin factor E1 DNA isolated from minicells (DNA replication). Proc Natl Acad Sci U S A. 1972 Jan;69(1):89–92. doi: 10.1073/pnas.69.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin D., Slater J. H. The influence of the growth environment on the stability of a drug resistance plasmid in Escherichia coli K12. J Gen Microbiol. 1979 Mar;111(1):201–210. doi: 10.1099/00221287-111-1-201. [DOI] [PubMed] [Google Scholar]
- Goebel W. Formation of complex Col E1 DNA by replication. Biochim Biophys Acta. 1971 Feb 25;232(1):32–42. doi: 10.1016/0005-2787(71)90488-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Dreisig H., Molin S., Nordström K., Uhlin B. E. DNA replication control: studies of plasmid R1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):419–425. doi: 10.1101/sqb.1979.043.01.048. [DOI] [PubMed] [Google Scholar]
- HIROTA Y., LIJIMA T. Acriflavine as an effective agent for eliminating F-factor in Escherichia coli K-12. Nature. 1957 Sep 28;180(4587):655–656. doi: 10.1038/180655a0. [DOI] [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. Elimination of resistance determinants from R-factor R1 by intercalative compounds. Antimicrob Agents Chemother. 1976 Jan;9(1):77–80. doi: 10.1128/aac.9.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Kinney T., Adams J. The maintenance of Plasmid-containing organisms in populations of Escherichia coli. J Gen Microbiol. 1981 Mar;123(1):129–141. doi: 10.1099/00221287-123-1-129. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Korn D. Cosegregation of a sex factor with the Escherichia coli chromosome during curing by acridine orange. J Mol Biol. 1969 Oct 28;45(2):385–395. doi: 10.1016/0022-2836(69)90113-2. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Isolation of catenated and replicating DNA molecules of colicin factor E1 from minicells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2839–2842. doi: 10.1073/pnas.68.11.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Requirement of a plasmid-encoded protein for replication in vitro of plasmid R6K. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5381–5385. doi: 10.1073/pnas.75.11.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Initiation of replication of plasmid ColE1 DNA by RNA polymerase, ribonuclease H, and DNA polymerase I. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):409–417. doi: 10.1101/sqb.1979.043.01.047. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. DNA replication of SV40-infected cells. VII. Formation of SV40 catenated and circular dimers. J Mol Biol. 1973 Jan 10;73(2):199–212. doi: 10.1016/0022-2836(73)90323-9. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Primrose S. B., Robinson A., Ellwood D. C. Maintenance of some ColE1-type plasmids in chemostat culture. Mol Gen Genet. 1980;180(3):579–584. doi: 10.1007/BF00268063. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Lanka E., Barth P. T. Plasmid RP4 specifies a deoxyribonucleic acid primase involved in its conjugal transfer and maintenance. J Bacteriol. 1981 Dec;148(3):769–781. doi: 10.1128/jb.148.3.769-781.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanka E., Scherzinger E., Günther E., Schuster H. A DNA primase specified by I-like plasmids. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3632–3636. doi: 10.1073/pnas.76.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Miller K. G., Englund P. T. Decatenation of kinetoplast DNA by topoisomerases. J Biol Chem. 1980 Jun 10;255(11):4976–4979. [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Fisher L. M., O'Dea M. H., Gellert M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1847–1851. doi: 10.1073/pnas.77.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Smith K., Sheehy R. J., Murphy E. A catenated intermediate in plasmid replication. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1460–1469. doi: 10.1016/0006-291x(73)91150-9. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Initiation of DNA replication in Bacillus subtilis. V. Role of DNA gyrase and superhelical structure in initiation. Mol Gen Genet. 1981;181(3):332–337. doi: 10.1007/BF00425607. [DOI] [PubMed] [Google Scholar]
- Orr E., Staudenbauer W. L. An Escherichia coli mutant thermosensitive in the B subunit of DNA gyrase: effect on the structure and replication of the colicin E1 plasmid in vitro. Mol Gen Genet. 1981;181(1):52–56. doi: 10.1007/BF00339004. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: the maturation of recombination intermediates. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Fietta A., Berti M., Silvestri L. G., Romero E. Relationships between curing of the F episome by rifampin and by acridine orange in Escherichia coli. Antimicrob Agents Chemother. 1973 Apr;3(4):456–462. doi: 10.1128/aac.3.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Suzuki K., Tomizawa J. I. Formation of catenated molecules by replication of colicin E1 plasmid DNA in cell extracts. J Mol Biol. 1976 Dec 15;108(3):569–582. doi: 10.1016/s0022-2836(76)80137-4. [DOI] [PubMed] [Google Scholar]
- Slack M. P., Wheldon D. B., Turk D. C. A rapid test for beta-lactamase production by Haemophilus influenzae. Lancet. 1977 Oct 29;2(8044):906–906. doi: 10.1016/s0140-6736(77)90836-4. [DOI] [PubMed] [Google Scholar]
- Smith G. R. DNA supercoiling: another level for regulating gene expression. Cell. 1981 Jun;24(3):599–600. doi: 10.1016/0092-8674(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Characterization of a plasmid mutation affecting maintenance, transfer and elimination by novobiocin. Mol Gen Genet. 1979 Jul 13;174(2):127–133. doi: 10.1007/BF00268350. [DOI] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J., Kline B. C. Mutation and identification of the F plasmid locus determining resistance to acridine orange curing. Plasmid. 1980 Nov;4(3):276–280. doi: 10.1016/0147-619x(80)90066-9. [DOI] [PubMed] [Google Scholar]
- von Wright A., Bridges B. A. Effect of gyrB-mediated changes in chromosome structure on killing of Escherichia coli by ultraviolet light: experiments with strains differing in deoxyribonucleic acid repair capacity. J Bacteriol. 1981 Apr;146(1):18–23. doi: 10.1128/jb.146.1.18-23.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


