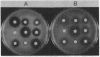
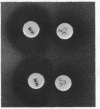
Abstract
Plasmid pBD9, which comprises two plasmids from Staphylococcus aureus, pE194 and pUB110, was joined to plasmid pBR322 by in vitro recombination to form plasmid pKH80. The ermC gene of plasmid pE194 confers inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. When pKH80 was transferred to Escherichia coli K-12, the bacteria became resistant to several of these antibodies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binns M. M., Davies D. L., Hardy K. G. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 1979 Jun 28;279(5716):778–781. doi: 10.1038/279778a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y., Weisblum B. A family of r-determinants in Streptomyces spp. that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1981 May;146(2):621–631. doi: 10.1128/jb.146.2.621-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Grandi G., Hahn J., Grandi R., Dubnau D. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 1980 Dec 20;8(24):6081–6097. doi: 10.1093/nar/8.24.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Shivakumar A. G., Dubnau D. Characterization of chimeric plasmid cloning vehicles in Bacillus subtilis. J Bacteriol. 1980 Jan;141(1):246–253. doi: 10.1128/jb.141.1.246-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K., Stahl S., Küpper H. Production in B. subtilis of hepatitis B core antigen and a major antigen of foot and mouth disease virus. Nature. 1981 Oct 8;293(5832):481–483. doi: 10.1038/293481a0. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B., Fahnestock S. R., Nomura M. Alteration of 23 S ribosomal RNA and erythromycin-induced resistance to lincomycin and spiramycin in Staphylococcus aureus. J Mol Biol. 1973 Feb 15;74(1):67–72. doi: 10.1016/0022-2836(73)90355-0. [DOI] [PubMed] [Google Scholar]
- Malke H., Holm S. E. Expression of streptococcal plasmid-determined resistance to erythromycin and lincomycin in Escherichia coli. Mol Gen Genet. 1981;184(2):283–285. doi: 10.1007/BF00272918. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Michel B., Palla E., Niaudet B., Ehrlich S. D. DNA cloning in Bacillus subtilis. III. Efficiency of random-segment cloning and insertional inactivation vectors. Gene. 1980 Dec;12(1-2):147–154. doi: 10.1016/0378-1119(80)90025-6. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Dubnau D. Studies on the synthesis of plasmid-coded proteins and their control in Bacillus subtilis minicells. Plasmid. 1979 Apr;2(2):279–289. doi: 10.1016/0147-619x(79)90046-5. [DOI] [PubMed] [Google Scholar]
- TAUBENECK U. Susceptibility of Proteus mirabilis and its stable L-forms to erythromycin and other macrolides. Nature. 1962 Oct 13;196:195–196. doi: 10.1038/196195b0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Systematic difference in the methylation of ribosomal ribonucleic acid from gram-positive and gram-negative bacteria. J Bacteriol. 1975 Aug;123(2):771–774. doi: 10.1128/jb.123.2.771-774.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]