Abstract
Disruption of the perforin gene results in primary immunodeficiency and an increased susceptibility to opportunistic pathogens. Perforin-deficient (PKO) mice fail to clear primary lymphocytic choriomeningitis virus (LCMV) Armstrong, resulting in persistent infection and functional exhaustion of virus specific CD8+ T cells. CD8+ T cell responses to Listeria monocytogenes (LM) challenge within the first week after LCMV infection were diminished in both WT and PKO mice, and correlated with enhanced bacterial clearance. However, bacterial challenge at later time points generated similar CD8 T cell responses in both groups of mice. The phenotype and function of pre-existing LM-specific memory CD8+ T cells were maintained in persistently infected PKO mice. Thus persistent LCMV infection, as a result of perforin deficiency, results in dysfunction of the virus-specific CD8+ T cell response but does not compromise the host's ability to maintain pre-existing memory CD8+ T cells or to generate new memory CD8+ T cell responses against other pathogens.
Keywords: Lymphocytic choriomeningitis virus, Listeria monocytogenes, CD8 T cells, immunity, immunodeficiency, perforin, memory
Introduction
Primary immunodeficiencies, although relatively rare, are often associated with persistent infections of opportunistic pathogens, such as adenovirus, bacillus Calmette-Guerin, Candida albicans, cytomegalovirus, Epstein-Barr virus, parainfluenza virus 3, Pneumocystis carinii, respiratory syncitial virus, and varicella (Stephan et al., 1993)(Buckley et al., 1997). The molecular basis of more then 120 primary immunodeficiencies has been determined (Notarangelo et al., 2006), many of which manifest themselves as defects in T cell development, effector functions and immunoregulation (Cunningham-Rundles and Ponda, 2005). Disruption of the perforin gene results in a primary immunodeficiency associated with impaired natural killer and cytotoxic T cell responses (Matloubian et al., 1999)(Stepp et al., 1999). Perforin-deficiency in humans is associated with the disease Familial Hemophagocytic Lymphohistiocytosis (FHL), which is uniformly fatal at an early age unless treated by haematopoietic stem cell transplant. Mortality is associated with dysregulated immune pathology triggered by viral infection (Almousa et al., 2005)(Katano et al., 2004). Perforin-deficient mice are similarly more susceptible to viral infections, including lymphocytic choriomeningitis virus (LCMV) infections (Walsh et al., 1994)(Matloubian et al., 1999).
The outcome of LCMV infections in mice depends on a number of factors including the mouse strain, age of the mouse, strain of the viral isolate, dose of virus, and route of infection (Roost et al., 1988). Differences in viral and host determinants have allowed for the development of distinct LCMV model systems for studying viral persistence and immunosuppression (Buchmeier et al., 1980)(Oldstone, 2002). In the carrier state model, wildtype (WT) mice are infected at birth or in utero with LCMV and become life long carriers of the virus (Buchmeier et al., 1980)(Cihak and Lehmann-Grube, 1978). This carrier state is associated with the clonal deletion of LCMV-specific T cells (Jamieson and Ahmed, 1988)(Volkert et al., 1975)(Oldstone, 2002). However, generalized immunosuppression is not observed as immune responses to many heterologous antigens remain unimpaired (Oldstone et al., 1973)(Oldstone, 2002).
In a second model, intravenous or intraperitoneal infections of adult WT mice with LCMV clone 13 (LCMV Cl13) or other highly virulent, fast growing LCMV strains, results in a vigorous expansion of virus-specific CD8+ T cells, however virus persists for 90−150 days in most organs (Wherry et al., 2003). LCMV Cl13 infection is associated with a virus specific immunosuppression mediated by the progressive, epitope-dependent, exhaustion and deletion of virus-specific CD8+ T cells virus-specific CD8+ T cells (Wherry et al., 2003). Infection of adult mice with fast growing strains of LCMV also result in a transient generalized immunosuppression (Mims and Wainwright, 1968)(Oldstone et al., 1973)(Lehmann-Grube, Niemeyer, and Lohler, 1972)(Ruedi, Hengartner, and Zinkernagel, 1990)(Guttler, Bro-Jorgensen, and Jorgensen, 1975) associated with the destruction and function disruption of antigen presenting cells (Sevilla et al., 2000)(Borrow, Evans, and Oldstone, 1995)(Sevilla et al., 2004).
In a third model, immunocompetent adult mice are infected with slow growing isolates of LCMV, such the LCMV Arm strain (LCMV Arm) (Oldstone, 2002). Like LCMV, CL13, LCMV Arm also stimulates a robust CD8+ T cell response (Badovinac, Tvinnereim, and Harty, 2000)(Badovinac, Porter, and Harty, 2002), which mediates viral clearance from the spleen within 8−10 days (Wherry et al., 2003)(Matloubian et al., 1993). However, LCMV Arm infection does not result in the attrition of virus specific CD8+ T cells (Fuller and Zajac, 2003)(Wherry et al., 2003)(Khanolkar, Fuller, and Zajac, 2004). Furthermore, LCMV Arm infection result in a very transient and weak generalized immunosuppression, if any (Roost et al., 1988), leaving APCs largly intact and unaffected (Sevilla et al., 2000)(Borrow, Evans, and Oldstone, 1995).
In contrast to WT mice, perforin-deficient (PKO) mice are unable to clear LCMV Arm (Walsh et al., 1994)(Fuller and Zajac, 2003). PKO mice on the B6 background often succumb to LCMV Arm (Walsh et al., 1994) (data not shown) while BALB/c PKO mice survive (Badovinac, Porter, and Harty, 2002). Whereas infection of WT mice by virulent strains of LCMV can be associated with both virus-specific and generalized immunosuppression, it is unknown whether and how persistent LCMV Arm infections of immunodeficient hosts affect immune responses against heterologous pathogens. Here we use LCMV infection of BALB/c PKO mice to examine CD8+ T cell responses to the heterologous bacterial pathogen, Listeria monocytogenes (LM) in a model of chronic viral infection due to primary immunodeficiency.
Results
Virus-specific T cell responses and the duration of LCMV infection
LCMV Arm is cleared from WT hosts between 10−12 days post infection (p.i.) (Wherry et al., 2003), however, high titers of LCMV persist in BALB/c PKO mice for at least 12 days (Badovinac, Porter, and Harty, 2002). To determine the full course of infection, BALB/c WT and BALB/c PKO mice were infected with 2×105 plaque forming units (PFU) of LCMV Arm and tested for viral clearance at various time points out to 234 days p.i. The spleens of BALB/c WT mice contained detectable virus until day 12, then fell below the limit of detection (Fig. 1A). In contrast, LCMV Arm remained detectable in the spleens from all BALB/c PKO mice out to 234 days p.i. (Fig. 1A). Consistent with this, high concentrations of LCMV Arm were detected in the brain, lung, and kidneys at day 216 in BALB/c PKO, but not BALB/c WT mice (Fig. 1B). As previously reported, BALB/c PKO mice do not succumb to LCMV Arm infection (Badovinac, Porter, and Harty, 2002). In addition, injection of BALB/c WT mice with virus recovered from chronically infected BALB/c PKO mice stimulated a robust LCMV-specific CD8 T cell response and was cleared with normal kinetics (data not shown), indicating that epitope escape and/or generation of variant viruses that have gained the capacity to mediate persistent infection in WT mice, as observed by Oldstone and colleagues (Ahmed and Oldstone, 1988) was unlikely to account for the persistent infection or survival. Thus, LCMV Arm establishes a long-term systemic infection in BALB/c PKO mice.
Figure 1.
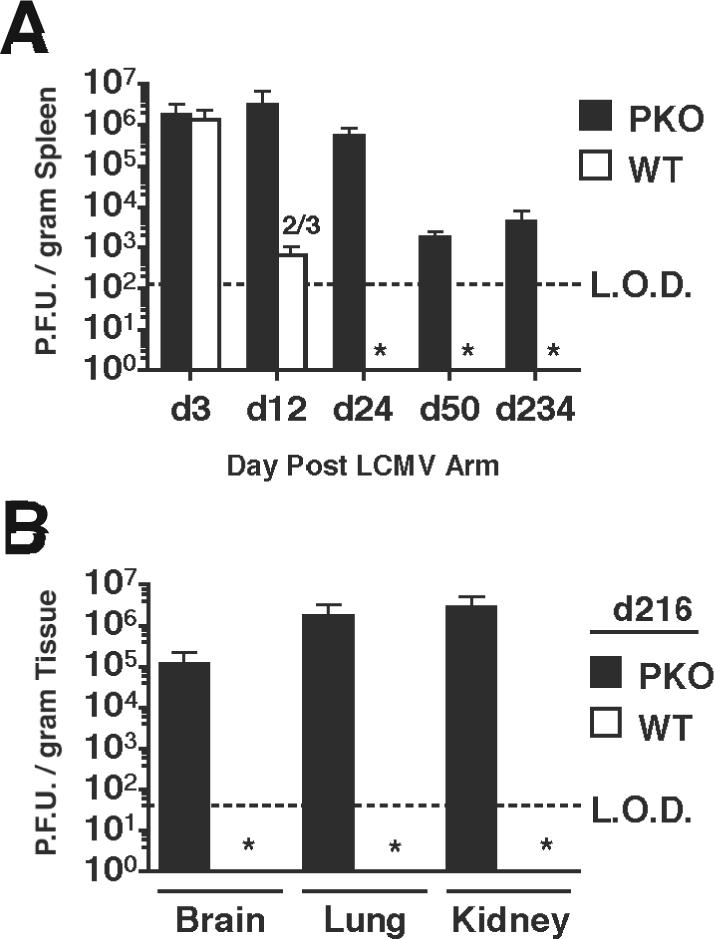
Virus titers in LCMV-infected mice. BALB/c WT and BALB/c PKO mice were infected intraperitoneally with 2×105 PFU of LCMV ARM. (A) Mean +/− SD PFU/gram of spleen from 3−4 mice/group were determined at the indicated days p.i. Data was pooled from 4 individual experiments. Dashed line indicates the limit of detection (LOD). Numbers indicate mice/group with viral titers above the limit of detection. * below limit of detection in all mice. (B) Mean +/− SD PFU/gram of brain, lung, and kidney from 3 mice/group in BALB/c PKO and BALB/c WT mice at day 216 p.i.
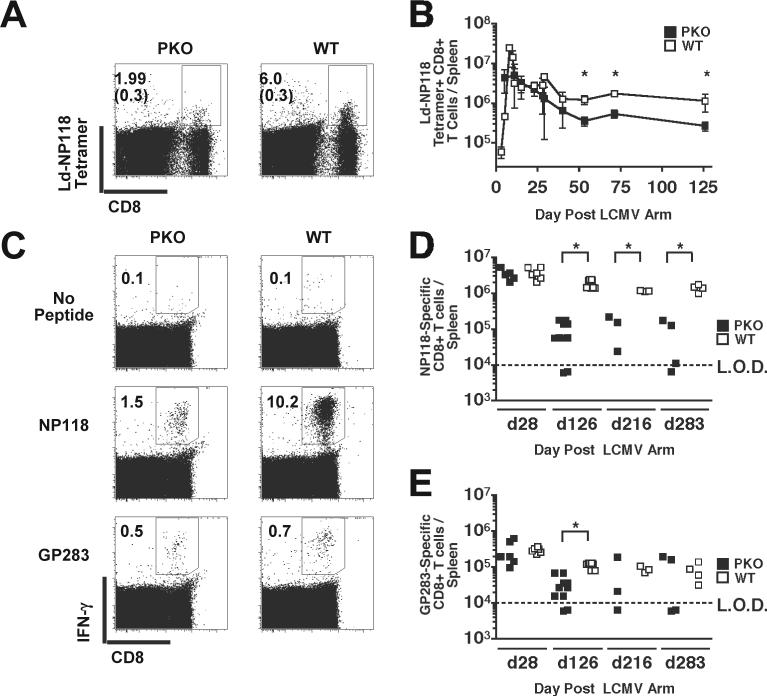
Persistent infection of WT mice with aggressive LCMV strains, such as LCMV Clone 13 (LCMV Cl13), is often associated with either functional exhaustion or deletion of virus-specific CD8+ T cells (Wherry et al., 2003); this exhaustion was reported to be perforin-dependent in H-2b PKO mice (Matloubian et al., 1999). To determine if chronic LCMV-infection in H-2d BALB/c PKO mice also results in the deletion of virus-specific CD8+ T cells, the number of NP118−126-specific CD8+ T cells/spleen was measured by MHC class I tetramer-staining. The fraction of NP118−126-tetramer positive CD8+ T cells was 3 to 4-fold lower in LCMV infected BALB/c PKO mice compared to WT mice at day 53 (Fig. 2A). As noted previously (Badovinac, Porter, and Harty, 2002), early after infection the NP118−126-specific CD8+ T cells/spleen in BALB/c PKO mice expanded in number and underwent contraction with similar onset and kinetics compared to those of BALB/c WT mice (Fig. 2B). However, the total number of NP118−126 specific CD8+ T cells/spleen in BALB/c PKO mice was reduced 2−5 fold compared to BALB/c WT mice at the peak of expansion and during the memory phase of the response (p values < 0.05) (Fig. 2B). Thus, persistent infection of BALB/c PKO mice is not associated with robust clonal deletion of NP118−126-specific CD8+ T cells. In addition, these data support findings demonstrating perforin is not required for the programmed contraction of virus-specific CD8+ T cells during persistent LCMV Arm infection (Badovinac, Porter, and Harty, 2002).
Figure 2.
BALB/c WT and BALB/c PKO mice were infected with 2×105 PFU of LCMV Arm i.p. and analyzed at various days p.i. (A) Detection of NP118−126 –specific CD8+ T cell responses in LCMV-infected mice by H-2Ld-NP118 tetramer staining. Representative dot plots from splenocytes harvested at day 53 p.i. Numbers represent percentages of H-2Ld-NP118 tetramer positive CD8+ T cells; background staining in uninfected PKO and WT control mice are in parenthesis. (B) Mean total number +/− SD of H-2Ld-NP118-specific CD8+ cells/spleen, from >3 mice/group, at the indicated day p.i. Data was pooled from 8 individual experiments. * p ≤ 0.05. (C) Functional assessment of virus-specific CD8+ T cell responses in LCMV-infected mice. CD8+ T cell responses to the dominant NP118−126 and the subdominant GP283−291 peptides were analyzed by peptide-stimulated ICS at various times p.i. Representative dot plots from unstimulated and peptide-stimulated splenocytes harvested at day 120 p.i. Total number of (D) NP118−126 –specific and (E) GP283−291 –specific CD8+ T cells/spleen at the indicated day p.i. Each symbol represents an individual mouse (n = 3−10 mice / time point). Data was pooled from 2 individual experiments. Dashed line indicates the LOD. * p ≤ 0.05.
To determine the impact of chronic infection on the function of LCMV-specific CD8+ T cell responses in BALB/c PKO mice, NP118−126-specific and GP283−291-specific CD8+ T cell responses were analyzed at various time points by peptide stimulated intracellular cytokine staining (ICS) for IFN-γ (Fig. 2C,D,E). At day 126 p.i. the fraction of CD8+ T cells from BALB/c PKO mice producing IFN-γ after NP118−126 and GP283−291 peptide stimulation was reduced compared to CD8+ T cells from WT mice (Fig. 2C). This corresponded to a 13.5-fold reduction in the total number of NP118−126-specific, IFÑγ producing CD8+ T cells/spleen in LCMV infected BALB/c PKO mice compared to BALB/c WT mice (Fig. 2D) after day 126 p.i. The total number of GP283−291-specific CD8+ T cells/spleen in BALB/c PKO mice was also reduced at days 126 p.i (Fig. 2E). In contrast, BALB/c PKO mice and BALB/c WT mice had similar numbers of NP118−126- and GP283−291-specific CD8+ T cells/spleen at day 28 p.i., prior to deletion. In addition, not all infected BALB/c PKO mice made detectable NP118−126- or GP283−291- specific CD8+ T cell responses after day 126 p.i. (Fig. 2E). The inability to detect GP283−291- and NP118−126-specific CD8+ T cells in some mice is consistent with functional exhaustion and/or deletion of virus-specific CD8+ T cells (Wherry et al., 2003). Furthermore, the greater reduction in the NP118−126-specific CD8+ T cells measured by ICS (Fig. 2C,D,E) compared to tetramer-staining (Fig. 2A,B) suggests functional inactivation of the NP118−126-specific CD8+ T cells in BALB/c PKO mice. Together these data demonstrate chronic LCMV Arm infection of BALB/c PKO mice results in substantial, but not complete exhaustion of virus-specific CD8+ T cells, and are consistent with findings using LCMV Arm-infected C57Bl/6 PKO mice (Fuller and Zajac, 2003).
Primary T cell responses to L. monocytogenes in LCMV-infected mice
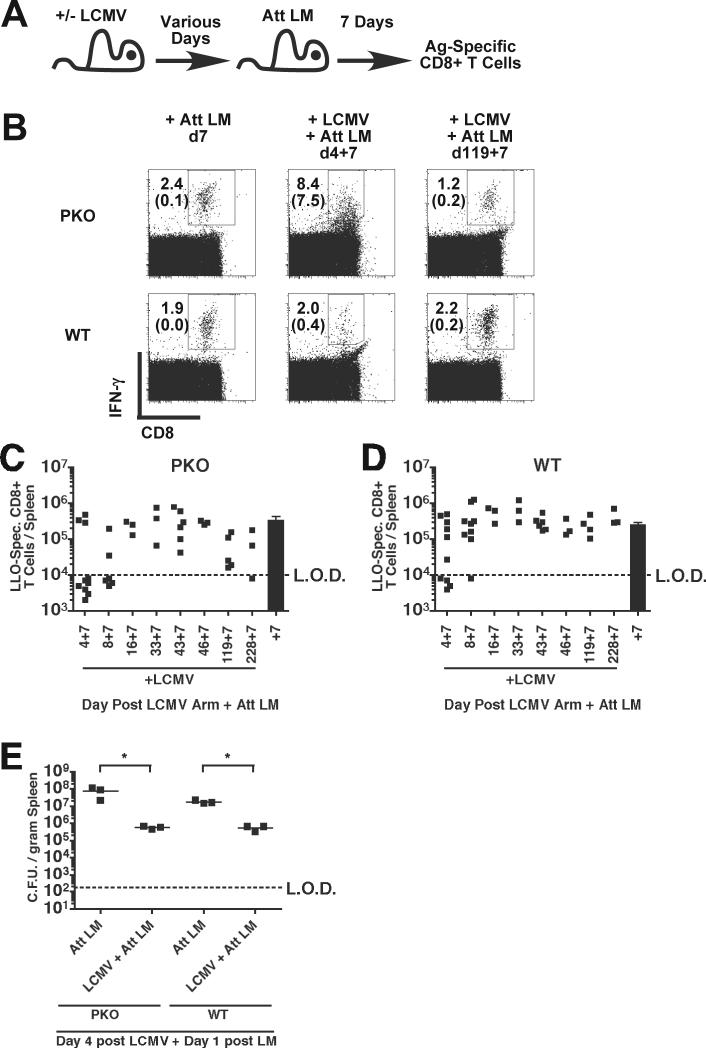
Prolonged infection of adult WT mice with some strains of LCMV results in generalized immunosuppression (Roost et al., 1988)(Mims and Wainwright, 1968)(Oldstone et al., 1973)(Lehmann-Grube, Niemeyer, and Lohler, 1972)(Ruedi, Hengartner, and Zinkernagel, 1990)(Guttler, Bro-Jorgensen, and Jorgensen, 1975). To determine immunocompetency of mice with chronic LCMV Arm infection, BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Arm i.p. and were subsequently infected with ∼0.1 LD50 of attenuated actA-deficient L. monocytogenes (Att LM) at various days after LCMV infection. The actA-deficient strain of LM was chosen as it is cleared similarly in WT and PKO mice (Badovinac, Tvinnereim, and Harty, 2000). CD8+ T cell responses to the LM antigen listeriolysin-O (LLO)91−99 (Pamer, Harty, and Bevan, 1991) were then determined by ICS 7 days later in LCMV-infected and LCMV-naïve control mice (Fig. 3A). LM infection at day 119 after LCMV infection stimulated similar expansion of LLO91−99-specific CD8+ T cells, based on frequency (Fig. 3B) and total numbers (Fig. 3C), in chronically infected BALB/c PKO, naïve controls, or BALB/c WT mice that had cleared LCMV. Consistent with previous findings, IFN-γ production by CD8+ T cells was detectable in the absence of in vitro peptide stimulation at day 11 p.i. in LCMV-infected PKO mice (Fig. 3B), and is likely due to persistence of viral antigen (Jordan et al., 2004)(Badovinac, Hamilton, and Harty, 2003). Despite the substantial fraction of CD8+ T cells from LCMV-infected BALB/c PKO mice that produce IFN-γ in vitro in the absence of peptide stimulation at day 11 post LCMV Arm infection (Fig. 3B), LLO91−99-specific CD8+ T cell responses were similar in all groups of mice at this and all time points post LCMV Arm infection (Fig. 3C,D) (p values >0.05). However, in both LCMV-infected BALB/c PKO and LCMV-infected BALB/c WT mice, the fraction of mice with detectable LLO91−99-specific CD8+ T cell responses was reduced in mice infected with Att LM 4 or 8 days after LCMV infection compared to either control LCMV-naïve mice or mice LCMV-infected mice challenged at days 16−288 post LCMV infection (Fig. 3C,D). In BALB/c PKO mice challenged with Att LM at day 4 post LCMV, 30% of mice mounted detectable LLO91−99-specific CD8+ T cell responses, whereas 60% of BALB/c WT mice had detectable responses. Although proximal LMCV infections inhibited CD8+ T cell responses to heterologous Att LM infections in both BALB/c WT and BALB/c PKO mice, these data demonstrate that chronically infected BALB/c PKO mice are not globally immunosuppressed and can mount vigorous antigen-specific (Ag-specific) CD8+ T cell responses despite a persistent LCMV infection.
Figure 3.
Primary L. monocytogenes –specific CD8+ T cell responses and bacterial clearance in LCMV-infected mice. (A) Experimental design. BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Arm i.p. and were subsequently infected with 3×106 colony forming units (CFU) of actA-deficient L. monocytogenes (Att LM) at the indicated day after LCMV infection. L. monocytogenes LLO91−99 –specific CD8+ T cell responses were determined 7 days after Att LM infection. (B) Representative dot plots from infected mice. Numbers represent the percentage of CD8+ T cells that produce IFN-γ after incubation with (top number) or without (lower number) LLO91−99 peptide. Total number of LLO91−99 – specific CD8+ T cells/spleen in (C) BALB/c PKO and (D) BALB/c WT mice. Each symbol represents an individual mouse (n = 3−10 mice / time point). Dashed line indicates the LOD. Solid bar represents mean responses (+/− SEM) in LCMV-naïve control mice. Data was pooled from 8 individual experiments. (E) BALB/c PKO and BALB/c WT mice were infected with LCMV Arm, 4 days later were infected with Att LM, and the number of bacteria per spleen determined 1 day after infection. Each symbol represents an individual mouse (n = 3 mice / time point). Lines indicate the mean CFU/gram spleen. * p ≤ 0.05.
The magnitude of LM-specific CD8+ T cell responses are dependent on the dose of bacteria inoculated (Badovinac, Porter, and Harty, 2002). Therefore, the failure to detect LLO91−99-specific CD8+ T cell responses in some LCMV infected mice after Att LM infection could result from enhanced bacterial clearance due to virus-induced activation of the innate immune system (Barton et al., 2007). To test this, BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Armstrong i.p., 4 days later infected with ∼0.1 LD50 Att LM, and bacterial clearance determined 1 day post Att LM infection in LCMV-infected and LCMV-naïve mice. The number of bacteria recovered from the spleens of LCMV infected mice was reduced 33-fold (BALB/c WT) and 104-fold (BALB/c PKO) compared to control mice (Fig. 3E). In contrast, bacterial recovery in LCMV-infected mice was similar to control mice when the challenge was initiated at day 12 and 24 post LCMV infection (data not shown). These data demonstrate that bacterial clearance is enhanced in both BALB/c PKO mice and BALB/c WT mice at early times after LCMV infection. Furthermore these data are consistent with the hypothesis that failure to prime LLO91−99-specific CD8+ T cell responses in some LCMV infected mice may be due to enhanced clearance of the bacterial challenge resulting in a suboptimal number of bacteria for CD8+ T cell priming.
Dendritic cell vaccination of LCMV-infected mice
To determine if LCMV Arm infection also regulates CD8+ T cell expansion independently from mechanisms mediating enhanced bacterial clearance, BALB/c PKO mice were infected with LCMV, subsequently vaccinated with LLO91−99–coated DCs or infected with Att LM, and CD8+ T cell responses analyzed at day 7 post vaccination (Fig. 4A). The magnitude of LLO91−99-specific CD8+ T cell responses after DC vaccination or Att LM infection of BALB/c PKO mice infected with LCMV 4 days prior to challenge were similar to those in LCMV-naïve BALB/c PKO mice (p values > 0.05)(Fig. 4B). The magnitude of CD8+ T cell expansion and the fraction of mice responding to DC or Att LM challenge at day 12 or day 18 post LCMV-infection were similar to control LCMV-naïve mice (p values > 0.05) (Fig. 4B). Furthermore, the magnitude of CD8+ T cell responses to DC vaccination were indistinguishable (p values > 0.05) from one another at each time point tested post LCMV infection. These data clearly demonstrate that LCMV-chronically infected BALB/c PKO mice can mount CD8+ T cell responses to DC vaccination similar in magnitude to control mice.
Figure 4.
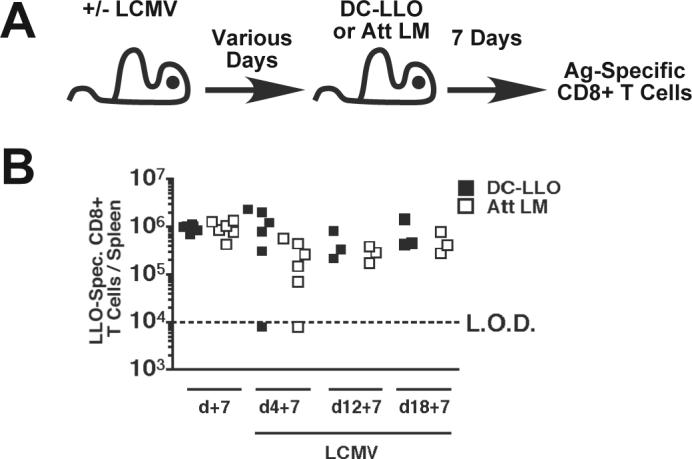
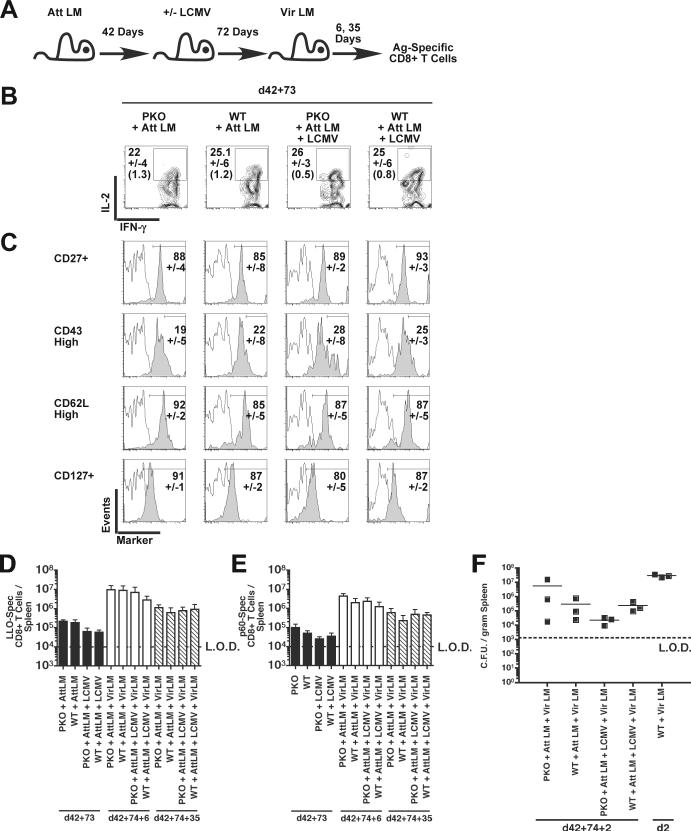
CD8+ T cell responses to peptide-coated dendritic cells and Att LM infection in LCMV-infected mice. (A) Experimental design. BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Arm i.p. At days 7, 12, or 18 post LCMV infection these mice and LCMV-naïve control mice were injected with 3×106 CFU Att LM or LLO91−99 – coated dendritic cells (DC-LLO). CD8+ T cell responses to LLO91−99 and NP118−126 were analyzed by ICS 7 days later. (B) Total number of LLO91−99 –specific CD8+ T cells/spleen at the indicated time point. Each symbol represents an individual mouse (n = 3−6 mice / time point). Data was pooled from 2 individual experiments. Dashed line indicates the LOD.
Kinetics of LLO91−99-specific CD8+ T cell responses in LCMV chronically infected mice
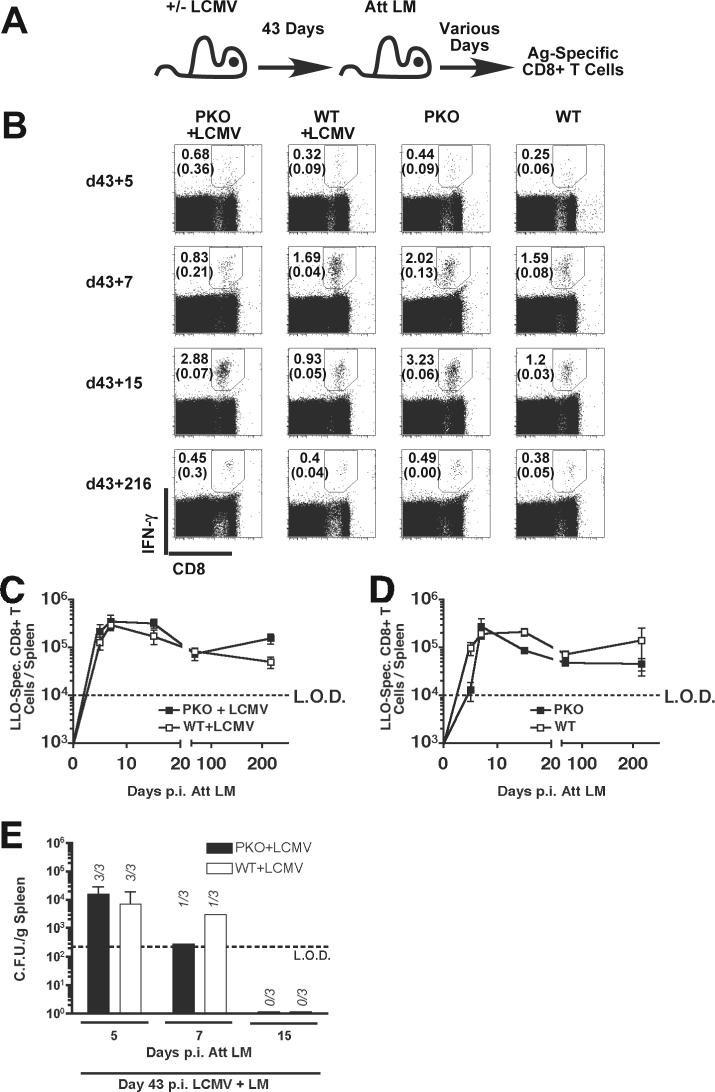
Ag-specific CD8+ T cell responses in normal mice are programmed early after LM infection to undergo rapid expansion followed by contraction to a stable number of memory CD8+ T cells (Wong and Pamer, 2003)(Harty and Badovinac, 2002), through a process controlled by early inflammatory signals including IFN-γ (Badovinac, Porter, and Harty, 2002). We next sought to determine if chronic LCMV-infection and the associated dysregulated virus-specific immune response in BALB/c PKO mice impacts the kinetics of LM-specific CD8+ T cell expansion, contraction, and memory generation. BALB/c PKO and BALB/c WT mice were infected with LCMV, 43 days later these mice and naïve control mice were infected with Att LM (Fig. 5B). During CD8+ T cell expansion at day 7, the peak of expansion, during contraction (day 15), and at the memory phase (day 216) the frequency and total numbers of LLO91−99-specific CD8+ T cells were similar in all groups of mice (Fig. 5B-D), demonstrating that chronic LCMV infection did not negatively impact the CD8+ T cell response to heterologous LM infection (p values > 0.05). Most persistently LCMV-infected PKO mice clear LM infection by day p.i. with similar kinetics to WT mice that have resolved LCMV infection (Fig. 5E). LCMV-infected WT mice and persistently LCMV-infected PKO had similar levels of LM at d5 post infection (p > 0.05). LM was cleared in 2 of 3 mice at 7 days p.i. and undetectable at d15 p.i. in both LCMV-infected PKO and WT mice. Thus, despite the dysregulation of LCMV-specific CD8+ T cell responses in LCMV chronically infected BALB/c PKO mice, the CD8+ T cell response program is normal following heterologous LM infection. Furthermore, LM-specific CD8+ T cells can mediate bacterial clearance, even in LCMV-persistently infected PKO mice.
Figure 5.
Generation of memory LLO91−99 –specific CD8+ T cell responses in LCMV-infected mice. (A) Experimental design. BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Arm i.p. and 43 days later were infected with 3×106 CFU of Att LM. CD8+ T cell responses were then analyzed at the indicated time points after infection by LLO91−99 –stimulated ICS. (B) Representative dot plots from infected mice. Numbers represent the percentage of CD8+ T cells that produce IFN-γ after incubation with (top number) or without (lower number) LLO91−99. Mean, +/− SEM, total number of LLO91−99 – specific CD8+ T cells/spleen of 4−5 mice/group in mice that were (C) infected with LCMV or (D) were not infected with LCMV. Data was pooled from 2 individual experiments. Dashed line indicates the LOD. (E) The mean (+/− SD) number of bacteria per spleen was determined 5, 7, 15 days after LM infection in BALB/c PKO and BALB/c WT mice infected with LCMV 43 days prior to LM infection. Numbers indicate mice/group with detectable LM loads (n = 3 mice / group). Dashed line indicates the limit of detection (LOD).
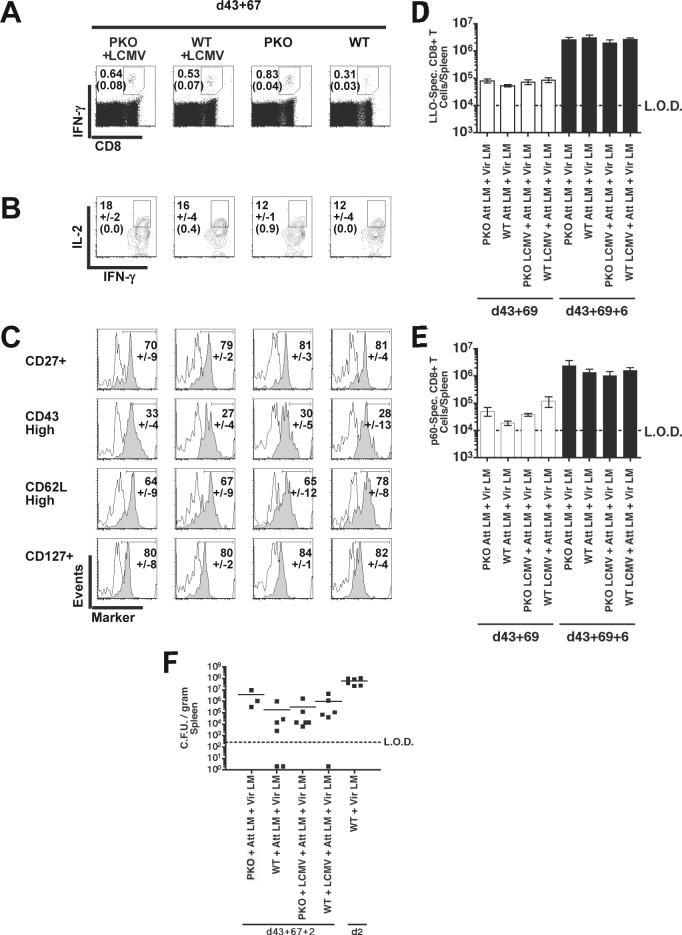
As primary antigen-specific CD8+ T cell populations progress into memory they undergo gradual phenotypic changes, including acquiring the ability to make IL-2 upon stimulation, enhanced expression of CD27, CD127, and CD62L, and loss of the activation-associated CD43 glycoform (Wherry and Ahmed, 2004). To characterize the impact of chronic infection on differentiation of memory CD8+ T cells, BALB/c PKO and control BALB/c WT mice were infected with LCMV and Att LM, as above. Sixty-seven days later the LLO91−99–specific CD8+ T cells were identified by IFN-γ production, and analyzed for expression of IL-2, CD27, CD127, CD43, and CD62L (Fig. 6A). IL-2 production by LLO91−99-specific CD8+ T cells in chronically-infected BALB/c PKO mice was similar to LCMV-naïve BALB/c PKO mice as well as control LCMV-infected and LCMV-naive BALB/c WT mice (Fig. 6B). The fraction of memory antigen-specific CD8+ T cells scoring CD27+, CD43high, CD62Lhigh, and CD127+ (Fig. 6C) and total number of LM-specific memory CD8+ T cells (Fig. 6D, E) were similar amongst LCMV-infected and LCMV-naïve groups of BALB/c WT and BALB/c PKO mice. In conclusion, these data demonstrate that chronic LCMV infection of BALB/c PKO does not impact the phenotype or number of memory CD8+ T cells following heterologous Att LM infection. Consistent with their phenotype, LM-specific memory CD8+ T cells generated in chronically infected BALB/c PKO and control mice undergo equivalent expansion after secondary virulent L. monocytogenes (Vir LM) challenge (Fig. 6D, E). Both vaccinated LCMV-infected PKO and WT mice survived secondary challenge with a lethal dose of Vir LM (data not shown) and control infection in contrast to unvaccinated control mice (Fig. 6F) (p < 0.05). LM load was similar in all vaccinated groups of mice (p > 0.05), demonstrating persistent LCMV infection does not impact the ability of memory LM-specific CD8+ T cells to mediate bacterial clearance.
Figure 6.
Phenotype and expansion of memory LLO91−99 –specific CD8+ T cells generated in LCMV-infected mice. BALB/c PKO and BALB/c WT mice were infected with 2×105 PFU of LCMV Arm i.p., 43 days later LCMV-infected mice and naïve control mice were vaccinated with 3×106 CFU of Att LM. At day 69 after LM vaccination all groups were infected with 3×104 CFU of Vir LM i.v. LLO91−99 –stimulated CD8+ T cell were then analyzed at d67 post Att LM infection for IFN-γ expression by ICS in combination with staining for IL-2, CD27, the activation-associated glycoform of CD43, CD62L, and CD127. (A) Representative dot plots at d43+67 p.i. Numbers represent the percentage of CD8+ T cells that produce IFN-γ in the absence or presence of peptides. (B) Representative contour plots of IL-2 production by IFN-γ+ CD8+ T cells. Numbers represent the mean percentage +/-SD of IFN-γ+ CD8+ T cells staining positive for IL-2 or (isotype control). (C) Representative histograms of IFN-γ+ CD8+ T cells analyzed for CD27, CD43, CD62L, and CD127 expression (solid histograms) or isotype control staining (open histograms). Numbers represent the mean percentage +/-SD of IFN-γ+ CD8+ T cells staining positive for each phenotypic marker. CD8+ T cell responses were determined at day 6 post secondary challenge by ICS. Mean, +/− SD, total number of LLO91−99 –specific (D) and (E) p60217−225 –specific CD8+ T cells/spleen in mice at day 0 (open bars) and day 6 post Vir LM challenge. Data was pooled from 2 individual experiments (n = 3−6 mice / group). Dashed line indicates the LOD. (F) The mean number of bacteria per spleen was determined 2 days after Vir LM infection. Each symbol represents an individual mouse. Solid lines indicate the mean CFU/gram spleen. Data was pooled from 2 individual experiments (n = 3−6 mice / group). Dashed line indicates the limit of detection (LOD).
Function and fate of pre-existing memory CD8+ T in LCMV chronically-infected mice
Acute LCMV Arm infection of WT mice induces the attrition of pre-existing memory CD8+ T cells (Bahl et al., 2006)(McNally et al., 2001). However, the impact of chronic LCMV infection on pre-existing non-virus specific memory CD8+ T cell phenotype and function remains unclear. Therefore, BALB/c PKO and control BALB/c WT mice were vaccinated with Att LM to generate memory CD8+ T cells, infected with LCMV 42 days later, and LM-specific memory CD8+ T cells analyzed for memory markers and function at day 73 post LCMV infection (Fig. 7A). LLO91−99–specific memory CD8+ T cells from LCMV infected BALB/c PKO mice produced IL-2 (Fig. 7B) and expressed CD27, CD43, CD62L, and CD127 similar in proportions and intensities as those seen in the control groups of mice (Fig. 7C). Although the phenotype of pre-existing memory was not affected by chronic or acute LCMV-infection, the numbers of LLO91−99-specific CD8+ T cells/spleen in LCMV-infected BALB/c PKO and BALB/c WT mice were reduced ∼3.0 and 3.4 fold (Fig. 7D). Similarly, BALB/c PKO and BALB/c WT mice had reduced p60 217−225-specific memory CD8+ T cells/spleen, ∼3.8 and ∼1.5 fold respectively, at day 73 post LCMV infection (Fig. 7E). Thus, chronic LCMV infection can result in a perforin-independent attrition of memory Ag-specific CD8+ T cells. Despite this attrition, secondary expansion of LM-specific CD8+ T cells was robust in LCMV infected BALB/c PKO mice and resulted in generation of similar numbers of secondary memory cells in all groups of mice (Fig. 7D,E). Both vaccinated LCMV-infected PKO and WT mice survived secondary challenge with a lethal dose of Vir LM (data not shown) and reduced infection in contrast to unvaccinated control mice (Fig. 7F) (p < 0.05). LM load was similar in vaccinated PKO and WT mice (p > 0.05), demonstrating that neither acute nor persistent LCMV infection impacts the ability of memory LM-specific CD8+ T cells to mediate bacterial clearance.
Figure 7.
Phenotype and function of pre-existing LLO91−99 –specific memory CD8+ T cell in LCMV-infected mice. (A) Experimental design. BALB/c PKO and BALB/c WT mice were vaccinated with 3×106 CFU of Att LM and infected with 2×105 PFU of LCMV Arm i.p. 42 days later. LLO91−99 –specific memory CD8+ T cell were analyzed at day 73 post LCMV Arm infection for IFN-γ expression by ICS in combination with staining for IL-2, CD27, the activation-associated glycoform of CD43, CD62L, and CD127. (B) Representative contour plots of IFN-γ+ CD8+ T cells analyzed for IL-2 production. Numbers represent the mean percentage +/− SD of IL-2 producing IFN-γ+ CD8+ T cells or (isotype control). (C) Representative histograms of IFN-γ+ CD8+ T cells analyzed for expression of each phenotypic marker (solid histograms) or isotype control (open histograms). Numbers represent the mean percentage +/-SD of IFN-γ+ CD8+ T cells staining positive for each phenotypic marker. CD8+ T cell responses were determined at day 6 and 35 post secondary challenge by ICS. Mean, +/− SD, total number of (D) LLO91−99 –specific and (E) p60217−225 –specific CD8+ T cells/spleen in mice 1 day prior to Vir LM challenge (solid bars), at day 6 p.i. (open bars), and at day 35 p.i. (diagonally striped bars). Data is representative of duplicate experiments. n = 3 mice / time group. Dashed line indicates the limit of detection (LOD). (F) The mean number of bacteria per spleen was determined 2 days after Vir LM infection. Each symbol represents an individual mouse. Solid lines indicate the mean CFU/gram spleen (n = 3 mice / group). Dashed line indicates the limit of detection (LOD).
Discussion
Many gene mutations have been found to cause primary immunodeficiencies, including perforin gene mutations, which result in impaired NK and CD8+ T cell function and an increased susceptibility to viral infections. In addition, PKO mice serve as an excellent model of the human disease, FHL (Jordan et al., 2004)(Badovinac, Hamilton, and Harty, 2003). Furthermore, viral infections, like LCMV infections, can result in disruption of CD8+ T cell function and immunosuppression. However, the impact of primary immunodeficiency combined with persistent viral infections in responses to heterologous CD8+ T cell responses is poorly characterized. In this study we have investigated the impact of chronic LCMV infection on general immunocompetency and virus-specific CD8+ T cell responses in perforin-deficient hosts. Taken together our findings reveal three main points regarding CD8+ T cell responses in LCMV Arm chronically infected PKO mice. First, although chronic viral infection of PKO mice negatively impacted virus-specific CD8+ T cell number and function, these mice mounted normal CD8+ T cell responses to primary infections with heterologous pathogens. Second, following vaccination, memory CD8+ T cells specific for non-LCMV antigens were generated, maintained, and functioned normally in LCMV Arm chronically infected PKO mice. Finally, pre-existing memory CD8+ T cells undergo attrition following viral infection in chronically infected BALB/c PKO similar to infected WT mice, but maintain their memory phenotype and respond normally to secondary challenge. Thus, our data suggest that global immunosuppression is not always concurrent with persistent or prolonged LCMV infection of adult mice. Furthermore, persistent LCMV infection of immunodeficient hosts does not negatively impact heterologous immune responses.
Persistent viral infections often result in immunosuppression of CD8+ T cell responses and can be associated with functional disruptions (such as exhaustion) and deletion of CD8+ T cells (Liu et al., 2002)(Kostense et al., 2001)(Schlaak et al., 1999)(Wedemeyer et al., 2002)(Greten et al., 1998). In models of LCMV infection using fast growing strains, such as LCMV Cl13 and LCMV Docile, infection of adult mice results in prolonged viral loads, exhaustion and deletion of virus-specific CD8+ T cells (Wherry et al., 2003), and generalized immunosuppression. In agreement with these models and our previous results (Badovinac, Porter, and Harty, 2002)(Fuller and Zajac, 2003), data shown here demonstrates that LCMV Arm infection is not cleared from BALB/c PKO mice, that chronic infection is associated with a substantial, yet incomplete exhaustion of NP118−126-specific CD8+ T cells and furthermore the exhaustion of GP283−291-specific CD8+ T cells. In contrast to LCMV-specific CD8+ T cell responses, Listeria-specific CD8+ T cell responses were not suppressed by persistent LCMV infection. Resistance to LM infection of normal WT mice, in part, depends on functional CD8+ T cells (Harty and Bevan, 1992)(Badovinac, Porter, and Harty, 2002), however the disruption of CD8+ T cells responses after LCMV infection of PKO mice appear to be limited to virus-specific CD8+ T cells and not LM-specific cells.
These data suggest that neither the disruption of viral-specific CD8+ T cells alone, nor persistent LCMV-Arm infection in combination with perforin-deficiency, are sufficient for global immunosuppression. One explanation is that perforin is required for both clearance of LCMV and long lasting LCMV-induced immunosuppression. LCMV-induced immunosuppression has been linked to the infection and subsequent destruction and functional disruption of LCMV-infected DCs (Borrow, Evans, and Oldstone, 1995)(Sevilla et al., 2004) and requires CD8+ T cells. (Borrow, Evans, and Oldstone, 1995). And more generally, APC survival is limited after the initial activation of T cells (Hermans et al., 2000), however, a role for perforin in the destruction of APCs in vivo remains controversial (Ludewig et al., 2001)(Yang et al., 2006). It is possible that in the absences of perforin, CD8+ T cells in persistently infected PKO mice are unable to cause sufficient elimination of DC to mediate lasting immunosuppression. Furthermore, LCMV Arm targets and disrupts DCs to a limited extent, compared to fast growing LCMV strains, (Sevilla et al., 2000)(Borrow, Evans, and Oldstone, 1995). This could account for the failure of LCMV Arm to mediate generalized immunosuppression, even in chronically infected PKO mice. We show here that persistent LCMV Arm does not significantly suppress CD8+ T cell responses to LM up to 228 days post LCMV infection (Fig. 3C). It is possible that persistent LCMV Arm infection may be globally immunosuppressive only at very late time point after LCMV infection. However, this seems unlikely given that infection of mice with fast growing LCMV isolates are most immunosuppressive proximal to LCMV infection and that immunosuppression wanes with time (Ruedi, Hengartner, and Zinkernagel, 1990)(Mims and Wainwright, 1968). Immunosuppression in our model more closely resembles carrier state models of LCMV infection, where wildtype (WT) mice are infected prior to, or shortly after birth. Chronic LCMV Arm infection of PKO mice, like LCMV carrier mice, both manifest disruptions in LCMV-specific T cells responses, although most likely by different mechanisms (Wherry et al., 2003) (Jamieson and Ahmed, 1988), while remaining competent to respond to heterologous infections (Oldstone et al., 1973)(Oldstone, 2002). This again, is likely due to preservation of DCs and DC function in these models, in contrast to LCMV Cl13 infection of adult mice.
Previous studies of immunocompetency in LCMV- infected mice have focused on immune responses to primary heterologous challenges while the impact of LCMV infection, in the either presence or absence of an underlying primary immunodeficiencies on CD8+ T cell memory have remained unclear. We show here that LM-specific CD8+ T cell memory is generated, maintained, and functions normally following secondary infection in chronically infected immunodeficient BALB/c PKO mice. However, as has been shown previously, LCMV Arm infection of WT mice can result in a rapid ∼2-fold reduction in pre-existing memory CD8+ T cells in the spleen (McNally et al., 2001). We show here that the number of memory LM-specific CD8+ T cells is reduced 1.5 to 3.8-fold in the spleens 72 days after LCMV Arm infection of BALB/c PKO mice, and to a similar degree in WT mice. Thus, the attrition in LM-specific memory CD8+ T cell number after LCMV Arm infection is perforin-independent and also occurs in chronically infected mice. The remaining memory LM-specific CD8+ T cells in LCMV-infected mice are phenotypically and functionally similar to those in LCMV-naïve mice. In both LCMV-naïve and chronically infected groups of mice, memory CD8+ T cells protect from secondary Vir LM challenge and expand vigorously. These data suggest that despite a reduction in Ag-specific cell number, chronically infected BALB/c PKO mice mount normal CD8+ T cell responses upon secondary challenge. However, it is possible that the relatively high level of memory Ag-specific CD8+ T cells remaining are adequately stimulated by LM infections, whereas secondary response to other heterologous pathogens in chronically infected mice may be more sensitive to a small reduction in the number of memory cells. Although the factors regulating infection-induced lymphopenia have yet to decisively determined, the magnitude of the ensuing T cell response and type I interferon production following infection are likely to be key factors (Bahl et al., 2006)(McNally et al., 2001).
Taken together, these data demonstrate that persistent LCMV Arm infection disrupts specifically viral-specific CD8+ T cells without impacting primary or secondary Listeria-specific responses. The absence of perforin did not impact the LCMV-mediated disruption of CD8+ T cells or CD8+ T cell responses to heterologous infection in LCMV-chronically infected hosts. More generally, these data demonstrate the efficacy of Listeria-based vaccines vectors, even in chronically infected hosts with underlying primary immunodeficiency.
Materials and Methods
Mice
Eight- to 10-wk-old female WT BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). BALB/c PKO mice (White et al., 1999) were maintained by brother-sister mating. All mice where housed under specific pathogen-free conditions at the University of Iowa (Iowa City, IA) animal care unit and experiments followed approved institutional animal care and use protocols. Mice were transferred to the appropriate biosafety level prior to infection with LCMV and/or LM.
Abs, peptides, and tetramer
The following mAbs were used: IFN-γ–APC (clone XMG1.2), IL-2-PE (clone JES6−5H4), CD27-PE (clone LG.7F9), CD127-PE (clone A7R34), IgG Isotype control-PE from eBiosciences (San Diego, CA) and CD8α-PerCP and CD8α-FITC (clone 53−6.7), CD43-PE (clone 1B11), CD62L-PE (clone MEL-14), Thy1.2-FITC (clone 53−2.1), IgG Isotype control-PE from BD Pharminogen (San Jose, CA). Synthetic peptides representing defined H-2Kd-restricted listeriolysin O (LLO) 91−99 (Pamer, Harty, and Bevan, 1991), p60 217−225 (Harty and Pamer, 1995), LCMV Glycoprotein (GP)283−291) (van der Most et al., 1996) and H-2Ld-restricted LCMV Nucleoprotein (NP)118−126 (van der Most et al., 1996) were obtained from Biosynthesis (Lewisville, TX). MHC class I tetramers specific for NP118−126 were prepared as previously described (Altman et al., 1996) or obtained from the National Institute of Allergy and Infectious Diseases tetramer core (Atlanta, GA).
LCMV infections and titers
The Armstrong strain of LCMV was used at 2 × 105 PFU/mouse injected intraperitoneally (Badovinac, Hamilton, and Harty, 2003). Viral titers in homogenates of spleen, lung, kidney, and brain were determined by plaque assay on VERO cells (ATCC; Manassas, VA) (Shen et al., 1998).
LM infection of mice and colony forming unit (CFU) detection
Vaccinations and primary infections of mice used ∼0.1 LD50 (3 × 106 CFU) attenuated (actA-deficient) LM strain DP-L1942 (Brundage et al., 1993). Secondary infection of memory mice used ∼2−3 LD50 (3−6 × 104 CFU) virulent LM strain 10403s, as indicated. Bacteria were grown, injected, and quantified as described (Harty and Bevan, 1995). CFU of LM per gram spleen or liver were determined at the indicated times post infections (Harty and Bevan, 1995).
Quantification and Phenotypic analysis of Ag-specific CD8+ T cells
Intracellular cytokine staining (ICS) or MHC class I tetramer staining was performed as previously described (Badovinac, Tvinnereim, and Harty, 2000). For ICS, splenocytes were cocultured with 2 μL/mL GolgiPlug (BD PharMingen) in the presence or absence of specific peptide for 5.5 h. Cells were then washed, surface stained, and treated with Cytofix/Cytoperm (BD PharMingen) before cytokine staining. The percentage of IFN-γ+ CD8+ T cells in unstimulated samples from each mouse was subtracted from the peptide-stimulated value to determine the percentage of Ag-specific CD8+ T cells. Next, the total number of epitope-specific CD8+ T cells per spleen was calculated from the percentage of IFN-γ+ CD8+ T cells, the percentage of CD8+ T cells in each sample, and the total number of cells per spleen. For phenotypic analysis, cells were stained, as above, and the percentage of IFN-γ+ CD8+ T cells scoring positive for each marker calculated. Cells stained for CD62L expression were pretreated with 100mM TAPI-2 (Peptides International; Louisville, KY) for 45 min prior to peptide stimulation to prevent CD62L cleavage (Jabbari and Harty, 2006).
Peptide-coated dendritic cells
CD11c+ bone marrow derived dendritic cells (DCs) were generated as previously described (Hamilton and Harty, 2002). Bone marrow cells were depleted of RBC, subjected to complement depletion after incubation with mAbs 3.168 (CD8-specific), RL172 (CD4-specific), and RA3−3A1/6.1 (B220/CD45R-specific) and cultured for 5 days in medium supplemented with 1000 U/ml rGM-CSF (BD Pharmingen) and 25 U/ml rIL-4 (PeproTech, Rocky Hill, NJ). Loosely adherent cells were harvested and depleted of neutrophils by complement depletion with the Ly-6G-specific Ab RB6.8C5. LPS (500 ng/ml; Sigma-Aldrich, St. Louis, MO) was then added to cultures of the remaining cells for 1 day to induce maturation. Next, cultures were incubated with 1 μM peptide for 3 h, washed 3 times, and injected i.v. at 2 × 105 DC per mouse.
Statistical Analysis
When comparing three or more groups of data an ANOVA with Tukey post-test was used. A student's t-test was used when comparing two groups of data. p values ≤ 0.05 were considered significant.
Acknowledgements
This work was supported by National Institutes of Health Grants AI42767, AI46653, and AI50073 (to J.T.H.) and NIH 5 T32 AI007511-10, the Mechanisms of Parasitology Training Grant (to T.D.H.).
We thank S. Varga, J. Haring, K. Messingham, and R. Podyminogin for their assistance and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Oldstone MB. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988;167(5):1719–24. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almousa H, Ouachee-Chardin M, Picard C, Radford-Weiss I, Caillat-Zucman S, Cavazzana-Calvo M, Blanche S, de Saint Basile G, Le Deist F, Fischer A. Transient familial haemophagocytic lymphohistiocytosis reactivation post-CD34 haematopoietic stem cell transplantation. Br J Haematol. 2005;130(3):404–8. doi: 10.1111/j.1365-2141.2005.05615.x. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–6. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18(4):463–74. doi: 10.1016/s1074-7613(03)00079-7. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 2002;3(7):619–26. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma.[comment]. Science. 2000;290(5495):1354–8. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol. 2006;176(7):4284–95. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin H. W. t. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69(2):1059–70. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci U S A. 1993;90(24):11890–4. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MB. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, Roberts JL, Puck JM. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–87. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Cihak J, Lehmann-Grube F. Immunological tolerance to lymphocytic choriomeningitis virus in neonatally infected virus carrier mice: evidence supporting a clonal inactivation mechanism. Immunology. 1978;34(2):265–75. [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5(11):880–92. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170(1):477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP, Pardoll DM, Jacobson S, Schneck JP. Direct visualization of antigen-specific T cells: HTLV-1 Tax11−19- specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci U S A. 1998;95(13):7568–73. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttler F, Bro-Jorgensen K, Jorgensen PN. Transient impaired cell-mediated tumor immunity after acute infection with lymphocytic choriomeningitis virus. Scand J Immunol. 1975;4(4):327–36. doi: 10.1111/j.1365-3083.1975.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Harty JT. Quantitation of CD8+ T cell expansion, memory, and protective immunity after immunization with peptide-coated dendritic cells. J. Immunol. 2002;169(9):4936–44. doi: 10.4049/jimmunol.169.9.4936. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Influence of effector molecules on the CD8(+) T cell response to infection. Current Opinion in Immunology. 2002;14(3):360–5. doi: 10.1016/s0952-7915(02)00333-3. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J. Exp. Med. 1992;175(6):1531–8. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3(1):109–17. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Harty JT, Pamer EG. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J. Immunol. 1995;154(9):4642–50. [PubMed] [Google Scholar]
- Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164(6):3095–101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells. J Immunol Methods. 2006 doi: 10.1016/j.jim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Jamieson BD, Ahmed R. T-cell tolerance: exposure to virus in utero does not cause a permanent deletion of specific T cells. Proc Natl Acad Sci U S A. 1988;85(7):2265–8. doi: 10.1073/pnas.85.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–43. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- Katano H, Ali MA, Patera AC, Catalfamo M, Jaffe ES, Kimura H, Dale JK, Straus SE, Cohen JI. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood. 2004;103(4):1244–52. doi: 10.1182/blood-2003-06-2171. [DOI] [PubMed] [Google Scholar]
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172(5):2834–44. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- Kostense S, Ogg GS, Manting EH, Gillespie G, Joling J, Vandenberghe K, Veenhof EZ, van Baarle D, Jurriaans S, Klein MR, Miedema F. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31(3):677–86. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F, Niemeyer I, Lohler J. Lymphocytic choriomeningitis of the mouse. IV. Depression of the allograft reaction. Med Microbiol Immunol. 1972;158(1):16–25. doi: 10.1007/BF02122004. [DOI] [PubMed] [Google Scholar]
- Liu H, Andreansky S, Diaz G, Hogg T, Doherty PC. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J Immunol. 2002;168(7):3477–83. doi: 10.4049/jimmunol.168.7.3477. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Bonilla WV, Dumrese T, Odermatt B, Zinkernagel RM, Hengartner H. Perforin-independent regulation of dendritic cell homeostasis by CD8(+) T cells in vivo: implications for adaptive immunotherapy. Eur J Immunol. 2001;31(6):1772–9. doi: 10.1002/1521-4141(200106)31:6<1772::aid-immu1772>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67(12):7340–9. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Suresh M, Glass A, Galvan M, Chow K, Whitmire JK, Walsh CM, Clark WR, Ahmed R. A role for perforin in downregulating T-cell responses during chronic viral infection. J Virol. 1999;73(3):2527–36. doi: 10.1128/jvi.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J Virol. 2001;75(13):5965–76. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA, Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968;101(4):717–24. [PubMed] [Google Scholar]
- Notarangelo L, Casanova JL, Conley ME, Chapel H, Fischer A, Puck J, Roifman C, Seger R, Geha RS. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Meeting in Budapest, 2005. J Allergy Clin Immunol. 2006;117(4):883–96. doi: 10.1016/j.jaci.2005.12.1347. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Tishon A, Chiller JM, Weigle WO, Dixon FJ. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973;110(5):1268–78. [PubMed] [Google Scholar]
- Pamer EG, Harty JT, Bevan MJ. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes.[comment]. Nature. 1991;353(6347):852–5. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roost H, Charan S, Gobet R, Ruedi E, Hengartner H, Althage A, Zinkernagel RM. An acquired immune suppression in mice caused by infection with lymphocytic choriomeningitis virus. Eur J Immunol. 1988;18(4):511–8. doi: 10.1002/eji.1830180404. [DOI] [PubMed] [Google Scholar]
- Ruedi E, Hengartner H, Zinkernagel RM. Immunosuppression in mice by lymphocytic choriomeningitis virus infection: time dependence during primary and absence of effects on secondary antibody responses. Cell Immunol. 1990;130(2):501–12. doi: 10.1016/0008-8749(90)90290-8. [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Tully G, Lohr HF, Gerken G, Meyer zum Buschenfelde KH. The presence of high amounts of HBV-DNA in serum is associated with suppressed costimulatory effects of interleukin 12 on HBV-induced immune response. J Hepatol. 1999;30(3):353–8. doi: 10.1016/s0168-8278(99)80090-7. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192(9):1249–60. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113(5):737–45. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92(4):535–45. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, Durandy A, Griscelli C, Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993;123(4):564–72. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau LL, Southwood S, Sidney J, Chesnut RW, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157(12):5543–54. [PubMed] [Google Scholar]
- Volkert M, Bro-Jorgensen K, Marker O, Rubin B, Trier L. The activity of T and B lymphocytes in immunity and tolerance to the lymphocytic choriomeningitis virus in mice. Immunology. 1975;29(3):455–64. [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci U S A. 1994;91(23):10854–8. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169(6):3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78(11):5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, MacNeil A, Busch DH, Pilip IM, Pamer EG, Harty JT. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J Immunol. 1999;162(2):980–8. [PubMed] [Google Scholar]
- Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18(4):499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103(1):147–52. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]