Abstract
Phospholipase C (PLC)-β1 and PLC-β2 are regulated by the Gq family of heterotrimeric G proteins and contain C2 domains. These domains are Ca2+-binding modules that serve as membrane-attachment motifs in a number of signal transduction proteins. To determine the role that C2 domains play in PLC-β1 and PLC-β2 function, we measured the binding of the isolated C2 domains to membrane bilayers. We found, unexpectedly, that these modules do not bind to membranes but they associate strongly and specifically to activated [guanosine 5′-[γ-thio]triphosphate (GTP[γS])-bound] Gαq subunits. The C2 domain of PLC-β1 effectively suppressed the activation of the intact isozyme by Gαq(GTP[γS]), indicating that the C2-Gαq interaction may be physiologically relevant. C2 affinity for Gαq(GTP[γS]) was reduced when Gαq was deactivated to the GDP-bound state. Binding to activated Gαi1 subunits or to Gβγ subunits was not detected. Also, Gαq(GTP[γS]) failed to associate with the C2 domain of PLC-δ, an isozyme that is not activated by Gαq. These results indicate that the C2 domains of PLC-β1 and PLC-β2 provide a surface to which Gαq subunits can dock, leading to activation of the native protein.
Phosphatidylinositol-specific phospholipase Cs (PLCs) are Ca2+-dependent enzymes that catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate [PIns(4,5)P2] to generate the two second messengers diacylglycerol and inositol 1,4,5-trisphosphate (for reviews see refs. 1 and 2). These messengers then promote the activation of protein kinase C and the release of Ca2+ from intracellular stores. There are three known families of mammalian PLCs (γ, δ, and β), which differ in their regulation. PLC-γs are regulated by tyrosine kinase receptors whereas regulators of the PLC-δ family are not well defined. The four members of the PLC-β family, which we will focus on here, are activated by the subunits from the Gαq family of heterotrimeric G proteins as well as Gβγ subunits.
The mammalian PLC-βs are modular proteins containing an N-terminal pleckstrin homology (PH) domain, four elongation factor (EF) hand motifs, a catalytic X/Y domain, a C2 domain, and a long, 400-residue C-terminal extension that is unique to the PLC-β family. Although the roles of the EF hand motifs and C2 domain are unknown, the functions of the PH domain and C-terminal region have been investigated. Studies of the isolated PH domains of PLC-β1 and PLC-β2 show that they have a membrane-binding affinity on the order of the intact enzyme and help to anchor the protein to the membrane surface, allowing for lateral association to G protein subunits (3). The PH domains PLC-β1 and PLC-β2 also have a strong affinity for Gβγ and may play a key role in docking the Gβγ subunits, allowing for interactions with the catalytic domain (3).
In contrast, the C-terminal extension plays a key role in Gαq regulation because its deletion preserves intrinsic activity but abolishes activation by Gαq subunits (4–6). Truncation of the C terminus also reduces the affinity of the protein for activated Gαq subunits ≈25-fold, but the residual affinity between the two proteins is still very strong. PLC-βs are also GAPs (GTPase-activating proteins), and proteins derived from this C-terminal region have ≈20% of the total GAP activity of the intact enzyme (7).
In this study, we investigate the role of the C2 domains of PLC-β1 and PLC-β2. C2 domains have been identified and characterized in many proteins to date (for review see refs. 8 and 9). In general, C2 domains are ≈130 residues in length and consist of a compact β-sandwich of two four-stranded β-sheets. C2 domains generally are found in proteins that interact with lipid membranes that have been grouped into four major classes (see ref. 8): those that mediate vesicle transport, modify lipids, regulate GTPase activity, and phosphorylate proteins. Most, but not all, C2 domains bind Ca2+, and many are able to target their host protein to lipid membranes. It is notable that in some cases, such as synaptotagmins, C2 domains mediate calcium-dependent dimerization, binding to target proteins and to inositol polyphosphates.
The crystal structure of PLC-δ1 shows that its C2 domain, which binds Ca2+, is integrated with the catalytic core, and so simple deletion of this domain would be expected to produce an inactive protein. It was proposed that the function of the C2 domain of PLC-δ1 correctly orients the catalytic site to the lipid substrate (10). However, recent biochemical studies of mutated PLC-δ1 indicate that this may not be the case, leaving the function of the C2 domain of PLC-δ1 unclear (11). Sequence homology maps of the C2 domains of PLC-β, which we have studied here, show nonconservative replacements of some of the Asp residues required for Ca2+ binding.
Here, we have expressed the C2 domains of PLC-β1, PLC-β2, and PLC-δ1 and studied their ability to bind various membranes in a Ca2+-dependent manner by using fluorescence methods. Although only the C2-PLC-δ1 showed membrane binding, we found, surprisingly, that the C2 domains of PLC-β1 and PLC-β2 mediate specific association to Gαq subunits, indicating that these domains may mediate G protein signal transduction.
MATERIALS AND METHODS
Protein Preparation.
Preparation of recombinant PLC-β1, PLC- β2, Gαq, and Gβ1γ2 expressed in Sf9 cells and Gαi1 expressed in bacteria have been described (12). The integrity of the protein products was assessed electrophoretically and by PLC-β activity and Gαq[guanosine 5′-[γ-thio]triphosphate (GTP[γS])] activation as reported (13).
C2 domains were prepared by amplifying the coding sequences for C2-PLC-β1 (residues 663–802), C2-PLC-β2 (residues 688–805), and C2-PLC-δ1 (residues 615–756) by PCR and by inserting them into the corresponding sites in the expression vector pGEX (Pharmacia) fused to glutathione S-transferase (GST). Escherichia coli BL-21 (DE3) cells, transformed with pGEX-C2s expression vectors, were grown in super broth containing ampicillin (50 μg/ml). GST-C2 domain expression was induced with 1 mM isopropyl β-d-thiogalactopyranoside at 18°C, and the bacteria were harvested after 24 h. C2-β1 and C2-β2 were extracted as GST fusion proteins from the soluble fraction of the bacterial lysates by using glutathione-Sepharose 4B resin according to the manufacturer’s protocol (Pharmacia). Proteins were >95% pure, as judged by SDS/PAGE analysis.
Because C2-PLC-δ1 was found mainly in the insoluble fraction of the bacterial extracts, inclusion bodies were solubilized in 8 M urea and slowly diluted to 4 M urea before subjecting to glutathione-Sepharose chromatography. Renaturation was accomplished by using the method of Rozema and Gellman, which gave a better recovery than simple dilution methods (see ref. 14). Briefly, the solution was dialyzed into 4 M urea and diluted into 0.53 mM cetyltrimethylammonium bromide/4.8 mM β-cyclodextrin to promote refolding. The sample was left overnight and dialyzed later into appropriate buffers.
Removal of the GST tag was accomplished by incubating the GST-fused proteins with biotinylated thrombin, removing the thrombin with streptavidin-agarose, and then removing the free GST by using glutathione-Sepharose. After GST cleavage, only the C2-PLC-δ1 required renaturation. This was accomplished by redissolving into 4 M urea and then removing the urea initially by dilution and then by dialysis. Proper folding of the GST-cleaved C2 domains was confirmed by circular dichroism as described (3).
Protein Labeling.
All probes were purchased from Molecular Probes. Labeling with the amine-reactive probes, 7-methoxyl coumarin succinyl ester and dabsyl-succinyl ester, has been described (3, 13). The protein/probe labeling ratios were determined by absorption spectroscopy by using the calculated extinction coefficients for the protein and the coefficients provided by the probe manufacturer. The labeling ratio for all C2 proteins was found to be ≈1:1 (mol/mol).
Preparation of Membranes and Proteoliposomes.
Large, unilamellar vesicles were prepared by extrusion. G protein subunits were reconstituted onto the membranes by adding the detergent-solubilized proteins to a large excess of preformed, extruded vesicles as described (13).
RESULTS
Membrane-Binding Studies.
Binding of the C2 domains of PLC-β1, PLC-β2, and PLC-δ1 to lipid bilayers was measured using two types of fluorescence-based assays (see refs. 3 and 13). In the first, membranes were doped with the fluorescent detergent 6-lauroyl-2-(dimethylamino)naphthalene, whose head group is highly sensitive to the polarity of its environment. As proteins bind to the membrane surface and displace water, the emission intensity increases and the center of spectral mass shifts to higher energies. In the second type of assay, C2 domains were labeled with the fluorescent probe, 7-methoxycoumarin succinyl ester, and membrane binding of the labeled proteins to the nonfluorescent lipid was followed by the increase of coumarin fluorescence. Binding of C2-β1 and C2-β2 could not be detected even at lipid concentrations as high as 500 μM 1-palmitoyl 2-oleoyl phosphatidylcholine (POPC), POPC/1-palmitoyl 2-oleoyl phosphatidylserine (POPS) (1:2), or POPC/PIns(4,5)P2 (2%) (data not shown). Addition of 10 mM free Ca2+ did not influence binding. We did find that C2-δ1 bound weakly in a Ca2+-dependent manner to the anionic membranes, but at affinities that were too weak to be determined accurately by these methods (partition coefficient, defined as the ratio of the concentration of free and membrane-bound protein, Kp > 1 mM).
The C2 Domains of PLC-β1 and PLC-β2 Bind Specifically to Gαq Subunits.
Because the C2 domain of the PLC-β isozymes lies between the catalytic core and the C-terminal region that mediates activation by Gαq subunits, we tested the idea that the C2 domains may serve to link Gαq to the activation and catalytic domains of the protein. Association between the C2 domains and Gαq subunits was determined by labeling the Gαq with coumarin (C-) and measuring the transfer of its excited energy to a nonfluorescent energy-transfer acceptor covalently attached to the C2 domains, 4-[4-(dimethylamino)phenylazo]benzoic acid (DAB-). Because energy transfer is highly distance dependent, association of the two proteins is observed by the loss of donor fluorescence. We confirmed that activation of PLC-β1 by Gαq(GTP[γS]) did not change after fluorescent labeling.
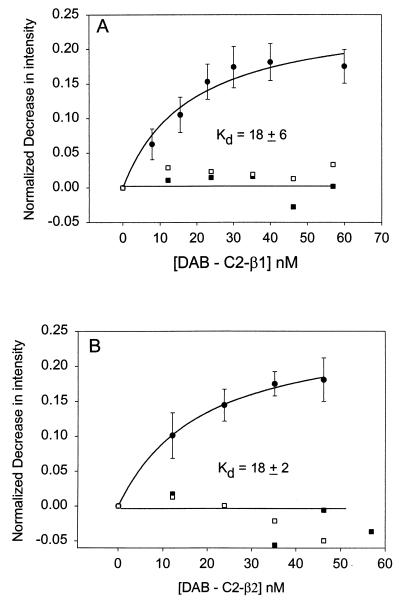
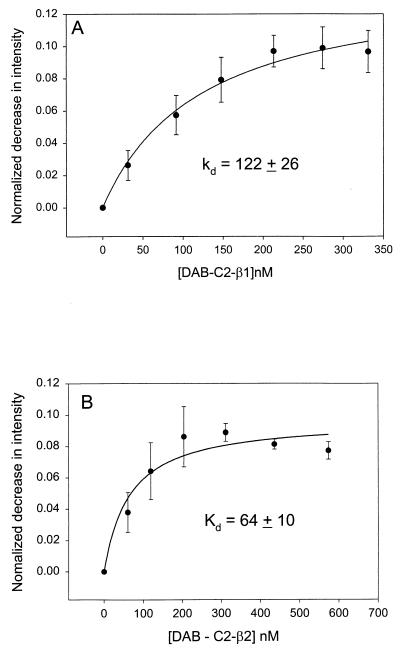
In Fig. 1 we show the increase in energy transfer from C-Gαq(GTP[γS]) reconstituted onto lipid bilayers as DAB-C2-PLC-β1 or DAB-C2-PLC-β2 was added. From these data, the apparent dissociation constants can be determined (Table 1). We find that both C2 domains bind with similar affinities that are independent of Ca2+, which is not surprising because the PLC-β domains are not expected to bind Ca2+ based on sequence (7). The extent of energy transfer (≈20% loss in donor intensity) is similar to that seen for the association between C-Gαq(GTP[γS]) and DAB-PLC-β1 and DAB-PLC-β2 (12). Similar to the full-length proteins, we find that the affinity for Gαq decreases when these subunits are deactivated to their GDP-bound forms (Fig. 2 and Table 1) although the decrease is lower than that observed for the native proteins. We also note that deactivation of Gαq to the GDP form reduces the amount of energy transfer to DAB-C2-β domains (compare Figs. 1 and 2), implying that either a portion of the DAB-C2-β domains does not bind to Gαq(GDP) or that their interaction is altered so that the energy-transfer distance between the coumarin and DAB probes is increased.
Figure 1.
Normalized decrease in the fluorescence intensity, obtained by dividing the zero-point fluorescence intensity and subtracting the background, of 2 nM C-Gαq(GTP[γS]) reconstituted on 120–150 μM POPC/POPS/POPE (1:1:1) membranes as DAB-C2-PLC-β1 (A, ●) or DAB-C2-PLC-β2 (B, ●) is added. Control studies substituted C-Gαi1(GTP[γS]) (□) or C-Gβγ (■) for C-Gαq(GTP[γS]).
Table 1.
Dissociation constants of the C2 domains of PLCs
| C2 domain | Gαq(GTP[γS]) | Gαq(GDP) | Gαil(GTP[γS]) | Gβγ |
|---|---|---|---|---|
| C2-PLC-β1 | 18 ± 2 | 122 ± 26 | >1,000 | >1,000 |
| C2-PLC-β2 | 18 ± 6 | 64 ± 10 | >1,000 | >1,000 |
| C2-PLC-δ1 | >1,000 | >1,000 | >1,000 | >1,000 |
Figure 2.
Normalized decrease in the fluorescence intensity of 2 nM deactivated C-Gαq(GDP) reconstituted on 120–150 μM POPC/POPS/POPE (1:1:1) membranes as DAB-C2-PLC-β1 (A, ●) or DAB-C2-PLC-β2 (B, ●) is added.
Several control studies were done to convince ourselves that the decrease in C-Gαq fluorescence was a result of energy transfer caused by molecular association between the proteins. First, the addition of unlabeled C2 domains or dialysis buffer did not alter the intensity of C-Gαq(GTP[γS]). Second, the change in intensity could be blocked completely by preincubation of activated or deactivated C-Gαq with an appropriate concentration (see below) of unlabeled, full-length PLC-β1 (Fig. 1).
Further studies were done to demonstrate that the associations between the PLC-β C2 domains and Gαq were specific. First, we substituted the PLC-β C2 domains with PLC-δ1 C2 domains and did not detect association (data not shown). This result is not surprising because the intact PLC-δ1 is not activated by Gαq(GTP[γS]). Second, we tested DAB-labeled N-terminal PH domains of PLC-β2, which is not expected to interact with Gαq (15), and we could not detect association (data not shown). Third, we replaced C-Gαq with C-Gβγ subunits and could not detect a change in fluorescence when DAB-C2 domains of PLC-β1 or PLC-β2 were added (Figs. 1 and 2); even the intensity of C-Gβγ decreases ≈20% upon association with DAB-Gαq(GDP), DAB-PLC-β2, DAB-PH-PLC-β1, or DAB-PH-β2 (3, 13). Finally, no association could be seen when C-Gαi1(GTP[γS]) was substituted for C-Gαq(GTP[γS]) (Fig. 1). Taken together, we conclude that energy transfer between C-Gαq(GTP[γS]) and DAB-C2 PLC-β1 and PLC-β2 reflects specific protein–protein associations.
We also used a second method to confirm direct binding between the proteins. Using glutathione-Sepharose, we measured the amount of C-Gαq(GTP[γS]) that adhered to the resin in the presence and absence of bound GST-PLC-β1-C2, with the nonbinding C-Gαi1(GTP[γS]) as a control. The results in Fig. 3 show that only C-Gαq(GTP[γS]) adheres to the resin only when GST-PLC-β1-C2 is bound.
Figure 3.
Interaction of C-Gαq(GTP[γS]) or C-Gαi1(GTP[γS]) with glutathione-Sepharose 4B resin (Amersham Pharmacia) in the presence or absence of bound GST-C2-PLC-β1. The G protein subunits were mixed with resin and washed. The amount of G protein that adhered to the resin was assessed by fluorescence after release of the proteins by the addition of excess glutathione.
Because the data in Figs. 1 and 2 represent affinities between a protein that is bound to the membrane surface and one that is free in solution, we have calculated the affinities by using a solution-state bimolecular association constant. The interaction between the intact enzymes and G protein occurs for proteins concentrated on membrane surfaces, which must be considered when comparing the Gαq affinities of C2 domains and their intact proteins. The apparent Kd for the association between C2-PLC-β1 and C2-PLC-β2 and Gαq(GTP[γS]) is 18 nM, which is 2- and 20-fold stronger than the solution value of the intact enzymes, respectively. However, association between Gαq subunits and intact PLC-β1 occurs on membrane surfaces, and their effective concentrations are higher because they are concentrated on a quasi-two-dimensional surface. Thus, at the lipid concentrations used here (i.e., 150 μM), the intact PLC-β1 and PLC-β2 will bind ≈120-fold and 10-fold more strongly to Gαq(GTP[γS]) than their isolated C2 domains. Interestingly, on this same basis we find that the C2 domains bind to deactivated Gαq(GDP) with an ≈10-fold-higher affinity than their native host proteins.
The C2 Domains of PLC-β1 and PLC-β2 Block Activation of PLC-β1 by Gαq(GTP[γS]).
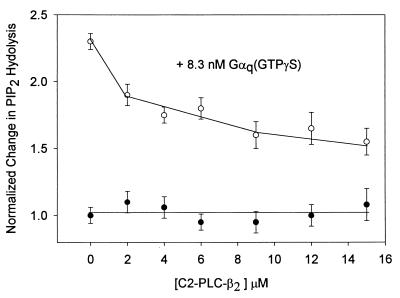
To determine whether the C2 domains of PLC-β1 and PLC-β2 are capable of competing with the native protein for activation by Gαq(GTP[γS]), we measured the change in the level of PLC-β1 activation by Gαq(GTP[γS]) on POPC/POPE/POPS (1:1:1) vesicles containing 2% PIns(4,5)P2 as C2 domains were added. The results in Fig. 4 show a systematic, but incomplete, reversal of PLC-β1 activation. Although this concentration range is in accord with the expected range, the lack of complete reversal is most likely due to nonspecific activation or the inability of C2 to correctly place itself on the membrane surface to disrupt the complex, and/or is indicative of higher-order interactions between the proteins that have been implicated in previous studies (see ref. 7).
Figure 4.
Reversal of the activation of PLC-β1 (0.5 nM), as measured by [3H]Ins(1,4,5)P3 production, by 8 nM Gαq(GTP[γS]) on POPC/POPS/POPE (1:1:1) vesicles with 2% PIns(4,5)P2, where n = 6 (see ref. 12).
DISCUSSION
PLC-β isozymes are primarily membrane-resident proteins that are activated through lateral association to G protein subunits (1, 2, 16). Here, we present data showing that the C2 domains of PLC-β1 and PLC-β2 serve as docking sites for Gαq subunits. The specificity of these interactions were confirmed by a number of controls that included unlabeled proteins, alternate G protein subunits, and PLC-δ C2 domains, all of which failed to bind. Competition between PLC-β1 and its C2 domain by activity studies and fluorescence suggest that they both bind to the same site on Gαq. Also, the affinity of the C2 domain of the PLC-βs for Gαq is sensitive to the GTP/GDP activation state, which is similar to that of the intact protein.
Previous studies have suggested that other regions besides the C-terminal tail of PLC-βs must interact with Gαq. Although loss of the C-terminal tail of PLC-β1 abolishes activation by Gαq (4–6), it reduces Gαq affinity by only 25-fold (12, 13). Our results here imply that the residual affinity of the truncated protein is due to the C2 domain. This, coupled with the finding that the C-terminal tail accounts for 20% of the GAP activity (7), suggests that the C2 domain serves to mediate productive interactions between the Gαq, the C-terminal tail, and the catalytic domain. The contribution of the C-terminal tail relative to the C2 domains to Gαq binding of the two PLC-β isozymes is indicated by the affinity of PLC-β2, which is 10-fold-lower than that of PLC-β1 (13), even though the Gαq affinities of their C2 domains are identical (Table 1). Thus, the difference is expected to be due to the tail region or the disposition of the C2 domains to other regions of the protein.
Our data offer insight into the interactions that occur between Gαq and the C2 domains of their PLC-β effectors. The absolute specificity for Gαq subunits as compared with Gαi1 implicates specific interactions to nonhomologous regions of the αq subunits, namely, residues 251–265 and 306–319. Peptides corresponding to these sequences have been shown to inhibit Gαq activation of PLC-β1 (16). Also, that deactivation of Gαq reduces its C2 domain affinity only 4- to 10-fold (Table 1) indicates that the interaction site(s) involves other regions of Gαq besides the switch region.
Here, we have found that in contrast to almost all other proteins containing this motif (see refs. 8 and 9), the C2 domains of PLC-β1, PLC-β2, and PLC-δ1 do not bind with measurable affinities to membranes or inositol phosphates. Membrane binding of the C2 domains of PLC-β1 and PLC-β2 was not detectable under a variety of conditions. The C2 domain of PLC δ1 bound only weakly to membranes containing anionic lipids or containing PIns(4,5)P2. Because we have found previously that membrane binding of PLC-β1 and PLC-β2 is driven by the N-terminal PH domain (3), the membrane targeting function found for the C2 domains of other proteins is not required for the PLC-β isozymes. Thus, the function of C2 domains is not membrane tethering but rather to mediate Gαq interactions between the C terminus and catalytic core, leading to allosteric activation.
Acknowledgments
We are grateful to Stuart McLaughlin for critically reading the manuscript and offering his advice and Dr. Daniel Raleigh for the use of his CD instrument. This work was supported by National Institutes of Health Grants GM53132 and GM43422 to S.S. and M.R.
ABBREVIATIONS
- PLC
phosphoinositide-specific phospholipase C
- PH
pleckstrin homology
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- GST
glutathione S-transferase
- POPC
1-palmitoyl 2-oleoyl phosphatidylcholine
- POPS
1-palmitoyl 2-oleoyl phosphatidylserine
- POPE
1-palmitoyl 2-oleoyl phosphatidylethanolamine
- PIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- C
7-methoxycoumarin-3-carboxylic acid
- DAB
4-[4-(dimethylamino)phenylazo]benzoic acid
References
- 1.Exton J H. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 2.Rhee S G, Bae Y S. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Pentyala S, Rebecchi M, Scarlata S. Biochemistry. 1999;38:1517–1527. doi: 10.1021/bi982008f. [DOI] [PubMed] [Google Scholar]
- 4.Wu D, Jiang H, Katz A, Simon M I. J Biol Chem. 1993;268:3704–3709. [PubMed] [Google Scholar]
- 5.Park D, Hhon D-Y, Lee C-W, Ryu S H, Rhee S G. J Biol Chem. 1993;268:3710–3714. [PubMed] [Google Scholar]
- 6.Lee S B, Shin S H, Hepler J R, Gilman A G, Rhee S G. J Biol Chem. 1993;268:25952–25957. [PubMed] [Google Scholar]
- 7.Paulssen R, Woodsen J, Lui Z, Ross E M. J Biol Chem. 1996;271:26622–26629. doi: 10.1074/jbc.271.43.26622. [DOI] [PubMed] [Google Scholar]
- 8.Nalefski E, Falke J J. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R L, Katan M. Structure. 1996;4:1387–1394. doi: 10.1016/s0969-2126(96)00146-3. [DOI] [PubMed] [Google Scholar]
- 10.Grobler J A, Hurley J H. Biochemistry. 1998;37:5020–5028. doi: 10.1021/bi972952w. [DOI] [PubMed] [Google Scholar]
- 11.Runnels L W, Scarlata S F. Biochemistry. 1998;37:15563–15574. doi: 10.1021/bi9811258. [DOI] [PubMed] [Google Scholar]
- 12.Runnels L W, Scarlata S F. Biochemistry. 1999;38:1488–1496. doi: 10.1021/bi9821519. [DOI] [PubMed] [Google Scholar]
- 13.Rozema D, Gellman SH. Biochemistry. 1996;35:15760–15771. doi: 10.1021/bi961638j. [DOI] [PubMed] [Google Scholar]
- 14.Morris A J, Scarlata S. Biochem Pharmacol. 1997;54:429–435. doi: 10.1016/s0006-2952(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 15.Runnels L W, Jenco J, Morris A, Scarlata S. Biochemistry. 1996;35:16824–16832. doi: 10.1021/bi961606w. [DOI] [PubMed] [Google Scholar]
- 16.Arkinstall S, Chabert C, Maundrell K, Peitsch M. FEBS Lett. 1995;364:45–50. doi: 10.1016/0014-5793(95)00351-9. [DOI] [PubMed] [Google Scholar]