Abstract
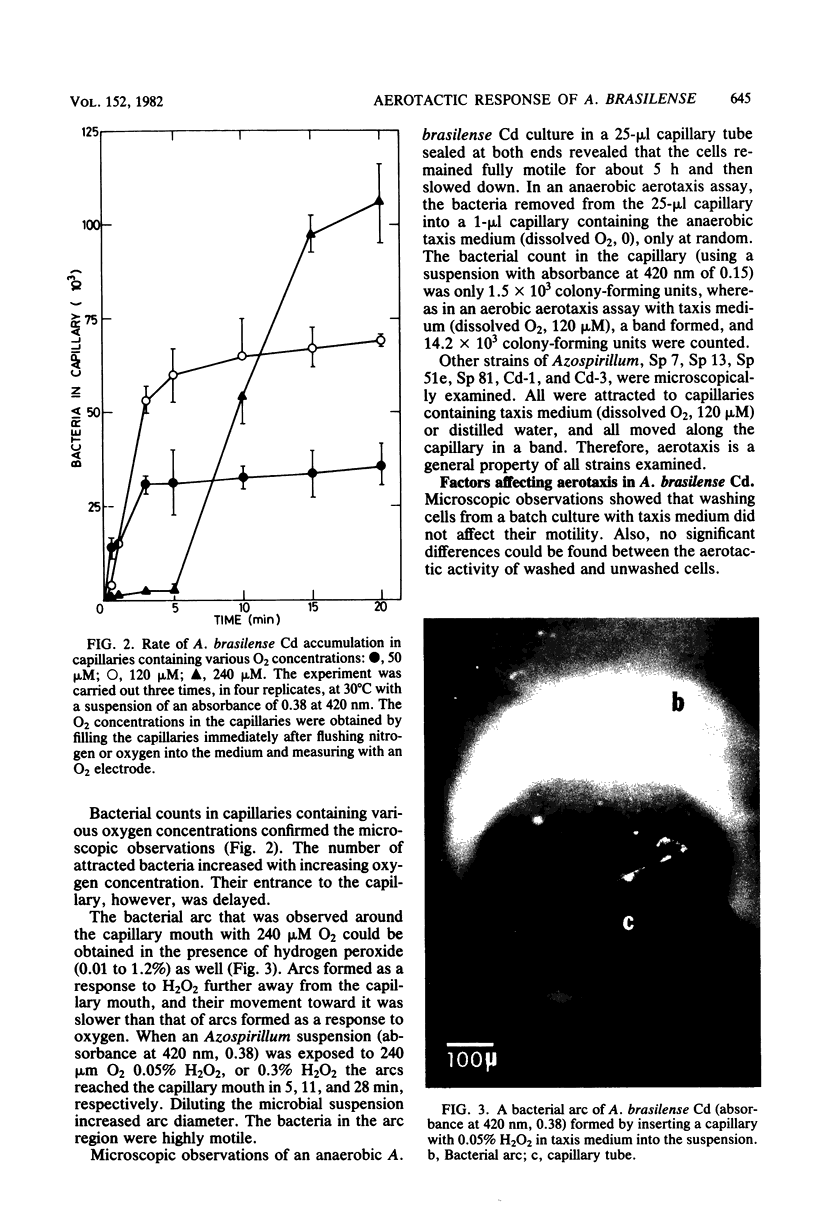
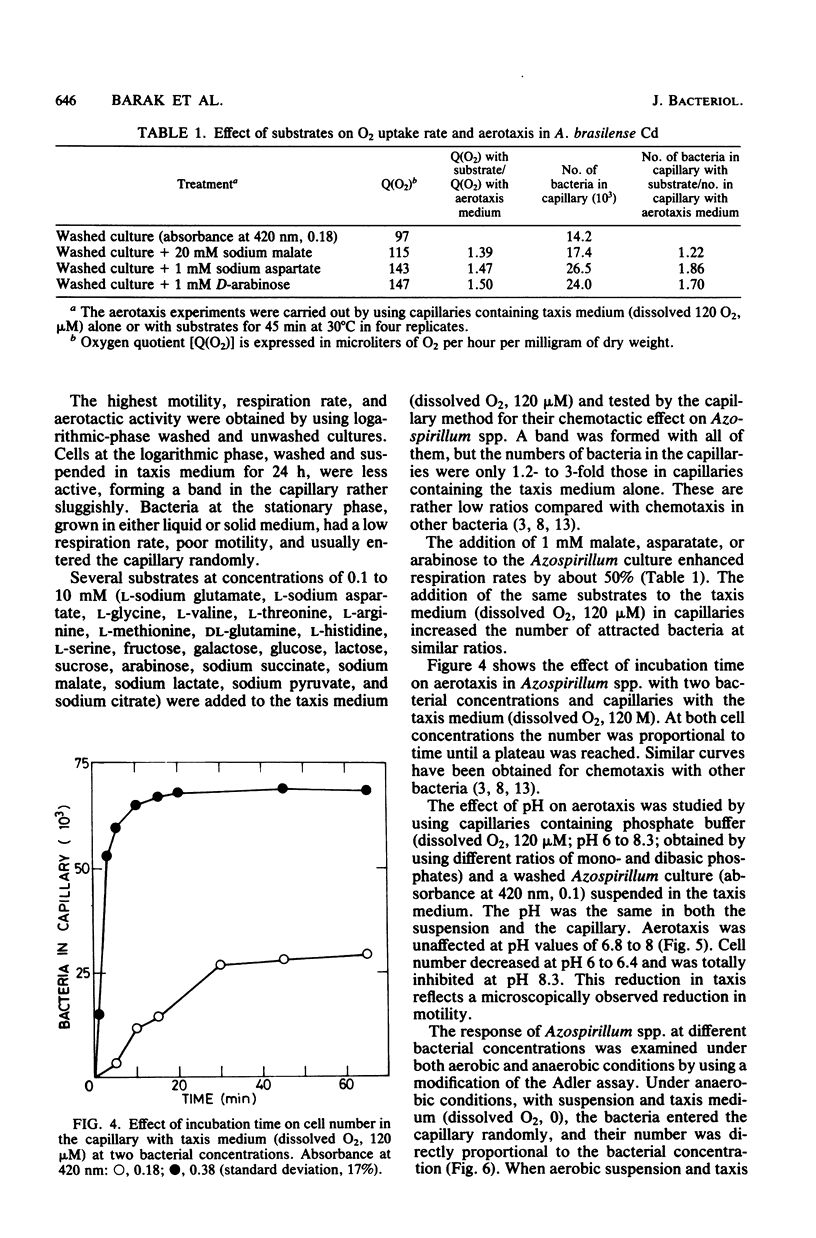
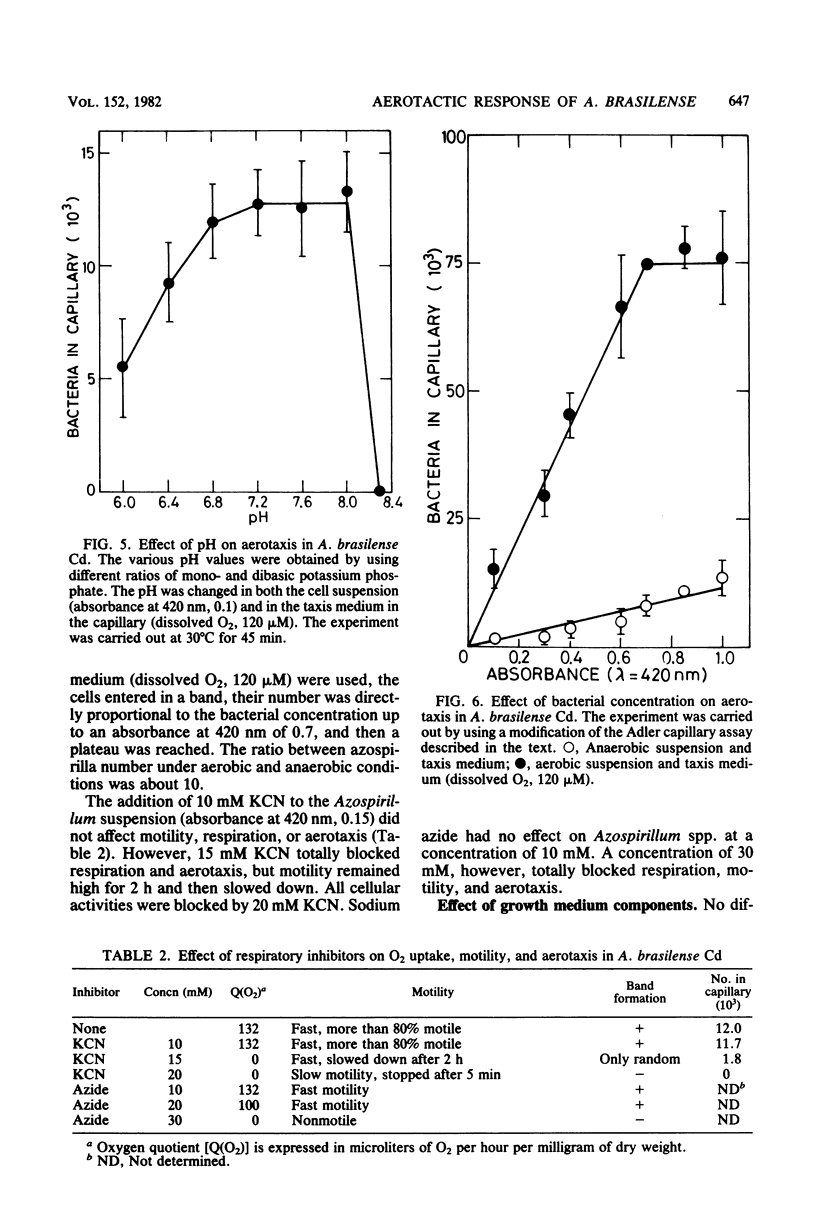
Five strains of Azospirillum brasilense and two of Azospirillum spp., from Israel, responded to self-created and preformed oxygen gradients by forming aerotactic bands in capillary tubes and actively moving toward a specific zone with low dissolved oxygen. Increasing the oxygen concentration in capillaries containing phosphate buffer increased the number of attracted bacteria and decreased band velocity. High O2 concentrations and H2O2 temporarily repulsed the bacteria, causing the formation of a bacterial arc around the capillary mouth. There was no band formation under anaerobic conditions, although the bacteria remained highly motile. Exogenous energy sources were unnecessary for aerotaxis in Azospirillum spp. The addition of oxidizable substrates to the capillary slightly enhanced aerotaxis, possibly by accelerating O2 consumption. Aerotactic band formation was affected by pH, bacterial concentration and age, incubation time, and respiratory inhibitors, but not by the lack of combined nitrogen in the growth medium. It is proposed that aerotaxis plays a role in the capacity of Azospirillum spp. to reach an environment suitable for N2 fixation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J. Effect of amino acids and oxygen on chemotaxis in Escherichia coli. J Bacteriol. 1966 Jul;92(1):121–129. doi: 10.1128/jb.92.1.121-129.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARACCHINI O., SHERRIS J. C. The chemotactic effect of oxygen on bacteria. J Pathol Bacteriol. 1959 Apr;77(2):565–574. doi: 10.1002/path.1700770228. [DOI] [PubMed] [Google Scholar]
- Eskew D. L., Focht D. D., Ting I. P. Nitrogen fixation, denitrification, and pleomorphic growth in a highly pigmented Spirillum lipoferum. Appl Environ Microbiol. 1977 Nov;34(5):582–585. doi: 10.1128/aem.34.5.582-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarre A. G., Straley S. C., Conti S. F. Chemotaxis toward amino acids by Bdellovibrio bacteriovorus. J Bacteriol. 1977 Jul;131(1):201–207. doi: 10.1128/jb.131.1.201-207.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo D. J., Taylor B. L. Aerotaxis in Salmonella typhimurium: role of electron transport. J Bacteriol. 1981 Feb;145(2):990–1001. doi: 10.1128/jb.145.2.990-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur I., Okon Y., Henis Y. Comparative studies of nitrogen-fixing bacteria associated with grasses in Israel with Azospirillum brasilense. Can J Microbiol. 1980 Jun;26(6):714–718. doi: 10.1139/m80-122. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Miller J. B., Warrick H. M., Koshland D. E., Jr Electron acceptor taxis and blue light effect on bacterial chemotaxis. J Bacteriol. 1979 Nov;140(2):567–573. doi: 10.1128/jb.140.2.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Drift C., Duiverman J., Bexkens H., Krijnen A. Chemotaxis of a motile Streptococcus toward sugars and amino acids. J Bacteriol. 1975 Dec;124(3):1142–1147. doi: 10.1128/jb.124.3.1142-1147.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]