Abstract
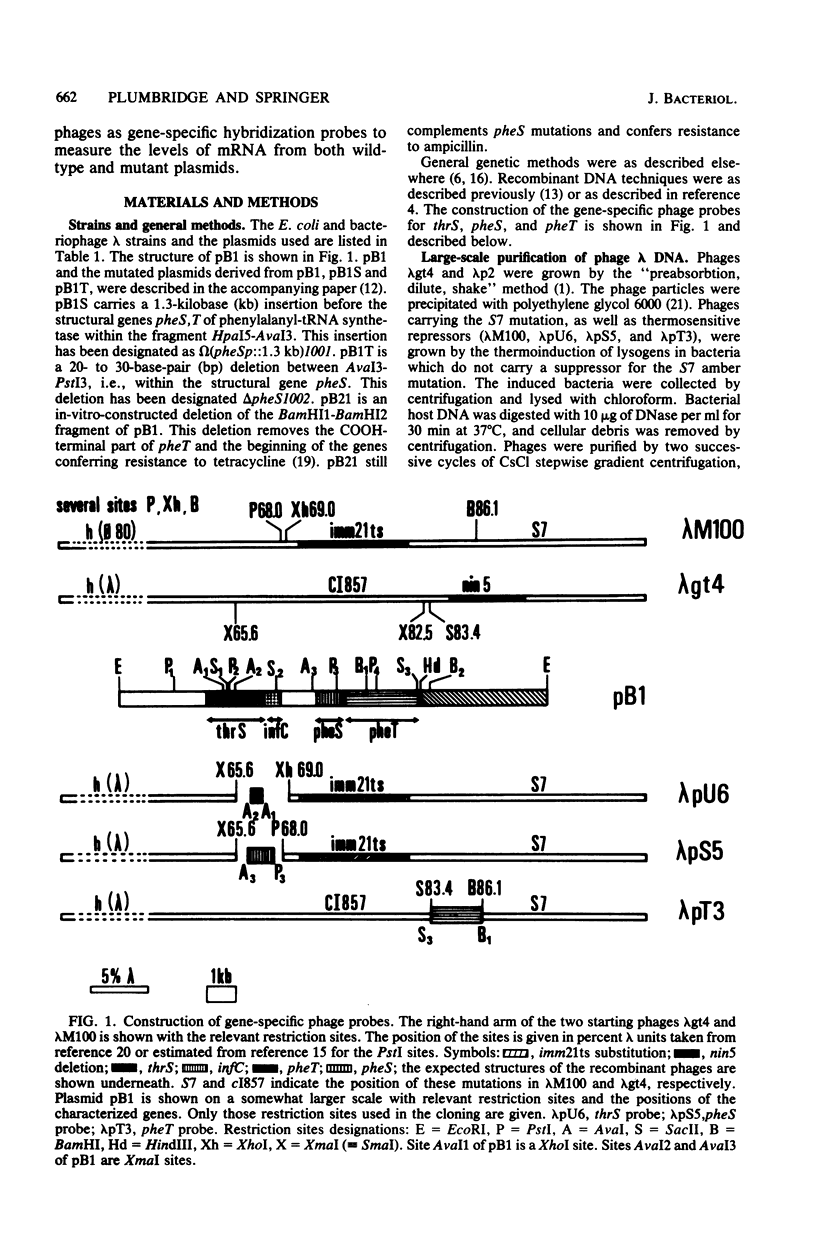
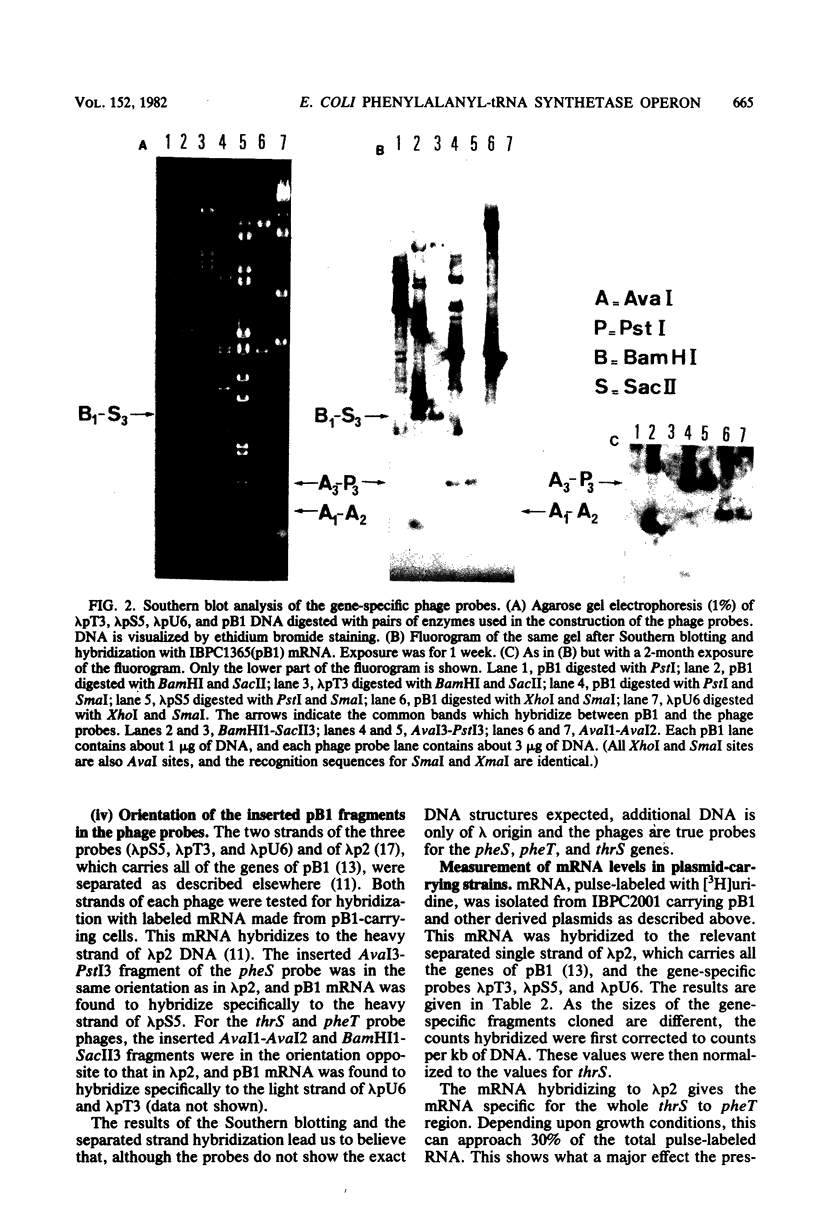
The construction of three lambda bacteriophages containing parts of the structural gene for threonyl-tRNA synthetase, thrS, and those for the two subunits of phenylalanyl-tRNA synthetases, pheS and pheT, is described. These phages were used as hybridization probes to measure the in vivo levels of mRNA specific to these three genes. Plasmid pB1 carries the three genes thrS, pheS, and pheT, and strains carrying the plasmid show enhanced levels of mRNA corresponding to these genes. Although the steady-state levels of threonyl-tRNA synthetase and phenylalanyl-tRNA synthetase produced by the presence of the plasmid differed by a factor of 10, their pulse-labeled mRNA levels were about the same. Mutant derivatives of pB1 were also analyzed. Firstly, a cis-acting insertion located before the structural genes for phenylalanyl-tRNA synthetase caused a major decrease in both pheS and pheT mRNA. Secondly, mutations affecting either structural gene pheS or pheT caused a reduction in the mRNA levels for both pheS and pheT. This observation suggests that autoregulation plays a role in the expression of phenylalanyl-tRNA synthetase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blumenthal R. M., Lemaux P. G., Neidhardt F. C., Dennis P. P. The effects of the relA gene on the synthesis of aminoacyl-tRNA synthetases and other transcription and translation proteins in Escherichia coli A. Mol Gen Genet. 1976 Dec 22;149(3):291–296. doi: 10.1007/BF00268530. [DOI] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on efficiency of translation of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):793–804. doi: 10.1128/jvi.26.3.793-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Escherichia coli phenylalanyl-tRNA synthetase operon: characterization of mutations isolated on multicopy plasmids. J Bacteriol. 1982 Nov;152(2):650–660. doi: 10.1128/jb.152.2.650-660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Genes for the two subunits of phenylalanyl-tRNA synthesis of Escherichia coli are transcribed from the same promoter. J Mol Biol. 1980 Dec 25;144(4):595–600. doi: 10.1016/0022-2836(80)90341-1. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981 Jun 25;291(5817):632–635. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Hennecke H. Specialized transducing phage for the initiation factor 3 gene in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3970–3974. doi: 10.1073/pnas.74.9.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Plumbridge J. A., Trudel M., Graffe M., Grunberg-Manago M. Transcription units around the gene for E. coli translation initiation factor IF3 (infC). Mol Gen Genet. 1982;186(2):247–252. doi: 10.1007/BF00331857. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]



