Abstract
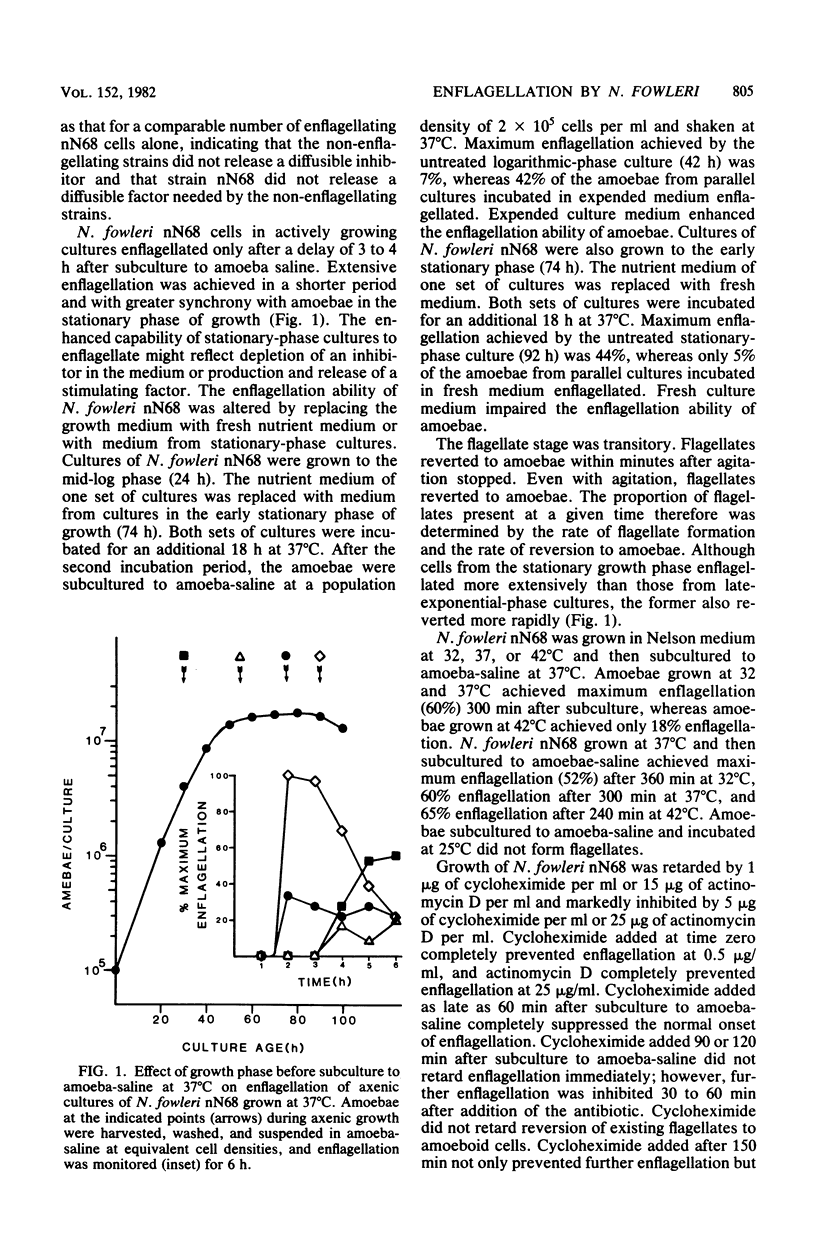
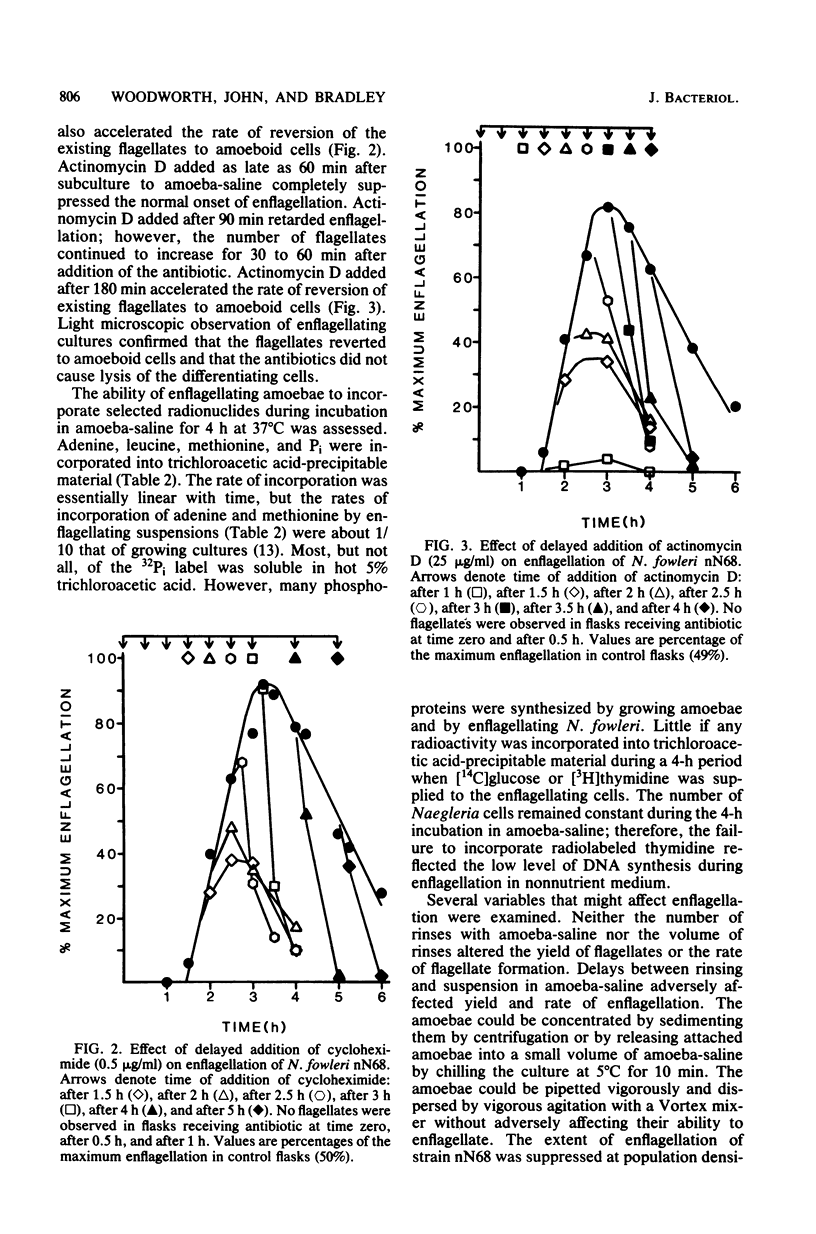
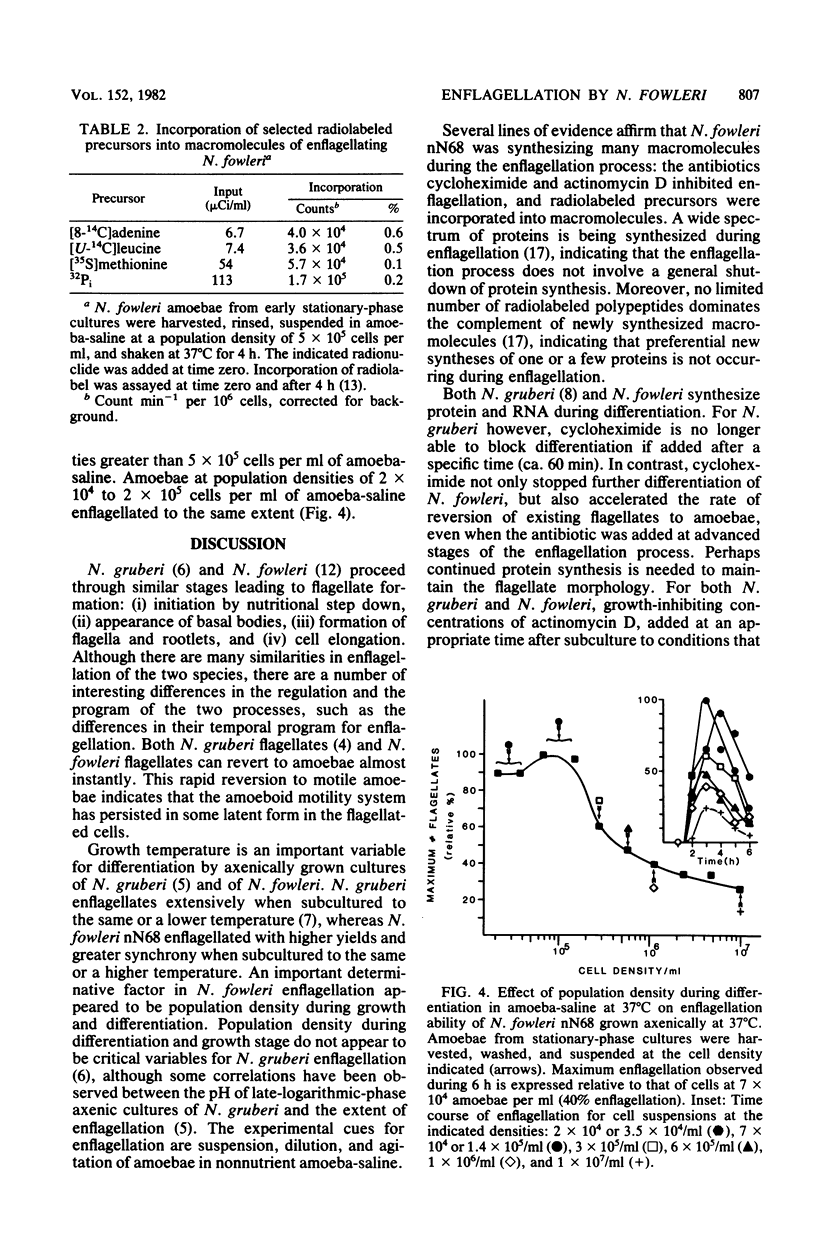
Naegleria fowleri is a pathogenic amoeboflagellate that can be evoked to transform from amoebae to flagellates by subculture to nonnutrient buffer. More than half of the amoebae of strains KUL, nN68, and Lovell became enflagellated 300 min after subculture to amoeba-saline, whereas no amoebae of strains NF66, NF69, and HB4 did. N. fowleri nN68 enflagellated best when grown at 32 or 37 degrees C and subcultured to amoeba-saline at 37 or 42 degrees C. Amoebae from the stationary phase of growth enflagellated more readily than did actively growing amoebae. Incubation in expended culture medium from stationary-phase cultures enhanced the capability of growing amoebae to enflagellate after subculture to amoebasaline. Enflagellation was more extensive when the population density in amoebasaline did not exceed 2 x 10(5) amoebae per ml. Cycloheximide at 1 microgram/ml and actinomycin D at 25 micrograms/ml inhibited growth of N. fowleri nN68. Cycloheximide at 0.5 microgram/ml and actinomycin D at 25 micrograms/ml completely prevented enflagellation when added at time zero. Cycloheximide at 0.5 microgram/ml, added 120 to 300 min after initiation of enflagellation, prevented further differentiation and caused existing flagellates to revert to amoeboid cells. Similarly, actinomycin D at 25 micrograms/ml, added 90 to 300 min after initiation of enflagellation, retarded differentiation and caused flagellates to revert. Radiolabeled precursors were incorporated into macromolecules during differentiation in nonnutrient buffer. Enflagellation of N. fowleri is a suitable model for studying regulation of a eucaryotic protist.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. Use of an axenic medium for differentiation between pathogenic and nonpathogenic Naegleria fowleri isolates. Appl Environ Microbiol. 1977 Apr;33(4):751–757. doi: 10.1128/aem.33.4.751-757.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma R. J., Rosenblum W. I., McGehee R. F., Jones M. M., Nelson E. C. Primary amoebic meningoencephalitis caused by Naegleria. Two new cases, response to amphotericin B, and a review. Ann Intern Med. 1971 Jun;74(6):923–931. doi: 10.7326/0003-4819-74-6-923. [DOI] [PubMed] [Google Scholar]
- Fulton C. Axenic cultivation of Naegleria gruberi. Requirement for methionine. Exp Cell Res. 1974 Oct;88(2):365–370. doi: 10.1016/0014-4827(74)90253-5. [DOI] [PubMed] [Google Scholar]
- Fulton C. Cell differentiation in Naegleria gruberi. Annu Rev Microbiol. 1977;31:597–629. doi: 10.1146/annurev.mi.31.100177.003121. [DOI] [PubMed] [Google Scholar]
- Fulton C., Dingle A. D. Appearance of the flagellate phenotype in populations of Naegleria amebae. Dev Biol. 1967 Feb;15(2):165–191. doi: 10.1016/0012-1606(67)90012-7. [DOI] [PubMed] [Google Scholar]
- Fulton C., Walsh C. Cell differentiation and flagellar elongation in Naegleria gruberi. Dependence on transcription and translation. J Cell Biol. 1980 May;85(2):346–360. doi: 10.1083/jcb.85.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight J. B., John D. T. Growth of Naegleria fowleri in several axenic media. Folia Parasitol (Praha) 1980;27(3):207–212. [PubMed] [Google Scholar]
- Jadin J. B., Hermanne J., Robyn G., Willaert E., Stevens W., Van Maercke Y. Trois cas de méningo-encéphalite amibienne primitive observés a Anvers (Belgique. Ann Soc Belges Med Trop Parasitol Mycol. 1971;51(2):255–266. [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Patterson M., Woodworth T. W., Marciano-Cabral F., Bradley S. G. Ultrastructure of Naegleria fowleri enflagellation. J Bacteriol. 1981 Jul;147(1):217–226. doi: 10.1128/jb.147.1.217-226.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Driessche E., Vandepitte J., Van Dijck P. J., De Jonckheere J., Van de Voorde H. Letter: Primary amoebic meningoencephalitis after swimming in stream water. Lancet. 1973 Oct 27;2(7835):971–971. doi: 10.1016/s0140-6736(73)92634-2. [DOI] [PubMed] [Google Scholar]
- Weik R. R., John D. T. Quantitation and cell size of Naegleria fowleri by electronic particle counting. J Parasitol. 1977 Feb;63(1):150–151. [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Amuso P. T., Chang S. L. Naegleria and water sports. Lancet. 1977 Jan 22;1(8004):199–200. doi: 10.1016/s0140-6736(77)91806-2. [DOI] [PubMed] [Google Scholar]
- Woodworth T. W., Keefe W. E., Bradley S. G. Characterization of proteins in flagellates and growing amebae of Naegleria fowleri. J Bacteriol. 1982 Jun;150(3):1366–1374. doi: 10.1128/jb.150.3.1366-1374.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


