Abstract
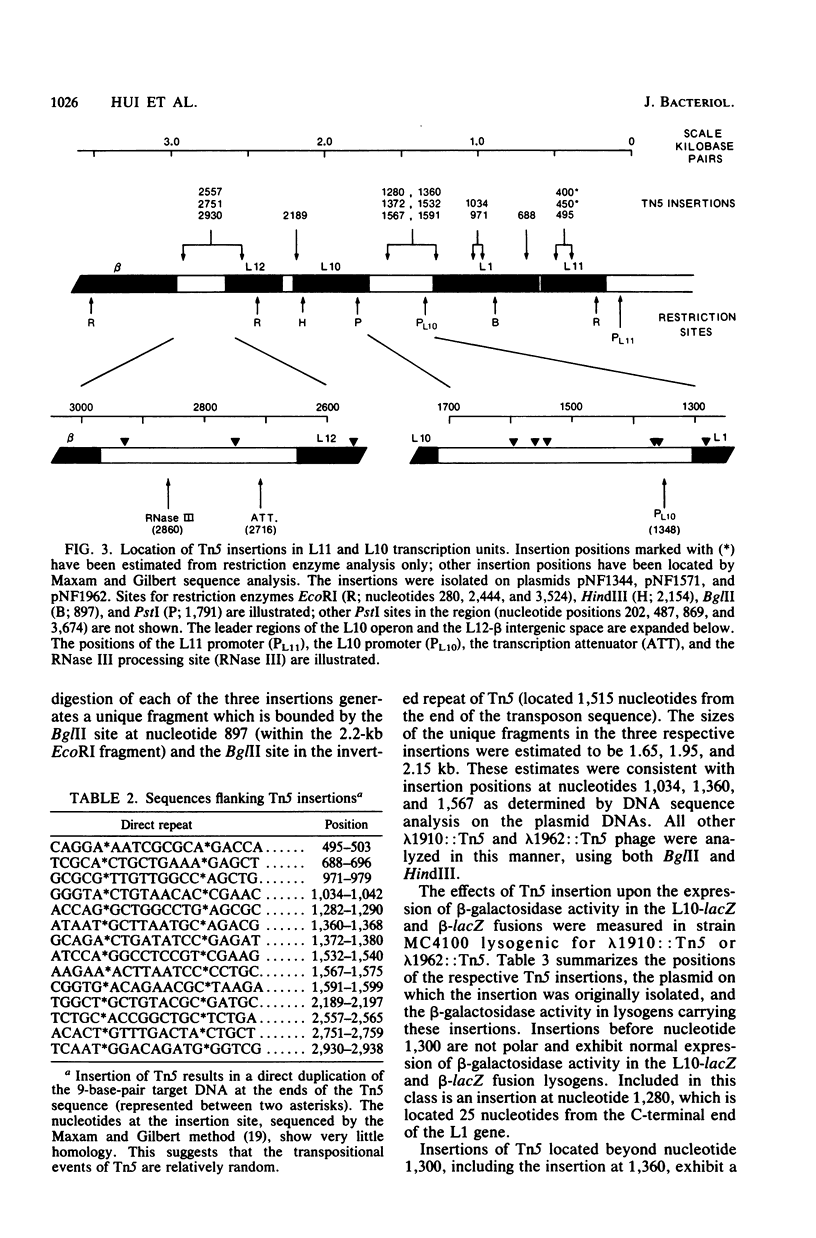
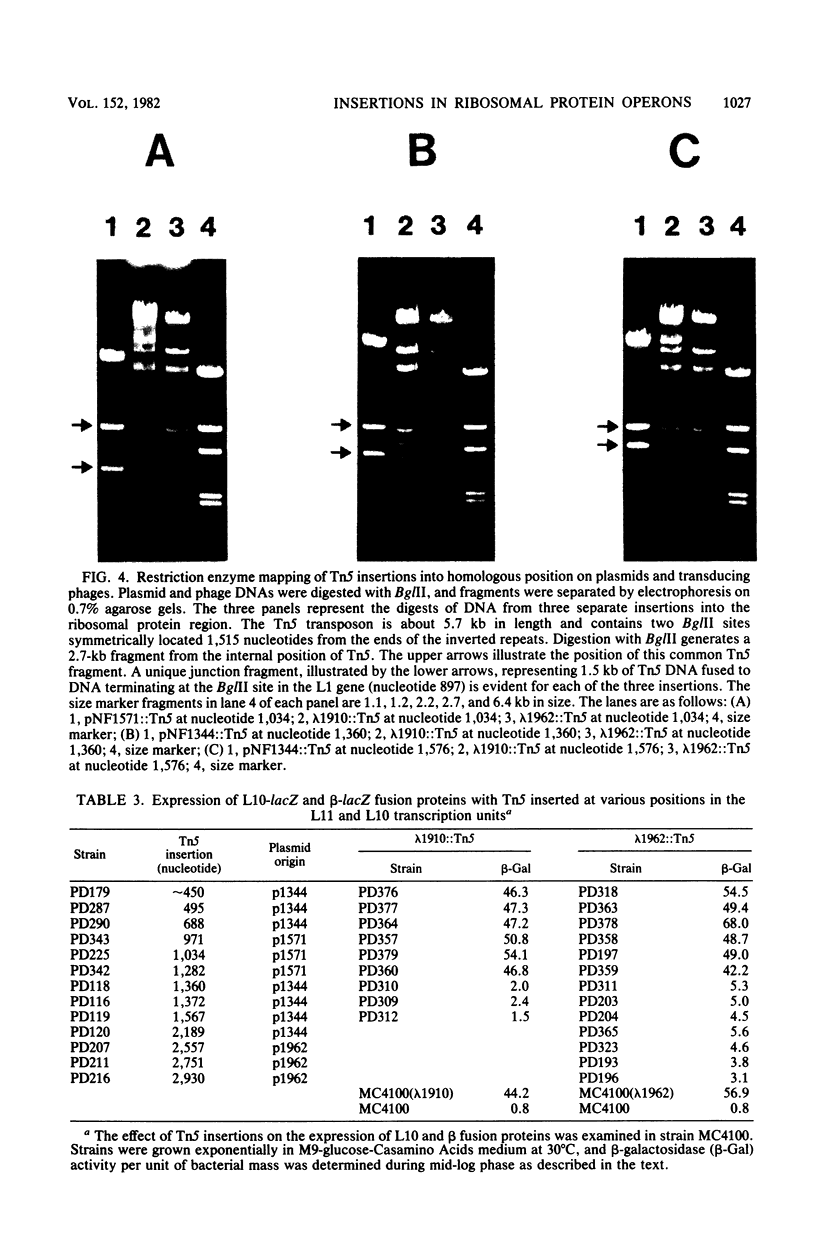
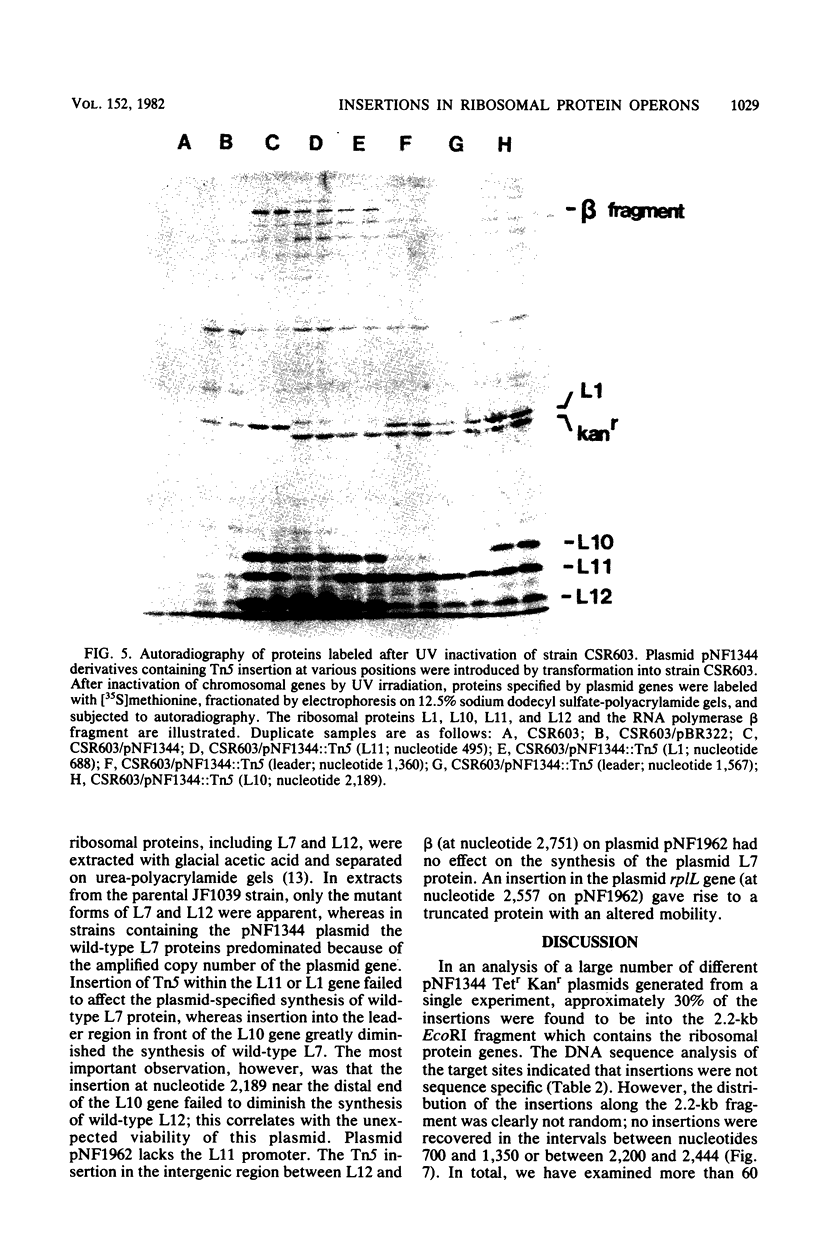
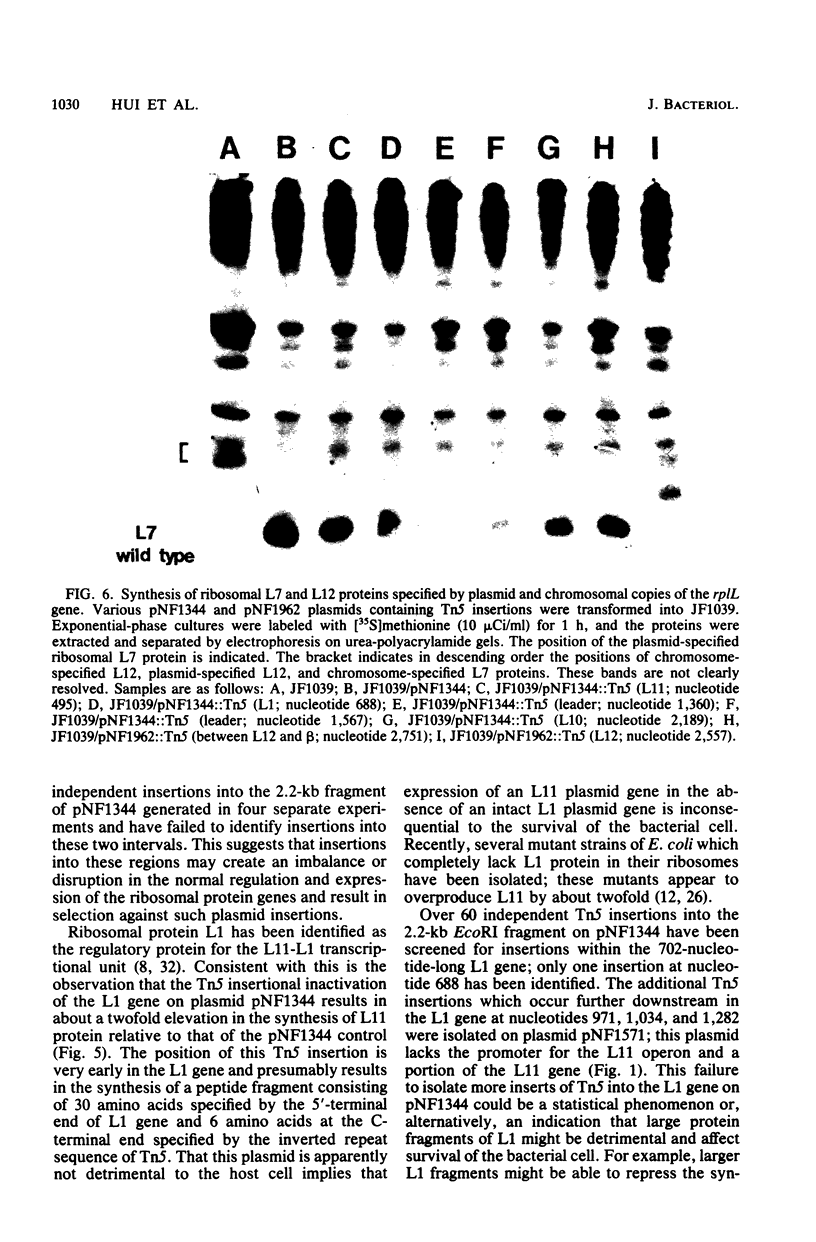
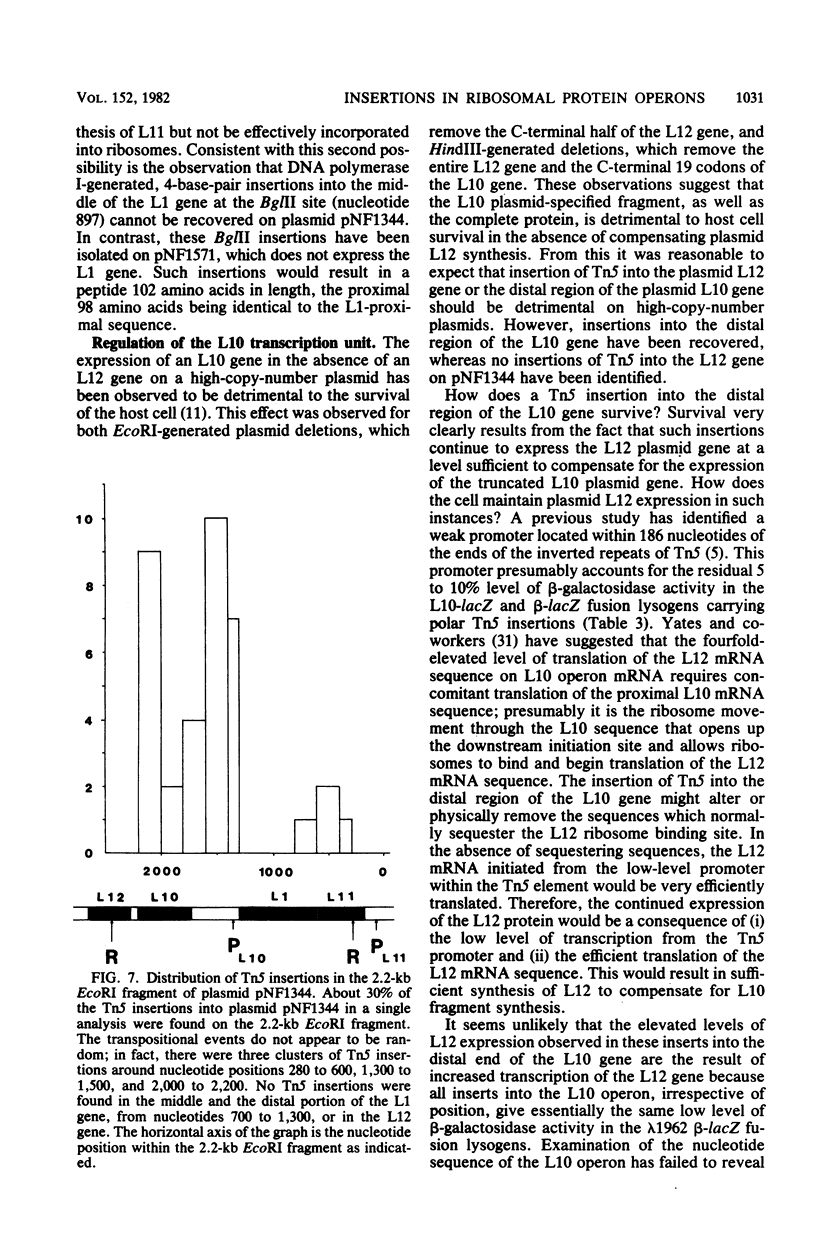
The genetic organization and interrelationships between the two ribosomal protein transcription units (the L11 and L10 operons) from near 89 min on the Escherichia coli chromosome were studied by using insertional mutations generated by the kanamycin-resistant transposable element Tn5. The polar effects of Tn5 insertions on the expression of the L11, L1, L10, and L12 ribosomal protein genes and the beta RNA polymerase subunit gene were examined (i) by the level of beta-galactosidase activity generated from L10-lacZ and beta-lacZ gene fusions, (ii) by direct sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the proteins specified by plasmid ribosomal protein genes in UV-irradiated maxicells, and (iii) by urea-polyacrylamide gel electrophoresis of plasmid- and chromosome-specified L12 protein. The results confirmed the organization of these genes into two transcription units as follows: PL11, rplK (L11), rplA (L1), PL10, rplJ (L10), rplL (L12), rpoB (beta). . .; they also localized the position of the PL10 promoter within an 80-nucleotide region near the end of the L1 gene. The results also support the idea that the translational regulatory proteins for the L11 and L10 operons are L1 and L10, respectively, and that the expression of the L12 gene is closely linked to L10 gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Characterization of promoter-cloning plasmids: analysis of operon structure in the rif region of Escherichia coli and isolation of an enhanced internal promoter mutant. J Bacteriol. 1980 Dec;144(3):904–916. doi: 10.1128/jb.144.3.904-916.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Matzura H. In vivo synthesis of a polycistronic messenger RNA for the ribosomal proteins L11, L1, L10 and L7/12 in Escherichia coli. Mol Gen Genet. 1981;183(2):277–282. doi: 10.1007/BF00270629. [DOI] [PubMed] [Google Scholar]
- Dean D., Nomura M. Feedback regulation of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3590–3594. doi: 10.1073/pnas.77.6.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Fill N. P. Transcriptional and post-transcriptional control of RNA polymerase and ribosomal protein genes cloned on composite ColE1 plasmids in the bacterium Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):7540–7547. [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Friesen J. D., Downing W. L., Dennis P. P. Post-transcriptional regulatory mutants in a ribosomal protein-RNA polymerase operon of E. coli. Cell. 1980 Apr;19(4):837–844. doi: 10.1016/0092-8674(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Nomura M. Regulation of ribosomal protein synthesis in an Escherichia coli mutant missing ribosomal protein L1. J Bacteriol. 1981 Mar;145(3):1445–1447. doi: 10.1128/jb.145.3.1445-1447.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Subramanian A. R. Selective separation procedure for determination of ribosomal proteins L7 and L12. Anal Biochem. 1975 Mar;64(1):121–129. doi: 10.1016/0003-2697(75)90413-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Expression of ribosomal genes in bacteria. Adv Genet. 1982;21:53–121. doi: 10.1016/s0065-2660(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Lindahl S., Yamamoto M., Nomura M. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J Mol Biol. 1977 Jan 5;109(1):23–47. doi: 10.1016/s0022-2836(77)80044-2. [DOI] [PubMed] [Google Scholar]
- Linn T., Goman M., Scaife J. G. Studies on the control of the genes for transcription and translation in Escherichia coli K12 I. tufB and rplA, K have separate promoters. J Mol Biol. 1979 Jun 5;130(4):405–420. doi: 10.1016/0022-2836(79)90431-5. [DOI] [PubMed] [Google Scholar]
- Linn T., Scaife J. Identification of a single promoter in E. coli for rplJ, rplL and rpoBC. Nature. 1978 Nov 2;276(5683):33–37. doi: 10.1038/276033a0. [DOI] [PubMed] [Google Scholar]
- Ma J. C., Newman A. J., Hayward R. S. Internal promoters of the rpoBC operon of Escherichia coli. Mol Gen Genet. 1981;184(3):548–550. doi: 10.1007/BF00352538. [DOI] [PubMed] [Google Scholar]
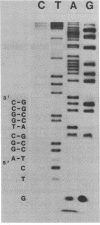
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Calos M. P., Galas D. J. Genetic and sequencing studies of the specificity of transposition into the lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):243–257. doi: 10.1101/sqb.1981.045.01.037. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Linn T. G., Hayward R. S. Evidence for co-transcription of the RNA polymerase genes rpoBC with a ribosomal protein gene of escherichia coli. Mol Gen Genet. 1979 Jan 31;169(2):195–204. doi: 10.1007/BF00271671. [DOI] [PubMed] [Google Scholar]
- Newman A., Hayward R. S. Cloning of DNA of the rpoBC operon from the chromosome of Escherichia coli K12. Mol Gen Genet. 1980 Feb;177(3):527–533. doi: 10.1007/BF00271493. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Hasenbank R., Dabbs E. R. Expression of the L11-L1 operon in mutants of Escherichia coli lacking the ribosomal proteins L1 or L11. Mol Gen Genet. 1981;181(2):164–168. doi: 10.1007/BF00268422. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R. Copies of proteins L7 and L12 and heterogeneity of the large subunit of Escherichia coli ribosome. J Mol Biol. 1975 Jun 15;95(1):1–8. doi: 10.1016/0022-2836(75)90330-7. [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Burgess R. R. Escherichia coli RNA polymerase binding and initiation of transcription on fragments of lambda rifd 18 DNA containing promoters for lambda genes and for rrnB, tufB, rplC,A, rplJ,L, and rpoB,C genes. Gene. 1979 Aug;6(4):331–365. doi: 10.1016/0378-1119(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Möller W., Laursen R., Wittmann-Liebold B. The primary structure of an acidic protein from 50-S ribosomes of Escherichia coli which is involved in GTP hydrolysis dependent on elongation factors G and T. Eur J Biochem. 1973 Apr 2;34(1):138–152. doi: 10.1111/j.1432-1033.1973.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Nomura M. Contranscription of genes for RNA polymerase subunits beta and beta' with genes for ribosomal proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3891–3895. doi: 10.1073/pnas.75.8.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Dean D., Strycharz W. A., Nomura M. E. coli ribosomal protein L10 inhibits translation of L10 and L7/L12 mRNAs by acting at a single site. Nature. 1981 Nov 12;294(5837):190–192. doi: 10.1038/294190a0. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. Feedback regulation of ribosomal protein synthesis in E. coli: localization of the mRNA target sites for repressor action of ribosomal protein L1. Cell. 1981 Apr;24(1):243–249. doi: 10.1016/0092-8674(81)90520-1. [DOI] [PubMed] [Google Scholar]