Abstract
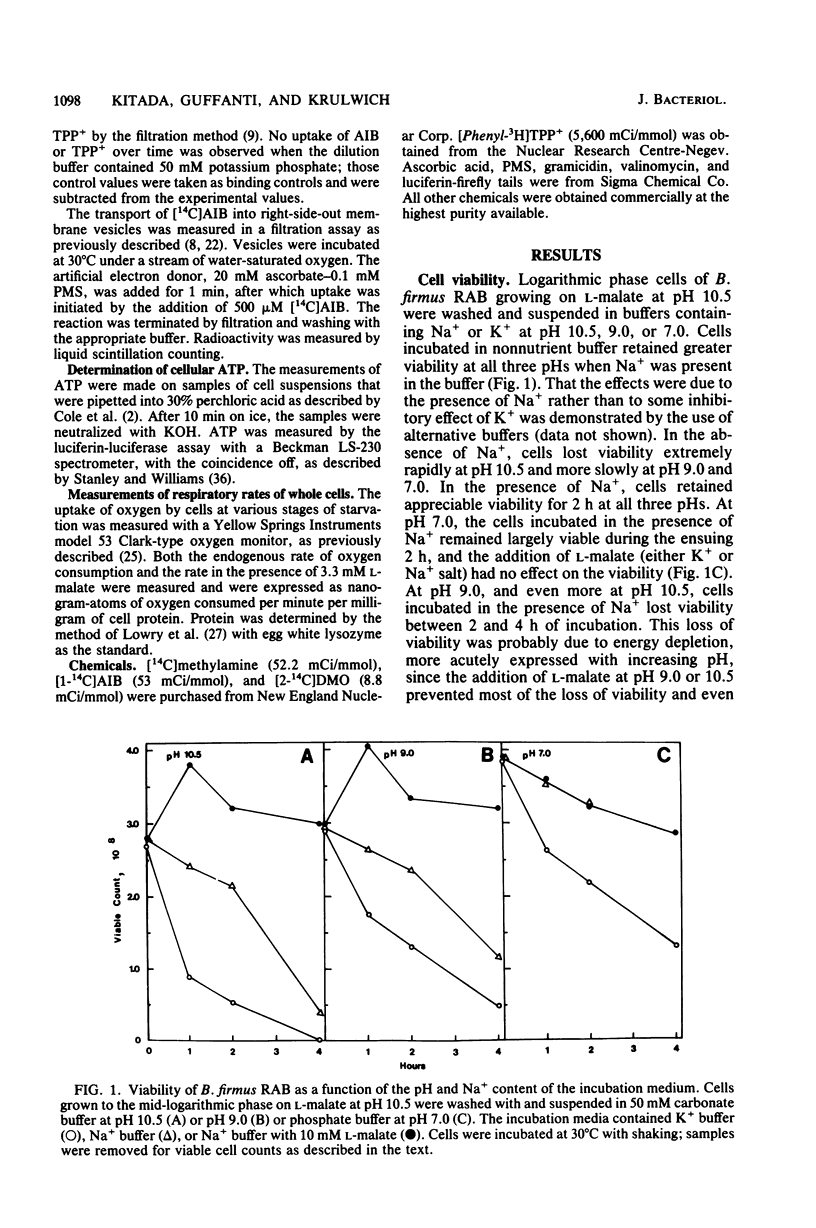
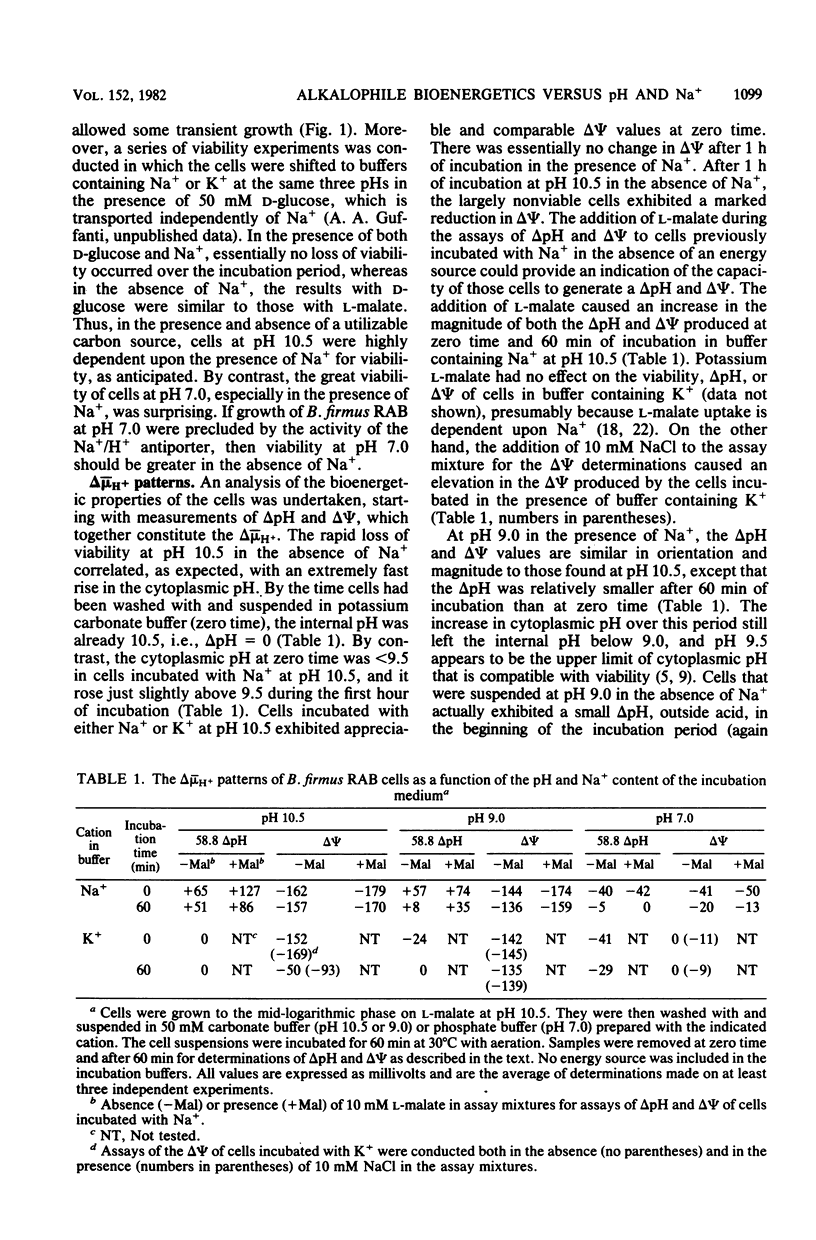
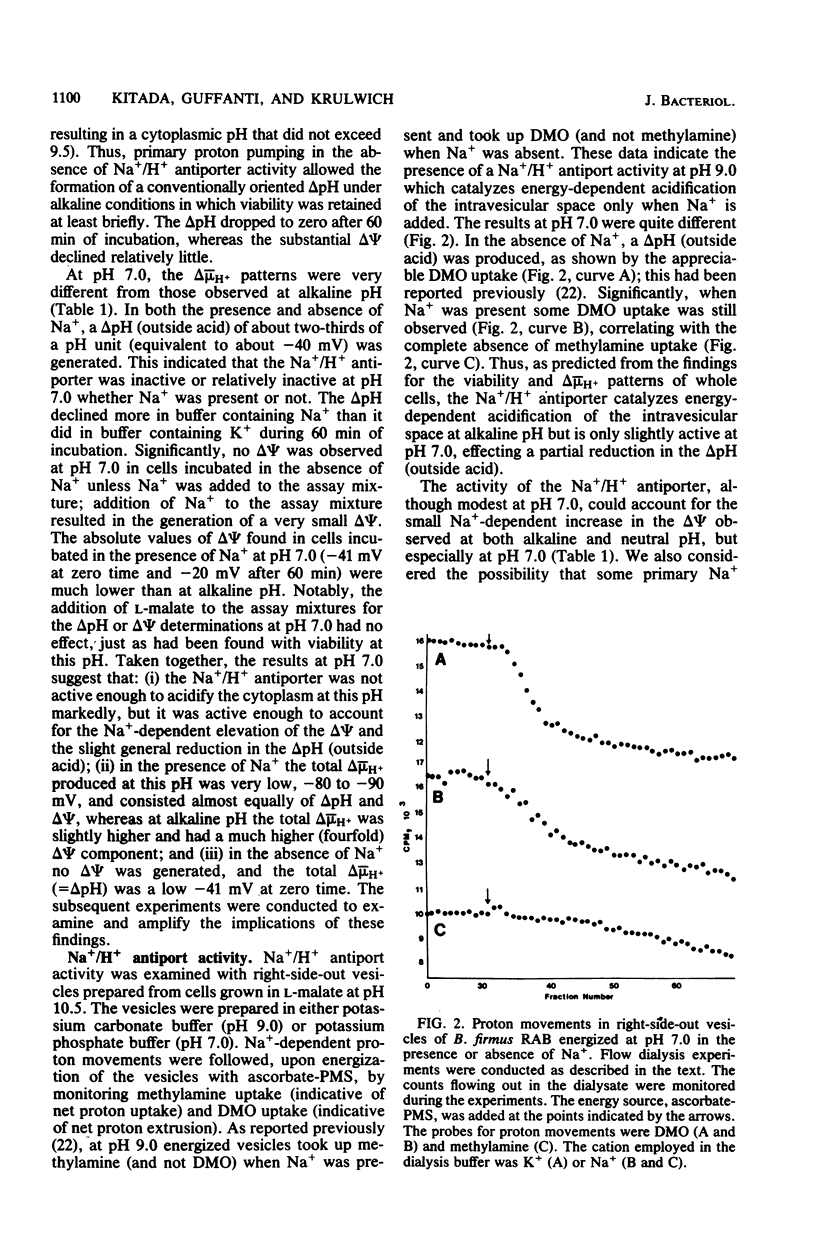
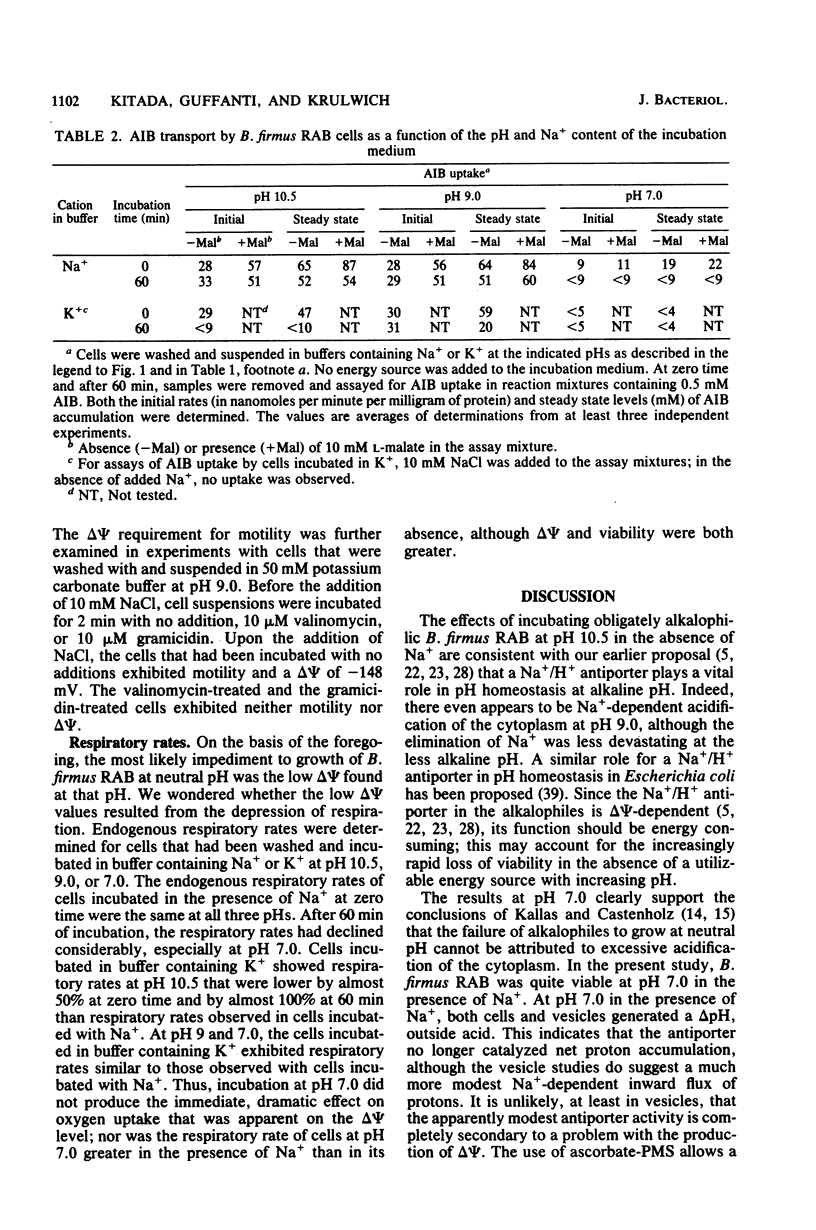
The bioenergetic properties and viability of obligately alkalophilic Bacillus firmus RAB have been examined upon incubation in alkaline and neutral buffers in the presence or absence of added Na+. At pH 10.5, cells incubated in the absence of Na+ exhibited an immediate rise in cytoplasmic pH from less than 9.5 to 10.5, and they lost viability very rapidly. Viability experiments in the presence or absence of an energy source further suggested that the Na+-dependent mechanism for pH homeostasis is an energy-requiring function. The Na+/H+ antiporter, which catalyzes the vital proton accumulation at alkaline pH, was only slightly operational at pH 7.0; both whole cells and vesicles exhibited net proton extrusion even in the presence of Na+. Moreover, cells incubated in buffer at pH 7.0 were actually more viable in the presence of Na+ than in its absence. Thus, the inability of B. firmus RAB to grow at neutral pH is not due to excessive acidification of the cytoplasm. Rather, the transmembrane electrical potential, delta psi, generated at pH 7.0 was found to be much lower than at alkaline pH. The very low delta psi compromised several cell functions, e.g., Na+/solute symport and motility, which in this and other alkalophiles specifically depend upon delta psi and Na+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Kaback H. R. Electrochemical proton gradient in Micrococcus lysodeikticus cells and membrane vesicles. J Bacteriol. 1980 May;142(2):651–658. doi: 10.1128/jb.142.2.651-658.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Blumenfeld H., Krulwich T. A. ATP synthesis by an uncoupler-resistant mutant of Bacillus megaterium. J Biol Chem. 1981 Aug 25;256(16):8416–8421. [PubMed] [Google Scholar]
- Guffanti A. A., Bornstein R. F., Krulwich T. A. Oxidative phosphorylation by membrane vesicles from Bacillus alcalophilus. Biochim Biophys Acta. 1981 May 13;635(3):619–630. doi: 10.1016/0005-2728(81)90118-3. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Cohn D. E., Kaback H. R., Krulwich T. A. Relationship between the Na+/H+ antiporter and Na+/substrate symport in Bacillus alcalophilus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1481–1484. doi: 10.1073/pnas.78.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-driven sodium pump in Streptococcus faecalis. Proc Natl Acad Sci U S A. 1982 May;79(9):2798–2802. doi: 10.1073/pnas.79.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse J. Constitutive inorganic pyrophosphatase of Escherichia coli. 1. Purification and catalytic properties. J Biol Chem. 1966 May 10;241(9):1938–1947. [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Internal pH and ATP-ADP pools in the cyanobacterium Synechococcus sp. during exposure to growth-inhibiting low pH. J Bacteriol. 1982 Jan;149(1):229–236. doi: 10.1128/jb.149.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Rapid transient growth at low pH in the cyanobacterium Synechococcus sp. J Bacteriol. 1982 Jan;149(1):237–246. doi: 10.1128/jb.149.1.237-246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium ion-stimulated alpha-[1-14C]aminoisobutyric acid uptake in alkalophilic Bacillus species. J Bacteriol. 1977 Sep;131(3):784–788. doi: 10.1128/jb.131.3.784-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium-ion stimulated amino acid uptake in membrane vesicles of alkalophilic Bacillus no. 8-1. J Biochem. 1980 Dec;88(6):1757–1764. doi: 10.1093/oxfordjournals.jbchem.a133150. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Van Brunt J., Harold F. M. ATP-linked calcium transport in cells and membrane vesicles of Streptococcus faecalis. J Biol Chem. 1978 Apr 10;253(7):2085–2092. [PubMed] [Google Scholar]
- Koyama N., Kiyomiya A., Nosoh Y. Na+-dependent uptake of amino acids by an alkalophilic Bacillus. FEBS Lett. 1976 Dec 15;72(1):77–78. doi: 10.1016/0014-5793(76)80816-2. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Bornstein R. F., Hoffstein J. A sodium requirement for growth, solute transport, and pH homeostasis in Bacillus firmus RAB. J Biol Chem. 1982 Feb 25;257(4):1885–1889. [PubMed] [Google Scholar]
- Krulwich T. A., Mandel K. G., Bornstein R. F., Guffanti A. A. A non-alkalophilic mutant of Bacillus alcalophilus lacks the Na+/H+ antiporter. Biochem Biophys Res Commun. 1979 Nov 14;91(1):58–62. doi: 10.1016/0006-291x(79)90582-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. J., Belkina S., Krulwich T. A. Alkalophiles have much higher cytochrome contents than conventional bacteria and than their own non-alkalophilic mutant derivatives. Biochem Biophys Res Commun. 1980 Jul 31;95(2):857–863. doi: 10.1016/0006-291x(80)90866-9. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Prince R. C., Dutton P. L., Knaff D. B., Krulwich T. A. The respiratory chain of Bacillus alcalophilus and its nonalkalophilic mutant derivative. J Biol Chem. 1981 Oct 25;256(20):10543–10549. [PubMed] [Google Scholar]
- Mandel K. G., Guffanti A. A., Krulwich T. A. Monovalent cation/proton antiporters in membrane vesicles from Bacillus alcalophilus. J Biol Chem. 1980 Aug 10;255(15):7391–7396. [PubMed] [Google Scholar]
- Matsuura S., Shioi J. I., Imae Y., Iida S. Characterization of the Bacillus subtilis motile system driven by an artificially created proton motive force. J Bacteriol. 1979 Oct;140(1):28–36. doi: 10.1128/jb.140.1.28-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Kihara M., Macnab R. M. Measurement of membrane potential in Bacillus subtilis: a comparison of lipophilic cations, rubidium ion, and a cyanine dye as probes. J Membr Biol. 1981;63(3):215–231. doi: 10.1007/BF01870983. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]
- Zilberstein D., Ophir I. J., Padan E., Schuldiner S. Na+ gradient-coupled porters of EScherichia coli share a common subunit. J Biol Chem. 1982 Apr 10;257(7):3692–3696. [PubMed] [Google Scholar]


