Abstract
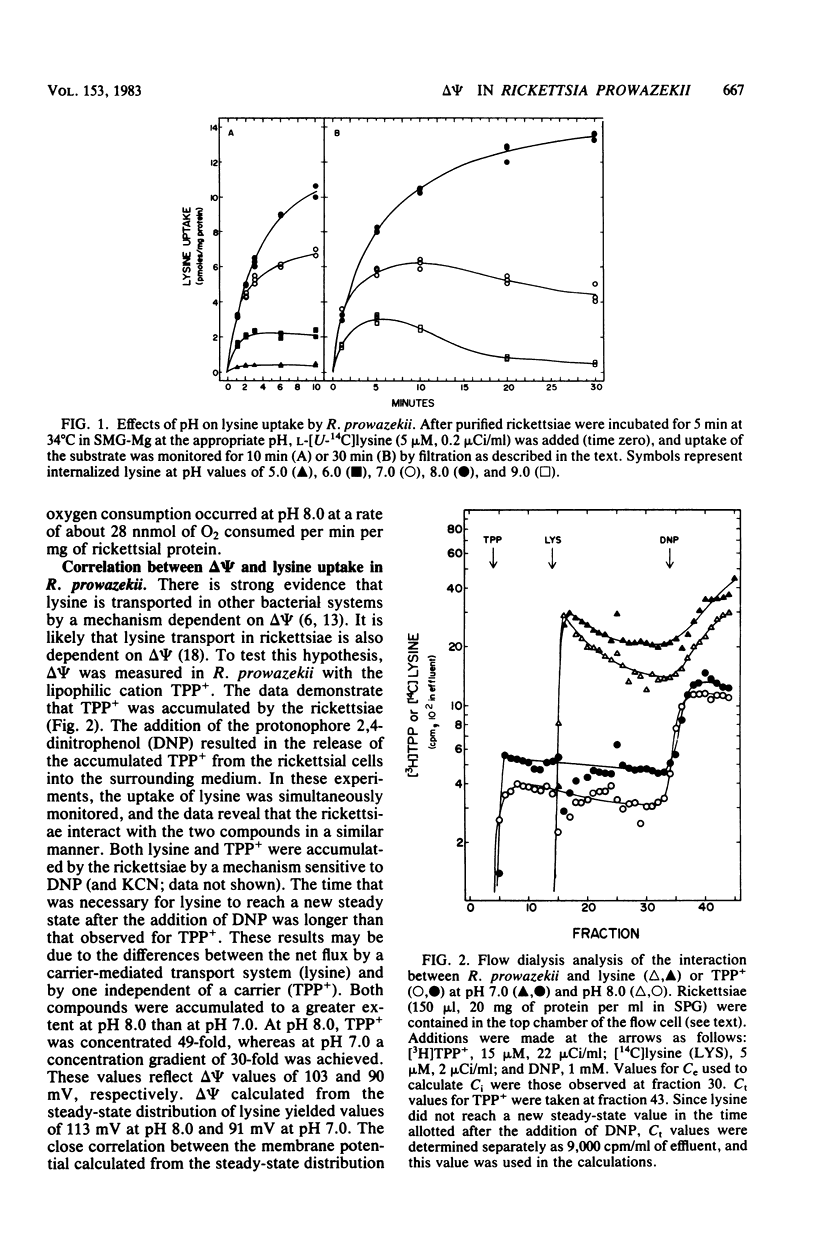
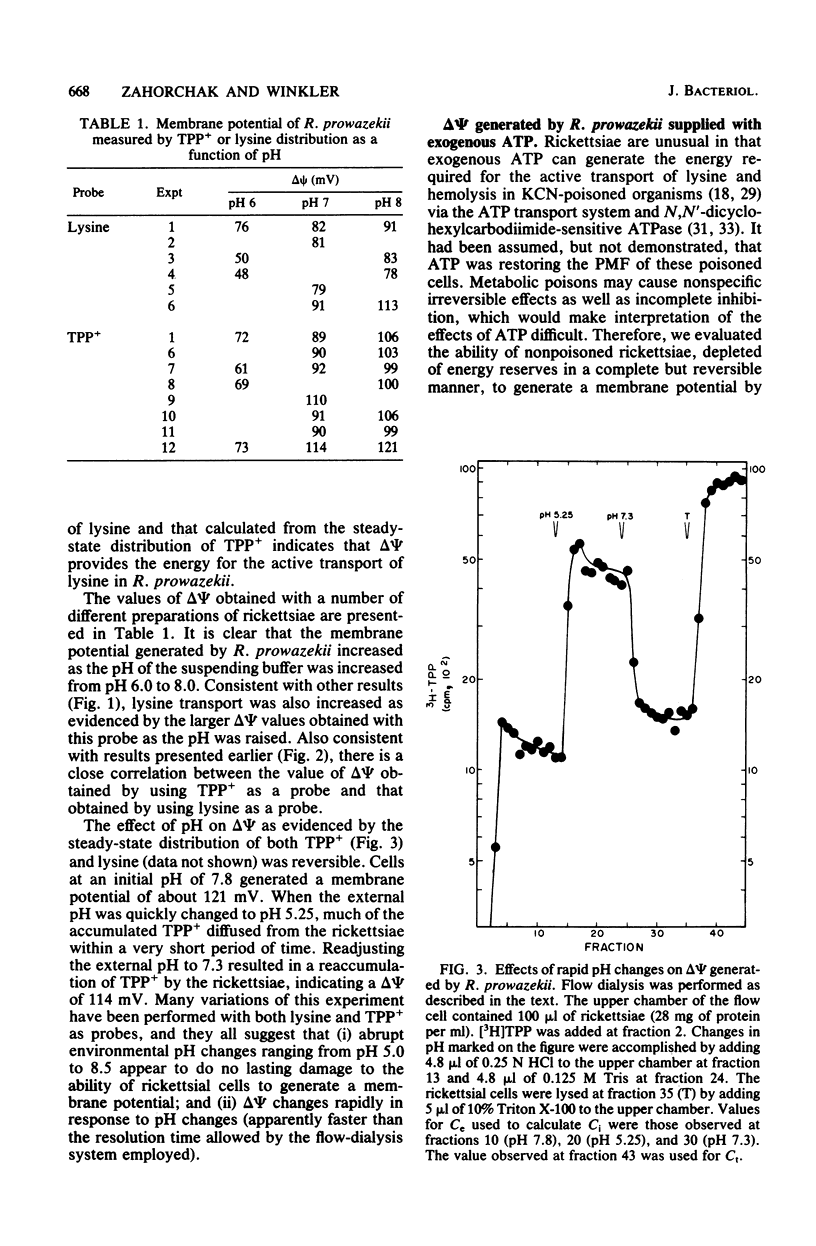
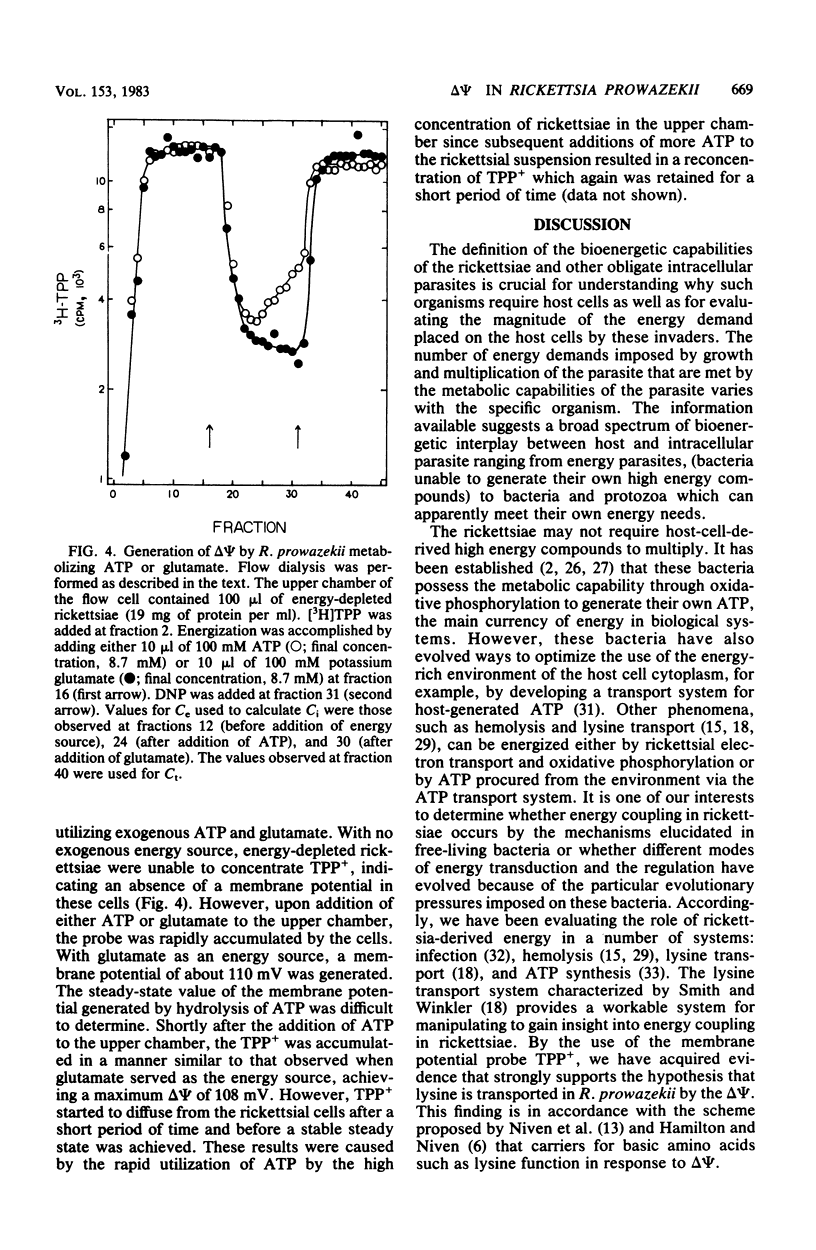
The transmembrane electrical potential (delta psi) generated by Rickettsia prowazekii metabolizing glutamic acid or ATP was determined by flow dialysis with the lipophilic cation tetraphenylphosphonium and with lysine. At pH 7.0, the rickettsiae generated a delta psi as measured by tetraphenylphosphonium distribution of 90 mV. Under similar conditions, cells of R.prowazekii concentrated lysine to a gradient indicating a delta psi of 90 mV. Energy-starved cells of R. prowazekii were able to utilize exogenously supplied ATP as well as glutamic acid to generate a delta psi of 110 mV at pH 8.0. Lysine transport was markedly affected by environmental pH, the optimum pH ranging from 8.0 to 8.5. delta psi as measured with tetraphenyl-phosphonium was similarly affected in this system, with values ranging from 70 mV at pH 6.0 to 100 mV at pH 8.0. Respiration rates were also affected by the external pH, with a maximum rate of 28 nmol of O2 consumed per min per mg of rickettsial protein occurring at pH 8.0. The pH effects were readily reversible and with a rapid onset.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maloney P. C. Coupling between H+ entry and ATP formation in Escherichia coli. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1496–1501. doi: 10.1016/0006-291x(78)91390-6. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Myers W. F., Provost P. J., Wisseman C. L., Jr Permeability properties of Rickettsia mooseri. J Bacteriol. 1967 Mar;93(3):950–960. doi: 10.1128/jb.93.3.950-960.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven D. F., Jeacocke R. E., Hamilton W. A. The membrane potential as the driving force for the accumulation of lysine by Staphylococcus aureus. FEBS Lett. 1973 Feb 1;29(3):248–252. doi: 10.1016/0014-5793(73)80030-4. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: effect of metabolic inhibitors upon hemolysis and adsorption. Infect Immun. 1973 Apr;7(4):550–555. doi: 10.1128/iai.7.4.550-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Characterization of a lysine-specific active transport system in Rickettsia prowazeki. J Bacteriol. 1977 Mar;129(3):1349–1355. doi: 10.1128/jb.129.3.1349-1355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Separation of inner and outer membranes of Rickettsia prowazeki and characterization of their polypeptide compositions. J Bacteriol. 1979 Feb;137(2):963–971. doi: 10.1128/jb.137.2.963-971.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial cell water and membrane permeability determined by a micro space technique. Appl Environ Microbiol. 1976 Jan;31(1):146–149. doi: 10.1128/aem.31.1.146-149.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]


