Abstract
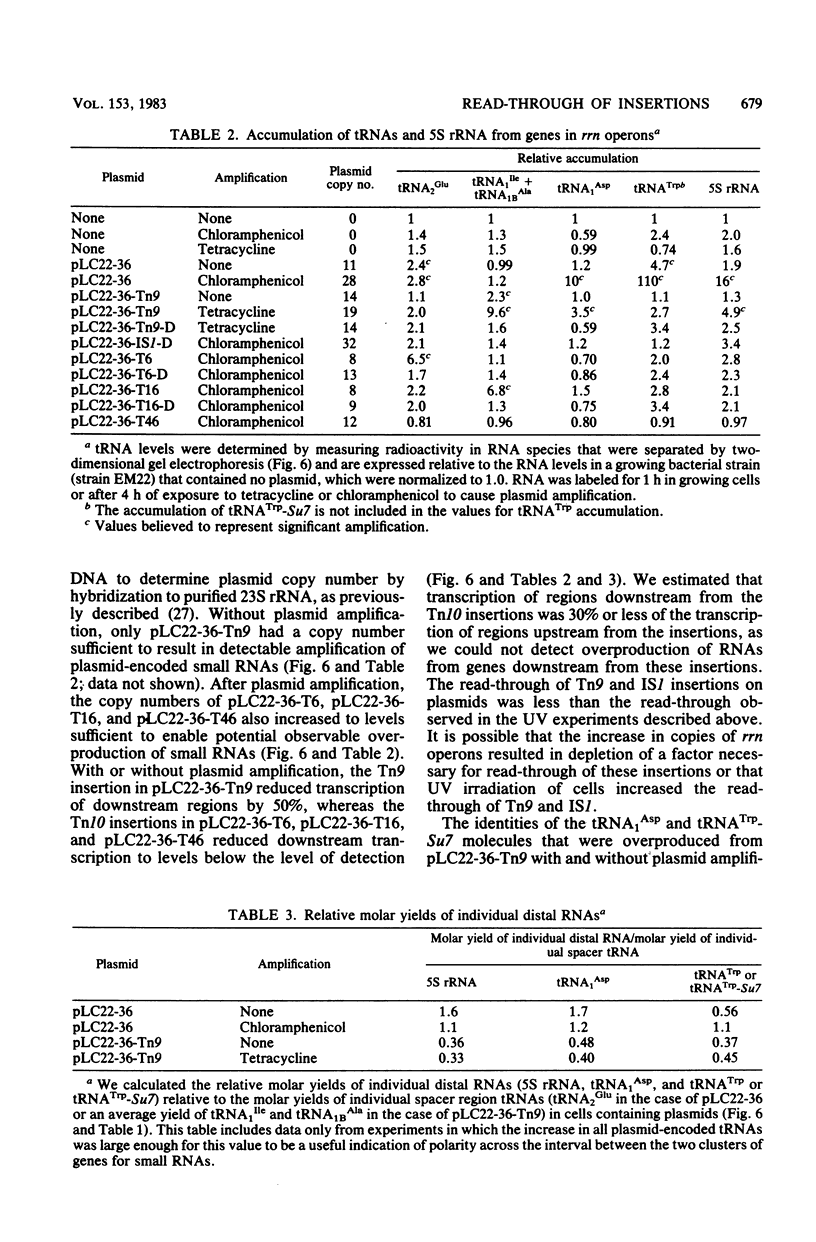
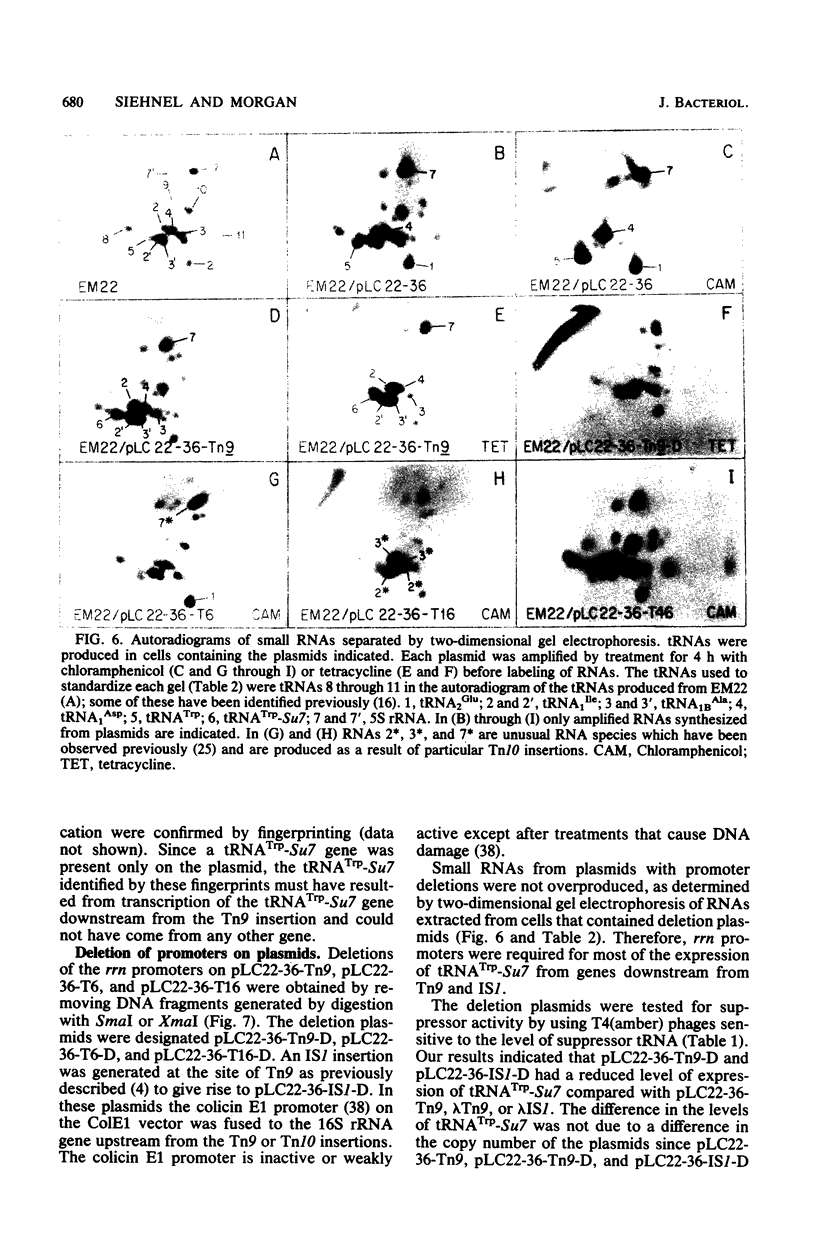
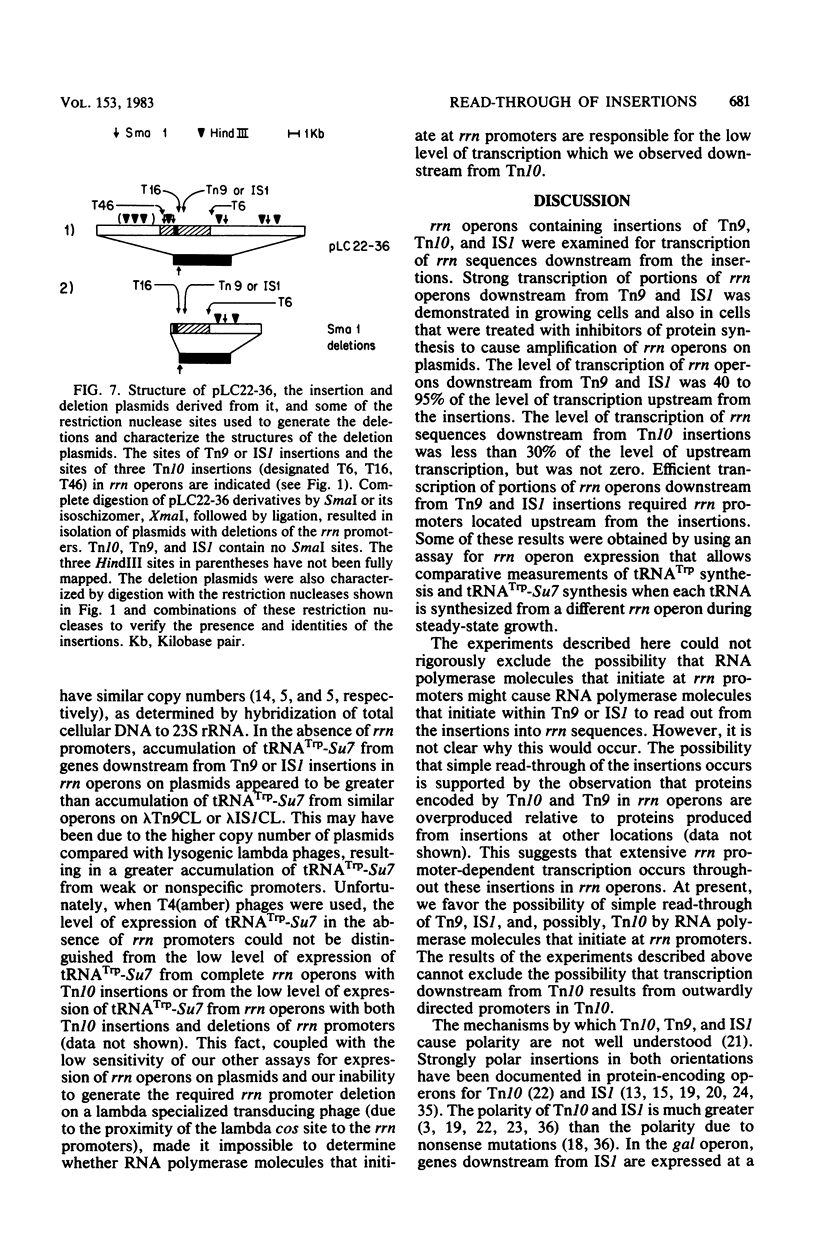
Transcription and translation are coupled in most Escherichia coli operons. As a consequence, ribosomes must be present on an mRNA molecule while transcription of the mRNA is in progress or else premature termination of transcription may result. This requirement is most clearly manifested when premature nonsense codons result in polarity in multicistronic operons. Polarity can also result from insertions of transposons and insertion sequences. However, since rRNA operons are not translated, some property of these operons must allow transcription to be uncoupled from translation. In this paper we demonstrate that transposon Tn9 and insertion sequence IS1 are nonpolar or incompletely polar in rRNA operons during normal growth. We also show that essentially all expression of rrn sequences distal to IS1 and Tn9 results from transcripts that originate at rRNA promoters. These results suggest either that rRNA operons possess some mechanism which reduces or prevents termination within rRNA operons or that Tn9 and IS1 can be very inefficient at blocking normal transcription. Insertions of Tn10 in rRNA operons are substantially but incompletely polar. We could not determine whether the residual downstream transcription observed results from promoters within Tn10 or from read-through of Tn10. We discuss the meaning of read-through of Tn9 and IS1 and the residual expression of genes downstream from Tn10 with regard to rRNA operon structure and previous experiments in which polarity of transposons or insertion sequences was observed in protein-encoding operons.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besemer J., Herpers M. Suppression of polarity of insertion mutations within the gal operon of E. coli. Mol Gen Genet. 1977 Mar 16;151(3):295–304. doi: 10.1007/BF00268793. [DOI] [PubMed] [Google Scholar]
- Brewster J. M., Morgan E. A. Tn9 and IS1 inserts in a ribosomal ribonucleic acid operon of Escherichia coli are incompletely polar. J Bacteriol. 1981 Dec;148(3):897–903. doi: 10.1128/jb.148.3.897-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calva E., Burgess R. R. Characterization of a rho-dependent termination site within the cro gene of bacteriophage lambda. J Biol Chem. 1980 Nov 25;255(22):11017–11022. [PubMed] [Google Scholar]
- Chandler M. G., Pritchard R. H. The effect of gene concentration and relative gene dosage on gene output in Escherichia coli. Mol Gen Genet. 1975;138(2):127–141. doi: 10.1007/BF02428117. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Kintner C., Lund E. Specific binding of tRNAMet to 23S rRNA of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1071–1075. doi: 10.1073/pnas.75.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 1981 Feb 11;9(3):563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I. Suppression of polarity in the gal operon by ultraviolet irradiation. J Bacteriol. 1980 Dec;144(3):1205–1207. doi: 10.1128/jb.144.3.1205-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- Gartland W. J., Sueoka N. Two interconvertible forms of tryptophanyl sRNA in E. coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):948–956. doi: 10.1073/pnas.55.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. J., Starlinger P., Brachet P. Two kinds of insertions in bacterial genes. Mol Gen Genet. 1972;119(3):191–206. doi: 10.1007/BF00333858. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Nomura M. Expression of spacer tRNA genes in ribosomal RNA transcription units carried by hybrid Col E1 plasmids in E. coli. Cell. 1977 Aug;11(4):779–793. doi: 10.1016/0092-8674(77)90291-4. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H. Polarity of amber mutations and suppressed amber mutations in the galactose operon of E. coli. Mol Gen Genet. 1967;100(3):283–295. doi: 10.1007/BF00381824. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. Strong-polar mutations in the transferase gene of the galactose operon in E.coli. Mol Gen Genet. 1967;100(3):296–306. doi: 10.1007/BF00381825. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Lindahl L., Fallon A. M., Nomura M. Some rRNA operons in E. coli have tRNA genes at their distal ends. Cell. 1978 Feb;13(2):335–344. doi: 10.1016/0092-8674(78)90202-7. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A. Insertions of Tn 10 into an E. coli ribosomal RNA operon are incompletely polar. Cell. 1980 Aug;21(1):257–265. doi: 10.1016/0092-8674(80)90133-6. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Nomura M. Deletion analysis of the expression of rRNA genes and associated tRNA genes carried by a lambda transducing bacteriophage. J Bacteriol. 1979 Jan;137(1):507–516. doi: 10.1128/jb.137.1.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T. J., Tessman E. S., Tessman I. Suppression of polar effects of nonsense mutations by ultraviolet irradiation. J Bacteriol. 1979 Apr;138(1):122–125. doi: 10.1128/jb.138.1.122-125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes O., Gottesman M., Adhya S. Suppression of polarity of insertion mutations in the gal operon and N mutations in bacteriophage lambda. J Bacteriol. 1976 Jun;126(3):1108–1112. doi: 10.1128/jb.126.3.1108-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Mutations caused by the insertion of genetic material into the galactose operon of Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):93–105. doi: 10.1016/0022-2836(69)90298-8. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., Riggs P. D., Botstein D. Genetics of bacteriophage P22. III. The late operon. Virology. 1980 Oct 15;106(1):82–91. doi: 10.1016/0042-6822(80)90223-8. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]