Abstract
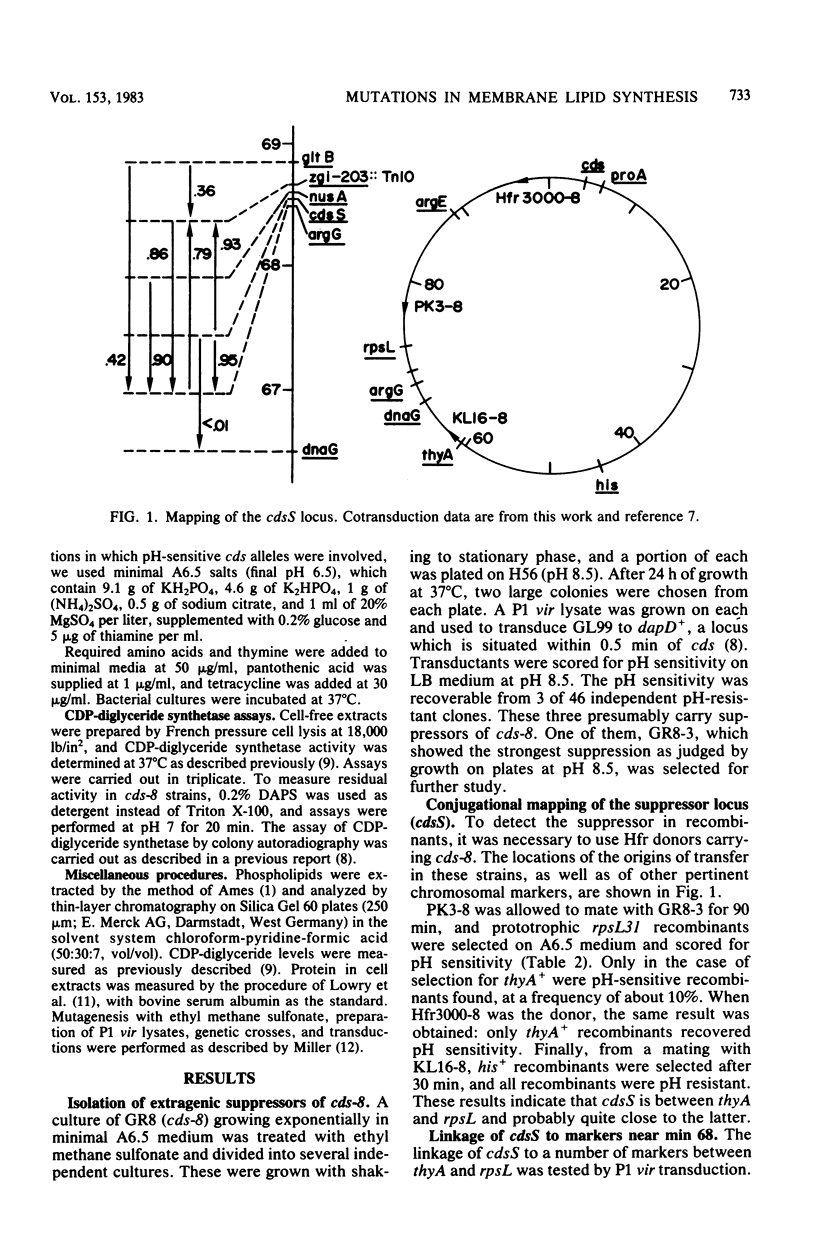
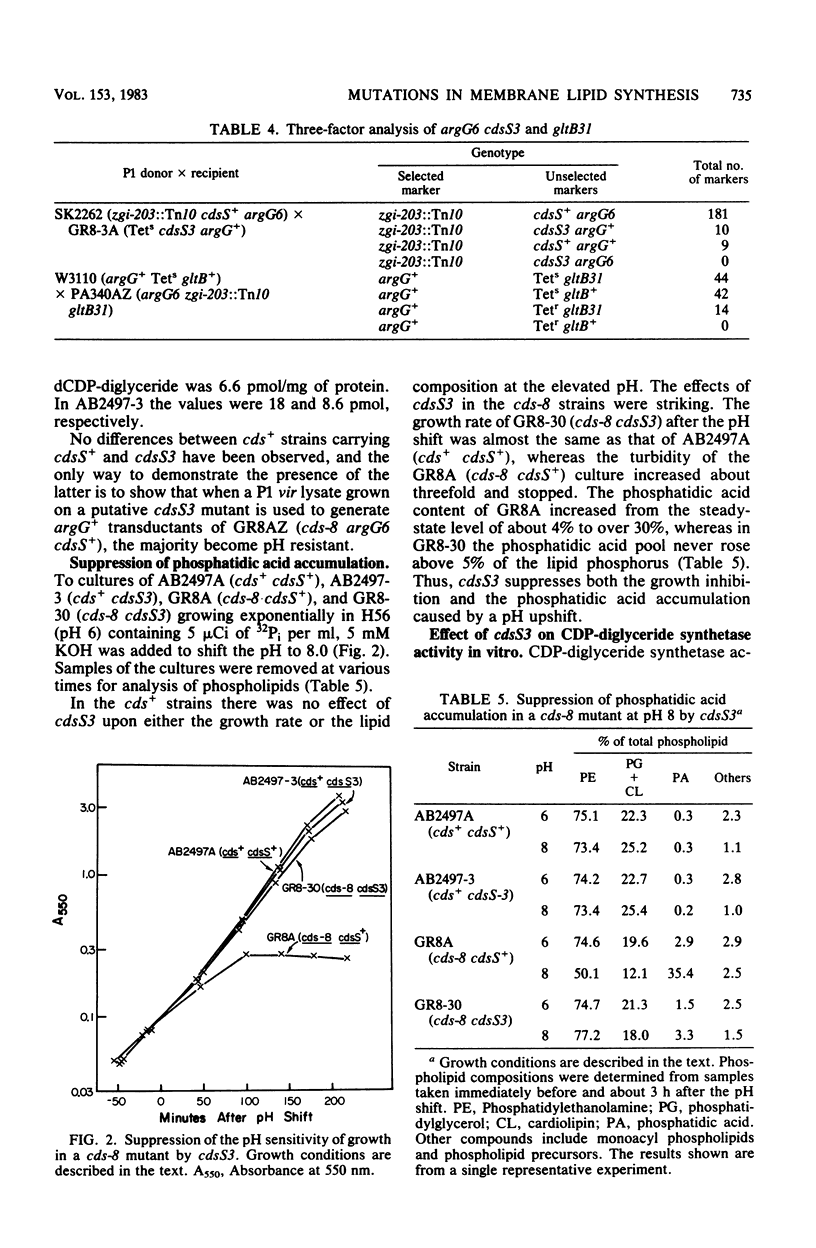
In Escherichia coli, mutations which lower the level of CDP-diglyceride synthetase are designated cds and map at min 4. The cds-8 mutation resulted in strikingly defective enzyme activity and also rendered cells pH sensitive for growth. Both the inhibition of growth and the massive accumulation of phosphatidic acid which occur in a cds-8 mutant at pH 8 were suppressed by mutations at a second locus, designated cdsS, which mapped between argG and gltB near min 68. The cdsS3 mutation by itself did not affect CDP-diglyceride synthetase activity in wild-type cells, but it caused a twofold stimulation of the residual activity present in strains harboring cds-8. Both the insensitivity to pH and the twofold stimulation of residual activity were lost by introduction of an F' strain carrying cdsS+ into a recA1 cds-8 cdsS3 host. When a culture of a cds-8 cdsS+ strain was shifted to pH 8, the residual specific activity of synthetase dropped by 75% within 100 min. In a cds-8 cdsS3 double mutant under the same conditions, the activity declined appreciably less, about to the level found in the cds-8 cdsS+ strain under permissive conditions (pH 6). Thus, it appears that mutations in the cdsS gene suppress the pH sensitivity of cds mutants by inhibiting the decay of residual CDP-diglyceride synthetase activity at the nonpermissive pH. The cdsS locus appears to be distinct from any known nonsense or missense suppressor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Cronan J. E., Jr Mutants of Escherichia coli defective in membrane phospholipid synthesis. Phenotypic suppression of sn-glycerol-3-phosphate acyltransferase Km mutants by loss of feedback inhibition of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1975 Sep 25;250(18):7153–7158. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Properties of wild type and Km defective sn-glycerol-3-phosphate acyltransferase activities. J Biol Chem. 1975 Sep 25;250(18):7147–7152. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J. R., Bell R. M. Biosynthesis in Escherichia coli of sn-glycerol 3-phosphate, a precursor of phospholipid. Kinetic characterization of wild type and feedback-resistant forms of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1978 Sep 25;253(18):6354–6363. [PubMed] [Google Scholar]
- Friedman D. I., Baron L. S. Genetic characterization of a bacterial locus involved in the activity of the N function of phage lambda. Virology. 1974 Mar;58(1):141–148. doi: 10.1016/0042-6822(74)90149-4. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Leonard J. M., Raetz C. R. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980 Feb 25;255(4):1623–1629. [PubMed] [Google Scholar]
- Ganong B. R., Raetz C. R. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982 Jan 10;257(1):389–394. [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Foulds J. Envelope composition and antibiotic hypersensitivity of Escherichia coli mutants defective in phosphatidylserine synthetase. J Biol Chem. 1977 Aug 25;252(16):5911–5915. [PubMed] [Google Scholar]


