Abstract
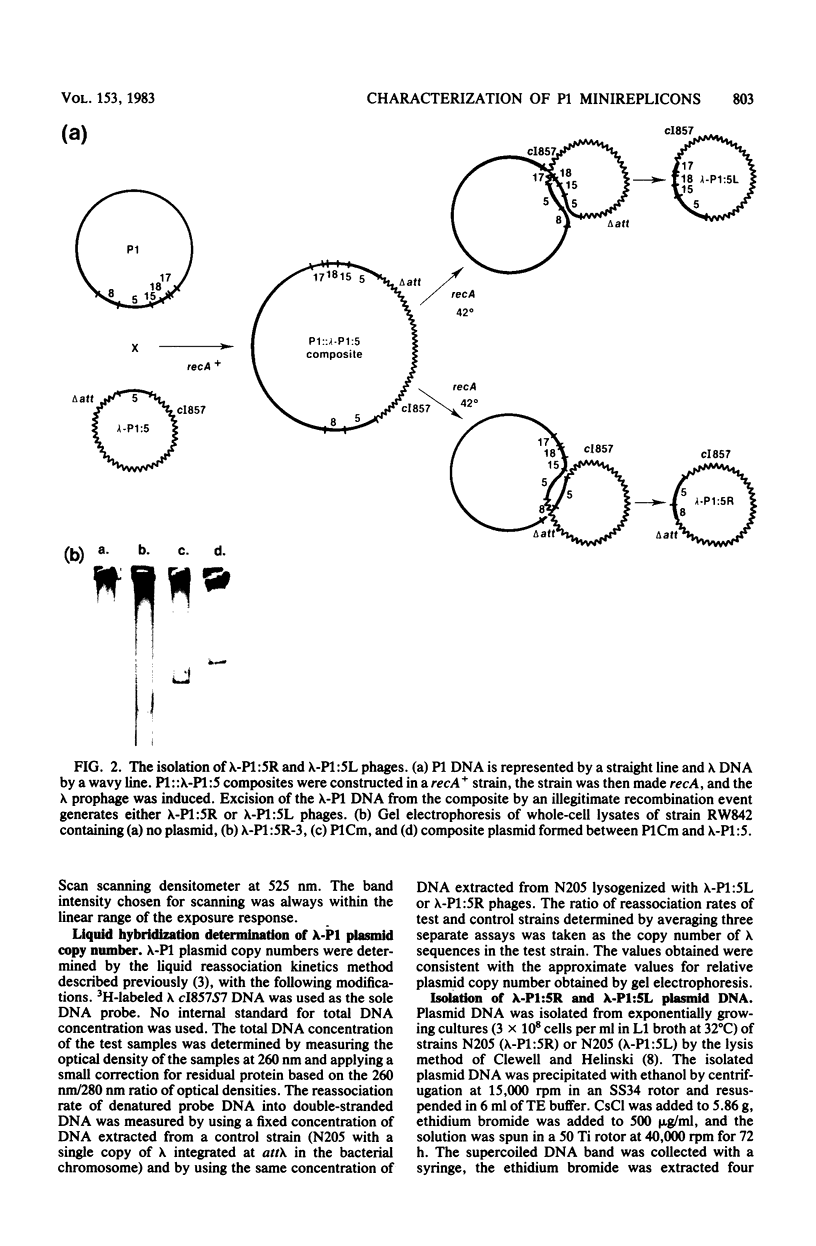
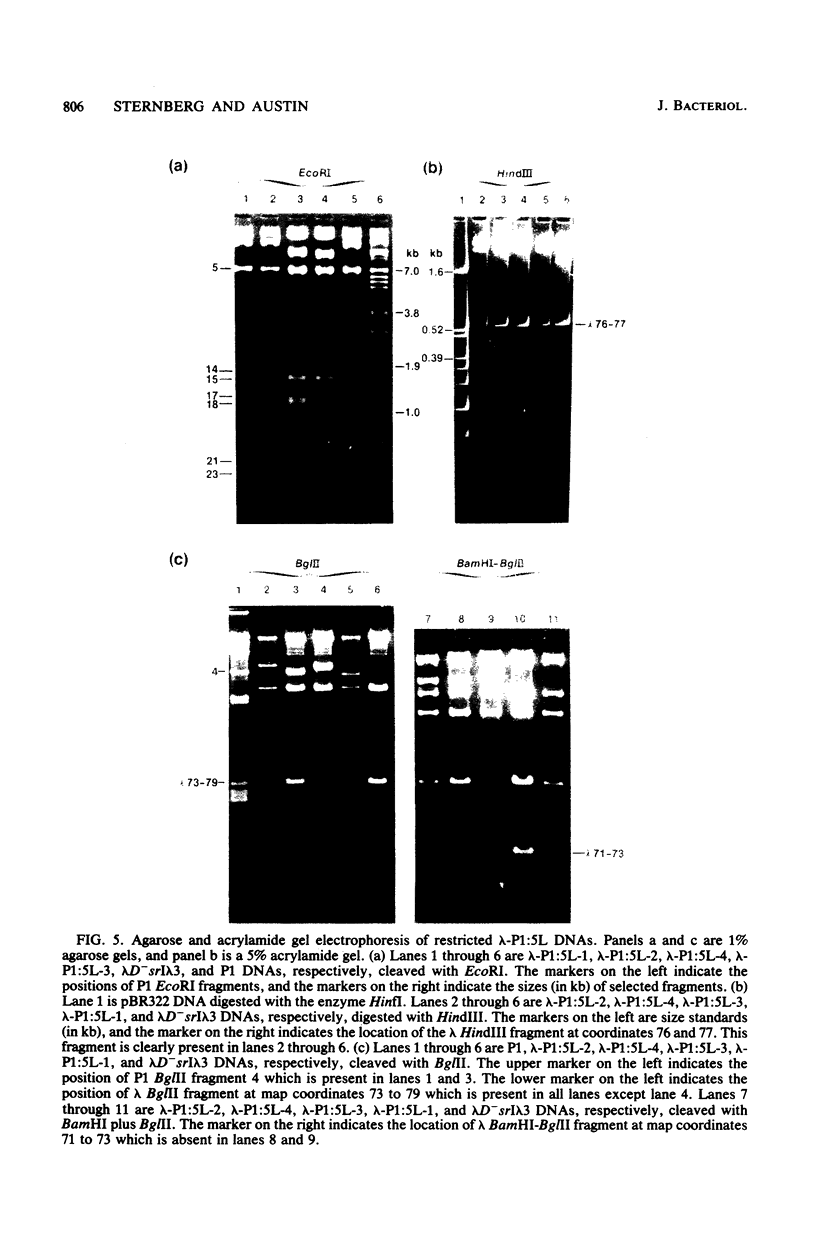
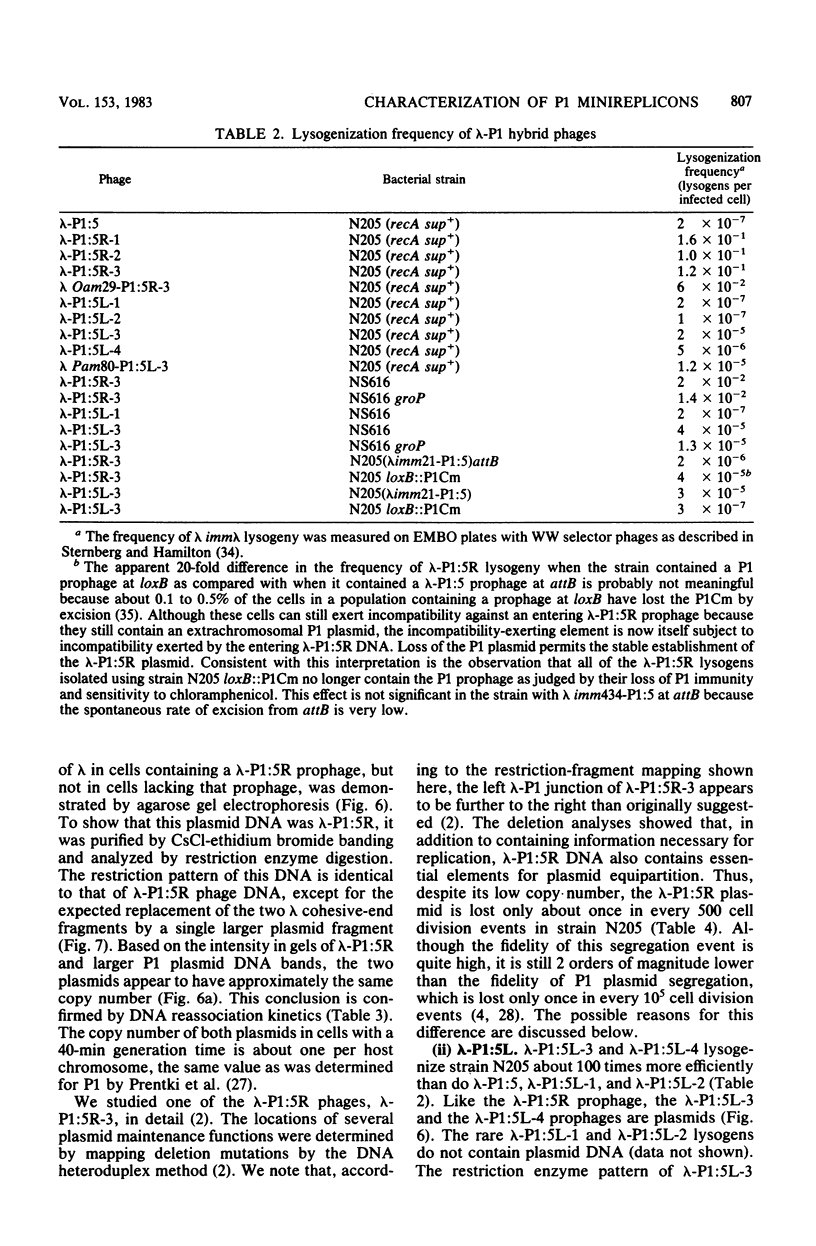
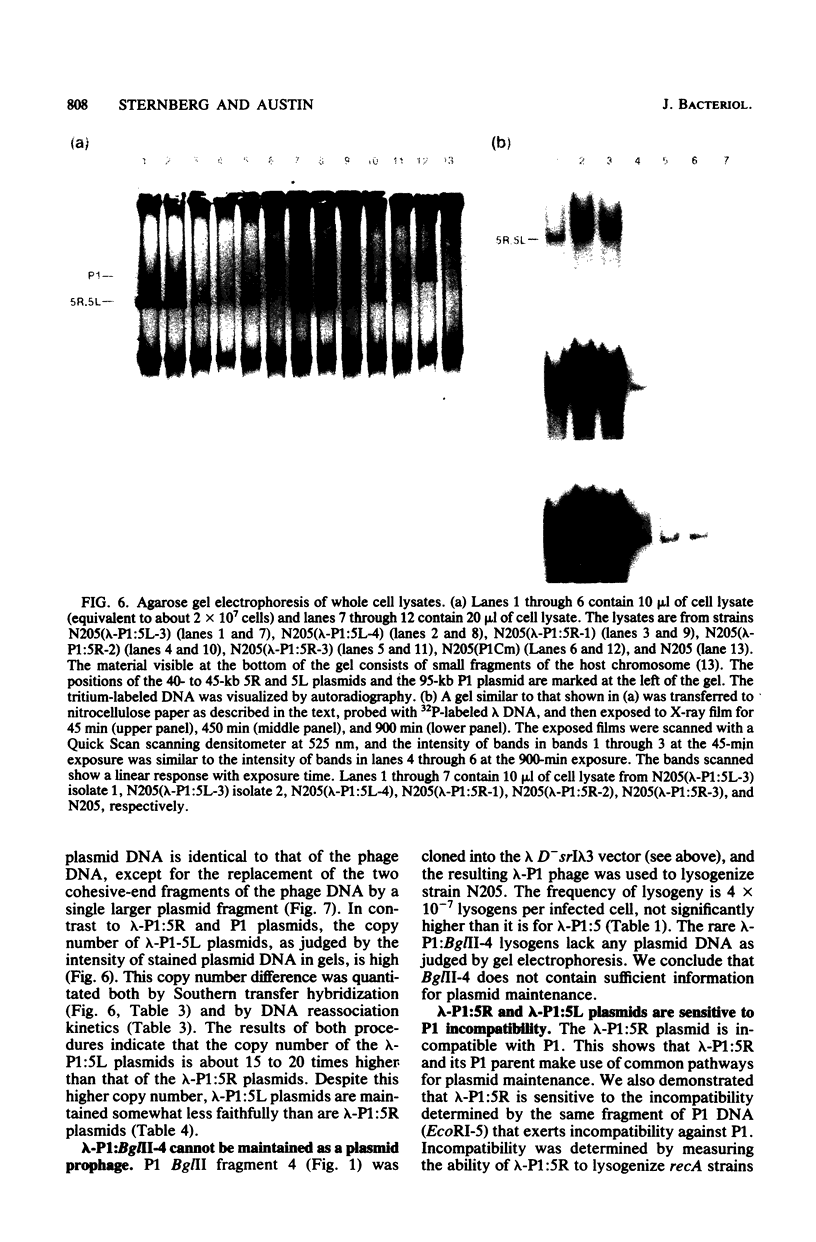
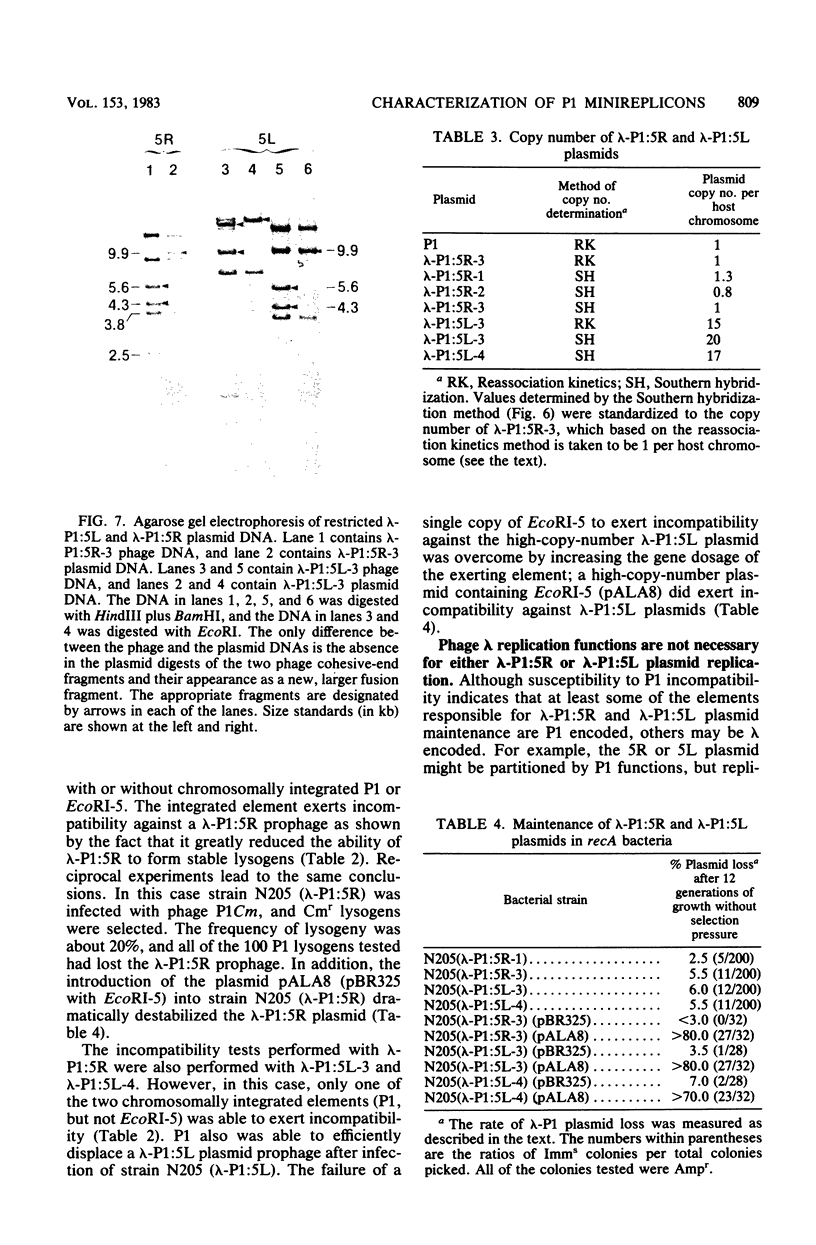
We have isolated two new classes of P1 miniplasmids, called lambda-P1:5R and lambda-P1:5L, by the in vivo extension of a cloned P1 fragment, EcoRI-5, which by itself is not capable of plasmid replication. The lambda-P1:5R plasmids contain EcoRI-5 plus a variable portion of the adjacent P1 EcoRI fragment 8. They have a copy number like that of P1 (about 1 per host chromosome), are faithfully segregated at cell division, and are subject to incompatibility exerted by either a single copy of P1 or a single copy of EcoRI-5. In contrast, the lambda-P1:5L plasmids contain EcoRI-5 and a portion of adjacent P1 DNA that includes at least P1 EcoRI fragments 15, 18, and 23 and a part of fragment 17. These plasmids have a copy number of about 15 per cell chromosome. Despite this they are segregated to daughter cells somewhat less faithfully than are lambda-P1:5R plasmids. They are sensitive to incompatibility exerted by a single copy of P1, but not to incompatibility exerted by a single copy of EcoRI-5. lambda-P1:5L plasmids are, however, sensitive to incompatibility exerted by multiple copies of EcoRI-5. These results show that the relative copy numbers of exerting and responding elements are important for the incompatibility phenotype and strongly suggest that lambda-P1:5L plasmids lack a repressor of replication that can be supplied in trans from P1 but not from EcoRI fragment 5. We suggest that P1 normally uses the 5R replicon and that the 5L replicon may be a backup system that ensures plasmid maintenance should the primary replication event fail to initiate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Hart F., Abeles A., Sternberg N. Genetic and physical map of a P1 miniplasmid. J Bacteriol. 1982 Oct;152(1):63–71. doi: 10.1128/jb.152.1.63-71.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Sternberg N., Yarmolinsky M. Miniplasmids of bacteriophage P1. I. Stringent plasmid replication does not require elements that regulate the lytic cycle. J Mol Biol. 1978 Apr 5;120(2):297–309. doi: 10.1016/0022-2836(78)90069-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Ziese M., Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981 Sep;25(3):729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
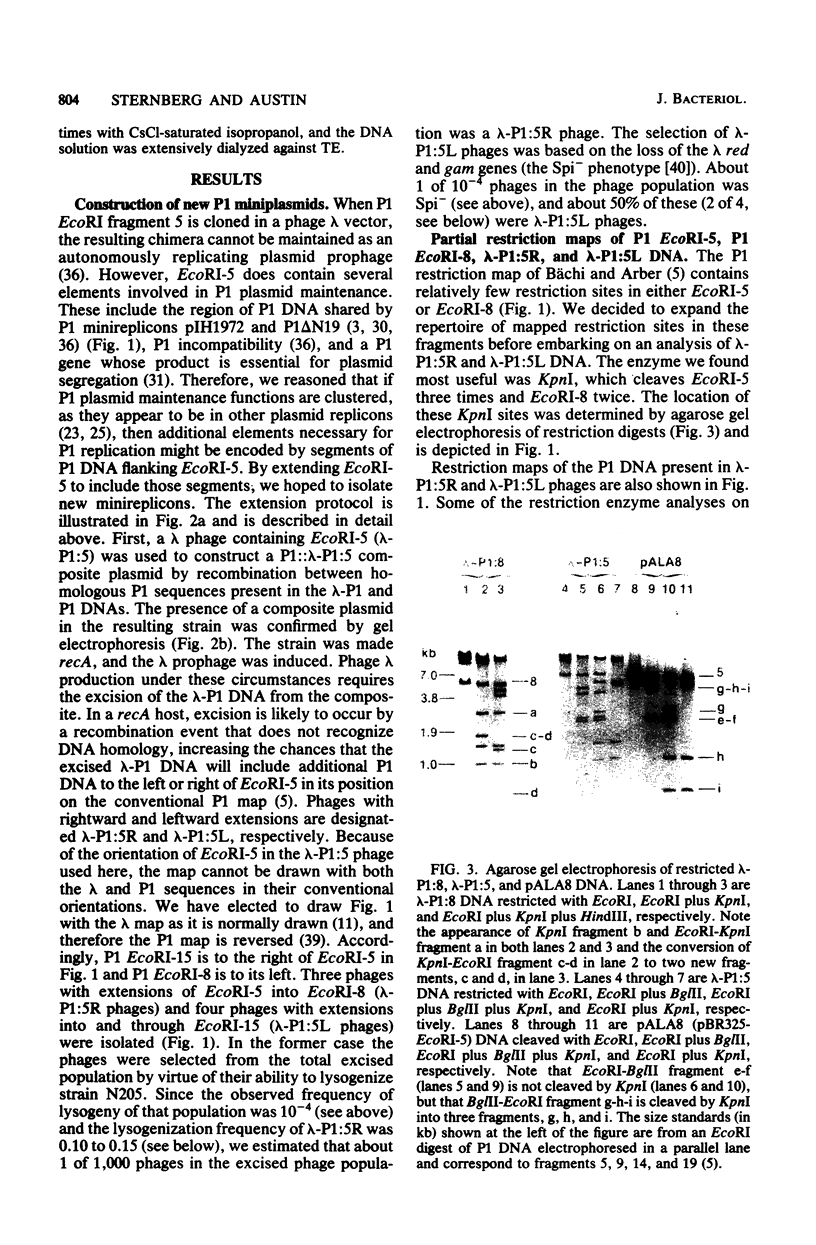
- Bächi B., Arber W. Physical mapping of BglII, BamHI, EcoRI, HindIII and PstI restriction fragments of bacteriophage P1 DNA. Mol Gen Genet. 1977 Jun 24;153(3):311–324. doi: 10.1007/BF00431596. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Danbara H., Timmis J. K., Lurz R., Timmis K. N. Plasmid replication functions: two distinct segments of plasmid R1, RepA and RepD, express incompatibility and are capable of autonomous replication. J Bacteriol. 1980 Dec;144(3):1126–1138. doi: 10.1128/jb.144.3.1126-1138.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
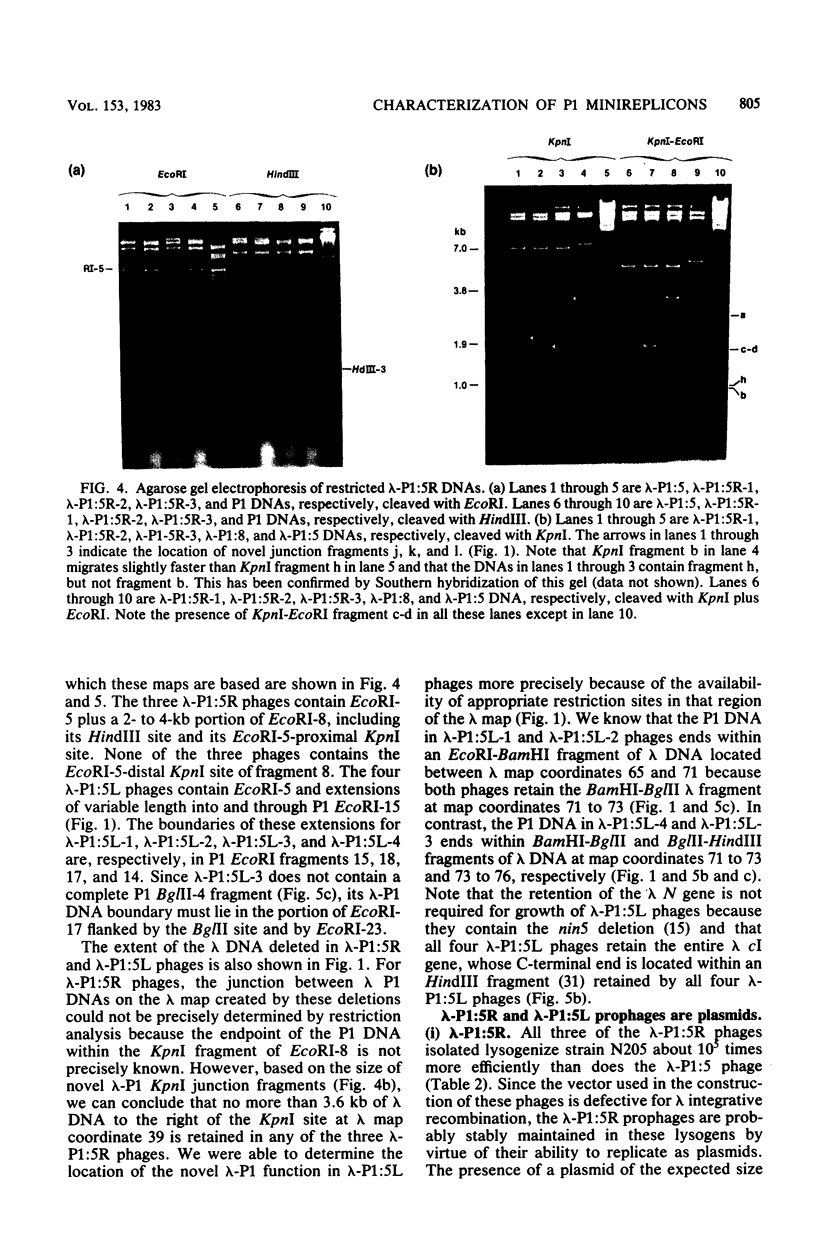
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Henning U. Tyrosine-incorporating amber suppressors in Escherichia coli K12. J Mol Biol. 1968 Apr 14;33(1):327–329. doi: 10.1016/0022-2836(68)90300-8. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda. Virology. 1976 Jul 1;72(1):147–153. doi: 10.1016/0042-6822(76)90319-6. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- KONDO E., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. IV. ACTIVE TRANSDUCING BACTERIOPHAGE P1 CM PRODUCED BY THE COMBINATION OF R FACTOR WITH BACTERIOPHAGE P1. J Bacteriol. 1964 Nov;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Gardner R. C. Second EcoRI fragment of F capable of self-replication. J Bacteriol. 1979 Jul;139(1):141–151. doi: 10.1128/jb.139.1.141-151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. E. Replication and incompatibility of F and plasmids in the IncFI Group. Plasmid. 1981 Jan;5(1):100–126. doi: 10.1016/0147-619x(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Uhlin B. E., Gustafsson P., Nordström K. Clustering of genes involved in replication, copy number control, incompatibility, and stable maintenance of the resistance plasmid R1drd-19. J Bacteriol. 1979 Apr;138(1):70–79. doi: 10.1128/jb.138.1.70-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mural R. J., Chesney R. H., Vapnek D., Kropf M. M., Scott J. R. Isolation and characterization of cloned fragments of bacteriophage P1 DNA. Virology. 1979 Mar;93(2):387–397. doi: 10.1016/0042-6822(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Prentki P., Chandler M., Caro L. Replication of prophage P1 during the cell cycle of Escherichia coli. Mol Gen Genet. 1977 Mar 28;152(1):71–76. doi: 10.1007/BF00264942. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sauer R. T. DNA sequence of the bacteriophage gama cI gene. Nature. 1978 Nov 16;276(5685):301–302. doi: 10.1038/276301a0. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Geller T., Hertman I. Identification of the P1 compatibility and plasmid maintenance locus by a mini P1/ac+-plasmid. Virology. 1979 Jul 15;96(1):32–37. doi: 10.1016/0042-6822(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Som T., Sternberg N., Austin S. A nonsense mutation in bacteriophage P1 eliminates the synthesis of a protein required for normal plasmid maintenance. Plasmid. 1981 Mar;5(2):150–160. doi: 10.1016/0147-619x(81)90016-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology. 1979 Jul 15;96(1):129–142. doi: 10.1016/0042-6822(79)90179-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D., Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol. 1981 Aug 25;150(4):487–507. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Powers M., Yarmolinsky M., Austin S. Group Y incompatibility and copy control of P1 prophage. Plasmid. 1981 Mar;5(2):138–149. doi: 10.1016/0147-619x(81)90015-9. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]