Abstract
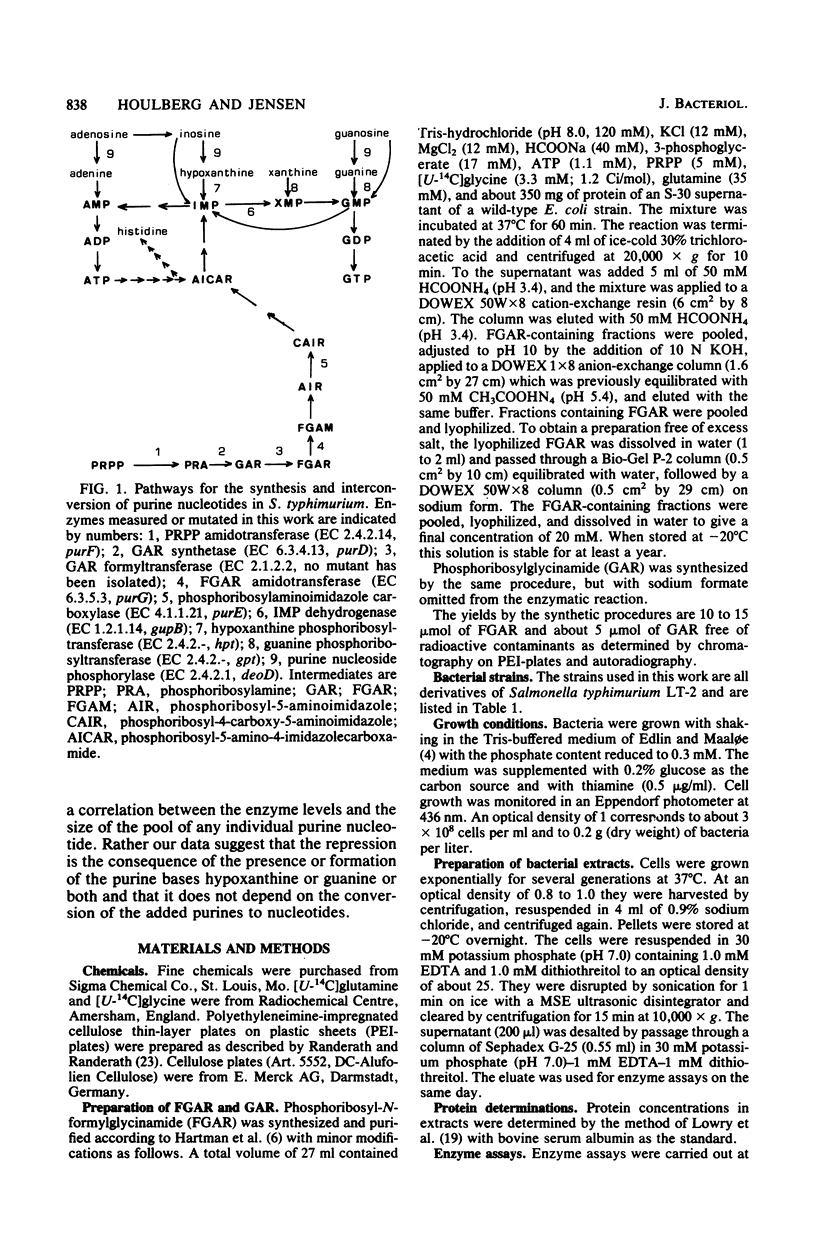
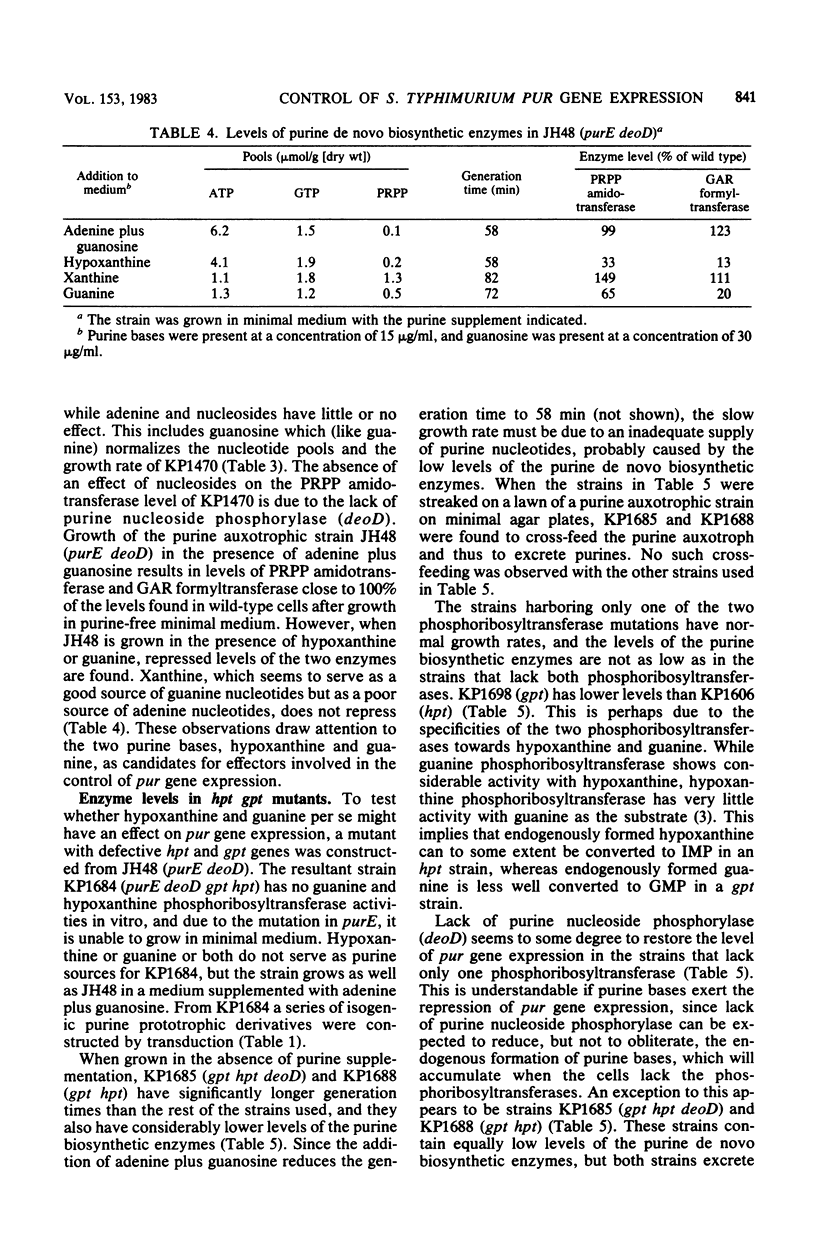
Data are presented which indicate that the repression of pur gene expression seen after the addition of preformed purines to cultures of Salmonella typhimurium is the consequence of the presence or the formation of the purine bases, hypoxanthine and guanine. This conclusion is based on the following observations. First, it was impossible to find a correlation between the size of any individual purine nucleotide pool and the level of the first four enzymes in the de novo biosynthetic pathway. Second, adenine plus guanosine served as a perfect source of purine nucleotides, but their presence caused no repression of pur gene expression if the cells lacked purine nucleoside phosphorylase activity. This enzyme is needed to convert adenine and guanosine to hypoxanthine and guanine, but not for their conversion to nucleotides. Third, addition of guanine to a strain lacking guanine phosphoribosyltransferase (gpt) resulted in a repression of the level of the purine de novo biosynthetic enzymes, a reduction of the growth rate, and a fall in the pools of ATP and GTP. Addition of hypoxanthine to a strain lacking hypoxanthine phosphoribosyltransferase (hpt) had a similar, although weaker, effect. If the cells lacked both hypoxanthine and guanine phosphoribosyltransferases (hpt gpt), their basal level of the purine de novo biosynthetic enzymes was repressed in minimal medium. Such cells grow slower than wild-type cells and excrete purines, probably due to the inability to salvage endogenously formed hypoxanthine and guanine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN J. M., HARTMAN S. C., LEVENBERG B. Biosynthesis of the purines. XI. Structure, enzymatic synthesis, and metabolism of glycinamide ribotide and (alpha-N-formyl)-glycinamide ribotide. J Biol Chem. 1956 Aug;221(2):1057–1070. [PubMed] [Google Scholar]
- Bagnara A. S., Finch L. R. Relationships between intracellular contents of nucleotides and 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Eur J Biochem. 1973 Jul 16;36(2):422–427. doi: 10.1111/j.1432-1033.1973.tb02927.x. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- Benson C. E., Hornick D. L., Gots J. S. Genetic separation of purine transport from phosphoribosyltransferase activity in Salmonella typhimurium. J Gen Microbiol. 1980 Dec;121(2):357–364. doi: 10.1099/00221287-121-2-357. [DOI] [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Jochimsen B., Koduri K. R. Microbial models and regulatory elements in the control of purine metabolism. Ciba Found Symp. 1977;(48):23–41. doi: 10.1002/9780470720301.ch3. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer J., Neuhard J. Metabolism of exogenous purine bases and nucleosides by Salmonella typhimurium. J Bacteriol. 1971 Apr;106(1):14–24. doi: 10.1128/jb.106.1.14-24.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Jensen B., Nygaard P. Phosphoribosylpyrophosphate synthetase of Escherichia coli, Identification of a mutant enzyme. Eur J Biochem. 1982 Aug;126(2):327–332. doi: 10.1111/j.1432-1033.1982.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F. Apparent involvement of purines in the control of expression of Salmonella typhimurium pyr genes: analysis of a leaky guaB mutant resistant to pyrimidine analogs. J Bacteriol. 1979 Jun;138(3):731–738. doi: 10.1128/jb.138.3.731-738.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Neuhard J., Schack L. RNA polymerase involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high, constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBC. EMBO J. 1982;1(1):69–74. doi: 10.1002/j.1460-2075.1982.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochimsen B., Nygaard P., Vestergaard T. Location on the chromosome of Escherichia coli of genes governing purine metabolism. Adenosine deaminase (add), guanosine kinase (gsk) and hypoxanthine phosphoribosyltransferase (hpt). Mol Gen Genet. 1975 Dec 30;143(1):85–91. doi: 10.1007/BF00269424. [DOI] [PubMed] [Google Scholar]
- Koduri R. K., Gots J. S. A DNA-binding protein with specificity for pur genes in Escherichia coli. J Biol Chem. 1980 Oct 25;255(20):9594–9598. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung H. B., Schramm V. L. Adenylate degradation in Escherichia coli. The role of AMP nucleosidase and properties of the purified enzyme. J Biol Chem. 1980 Nov 25;255(22):10867–10874. [PubMed] [Google Scholar]
- Levine R. A., Taylor M. W. Mechanism of adenine toxicity in Escherichia coli. J Bacteriol. 1982 Mar;149(3):923–930. doi: 10.1128/jb.149.3.923-930.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. A., Taylor M. W. Regulation of purE transcription in a purE::lac fusion strain of Escherichia coli. J Bacteriol. 1982 Mar;149(3):1041–1049. doi: 10.1128/jb.149.3.1041-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. A., Taylor M. W. Selection for purine regulatory mutants in an E. coli hypoxanthine phosphoribosyl transferase-guanine phosphoribosyl transferase double mutant. Mol Gen Genet. 1981;181(3):313–318. doi: 10.1007/BF00425604. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Messenger L. J., Zalkin H. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem. 1979 May 10;254(9):3382–3392. [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol. 1975 Mar;121(3):814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Gots J. S. purF-lac fusion and direction of purF transcription in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1156–1164. doi: 10.1128/jb.143.3.1156-1164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Thomulka K. W., Gots J. S. Isolation and characterization of purine regulatory mutants of Salmonella typhimurium with an episomal purE-lac fusion. J Bacteriol. 1982 Jul;151(1):153–161. doi: 10.1128/jb.151.1.153-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- Walker R. D., Duerre J. A. S-adenosylhomocysteine metabolism in various species. Can J Biochem. 1975 Mar;53(3):312–319. doi: 10.1139/o75-044. [DOI] [PubMed] [Google Scholar]


