Abstract
Glutamine phosphoribosylpyrophosphate amidotransferase is stable in growing cells, but is inactivated in an oxygen-dependent process at various rates in starving or antibiotic-treated cells. On the basis of studies of the purified enzyme, we suggested (D.A. Bernlohr and R.L. Switzer, Biochemistry 20:5675-5681, 1981) that the inactivation in vivo was regulated by substrate stabilization and a competition between stabilizing (AMP) and destabilizing (GMP, GDP, and ADP) nucleotides. This proposal was tested by measuring the intracellular levels of these metabolites under cultural conditions in which the stability of the amidotransferase varied. The results established that the stability of amidotransferase in vivo cannot be explained by the simple interactions observed in vitro. Metabolite levels associated with stability of the enzyme in growing cells did not confer stability under other conditions, such as ammonia starvation or refeeding of glucose-starved cells. The data suggest that a previously unrecognized event, possibly a covalent modification of amidotransferase, is required to mark the enzyme for oxygen-dependent inactivation.
Full text
PDF


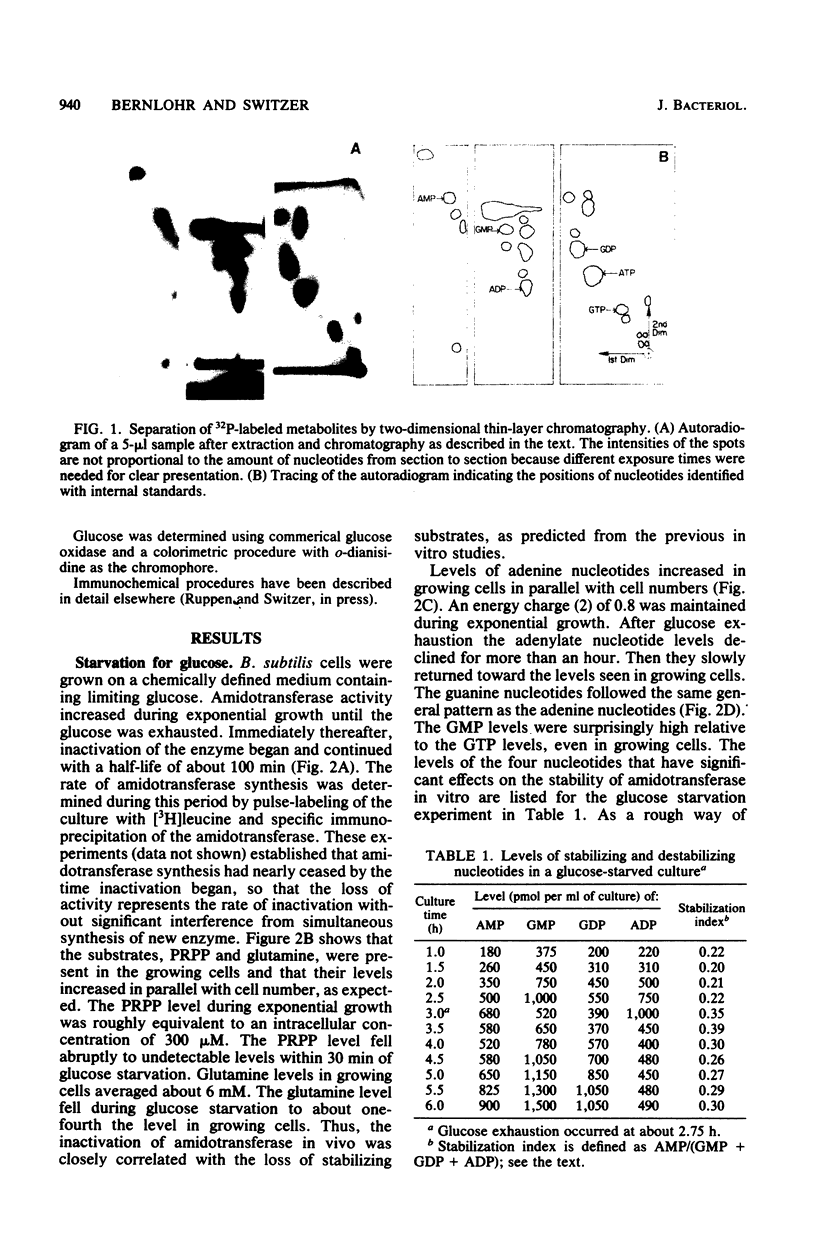


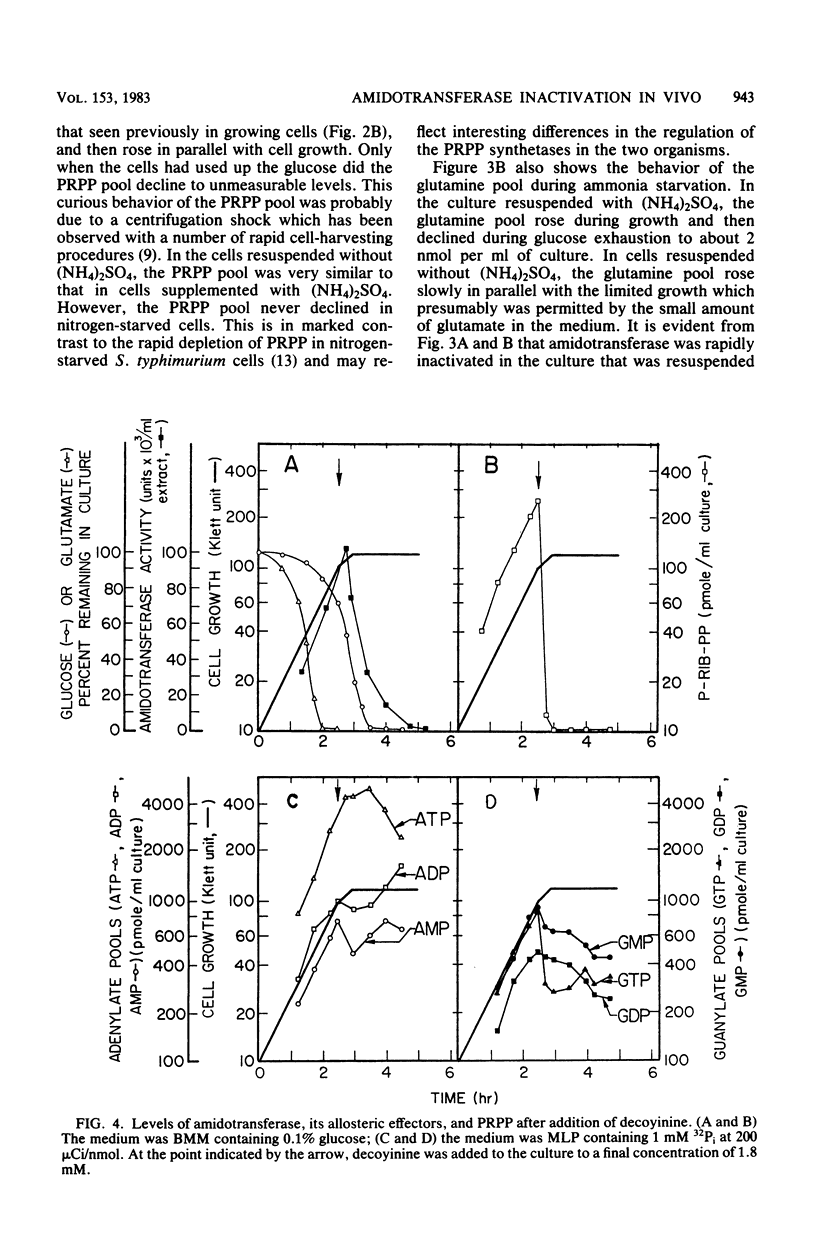

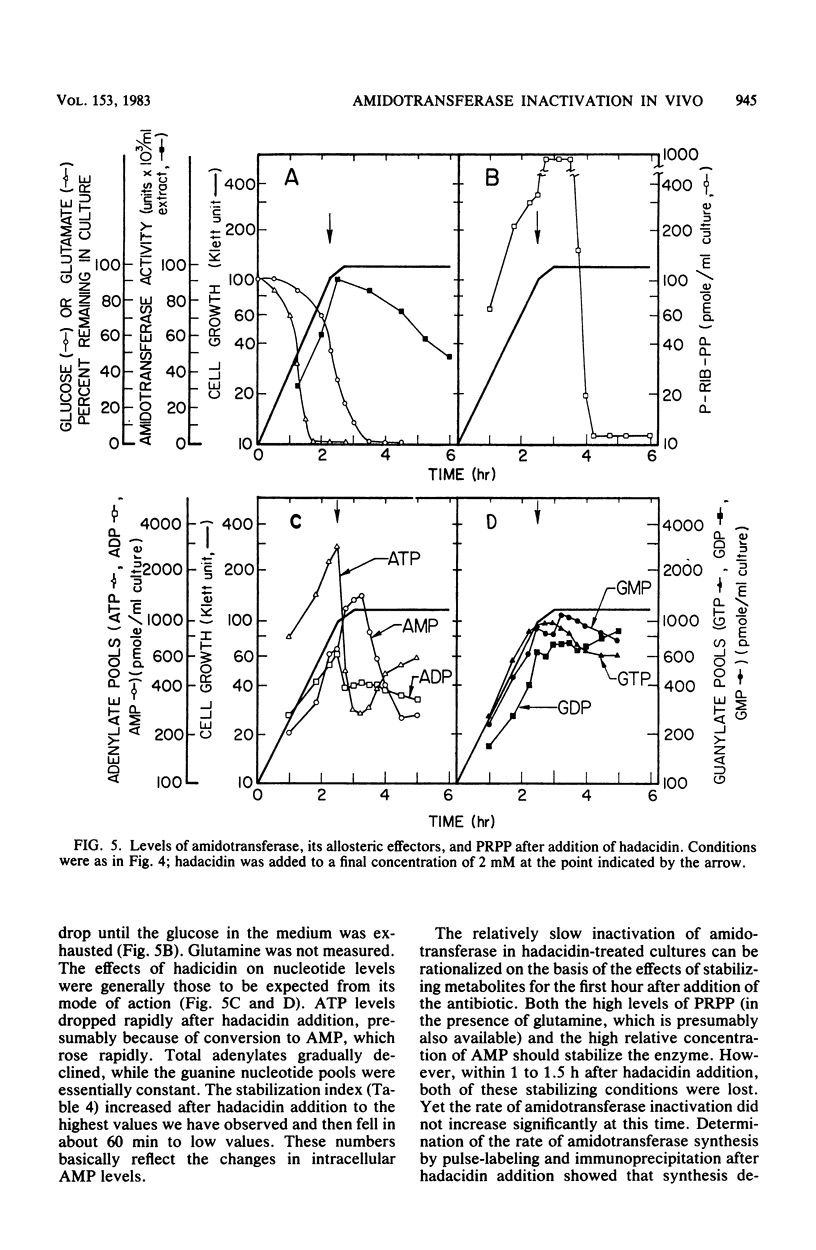
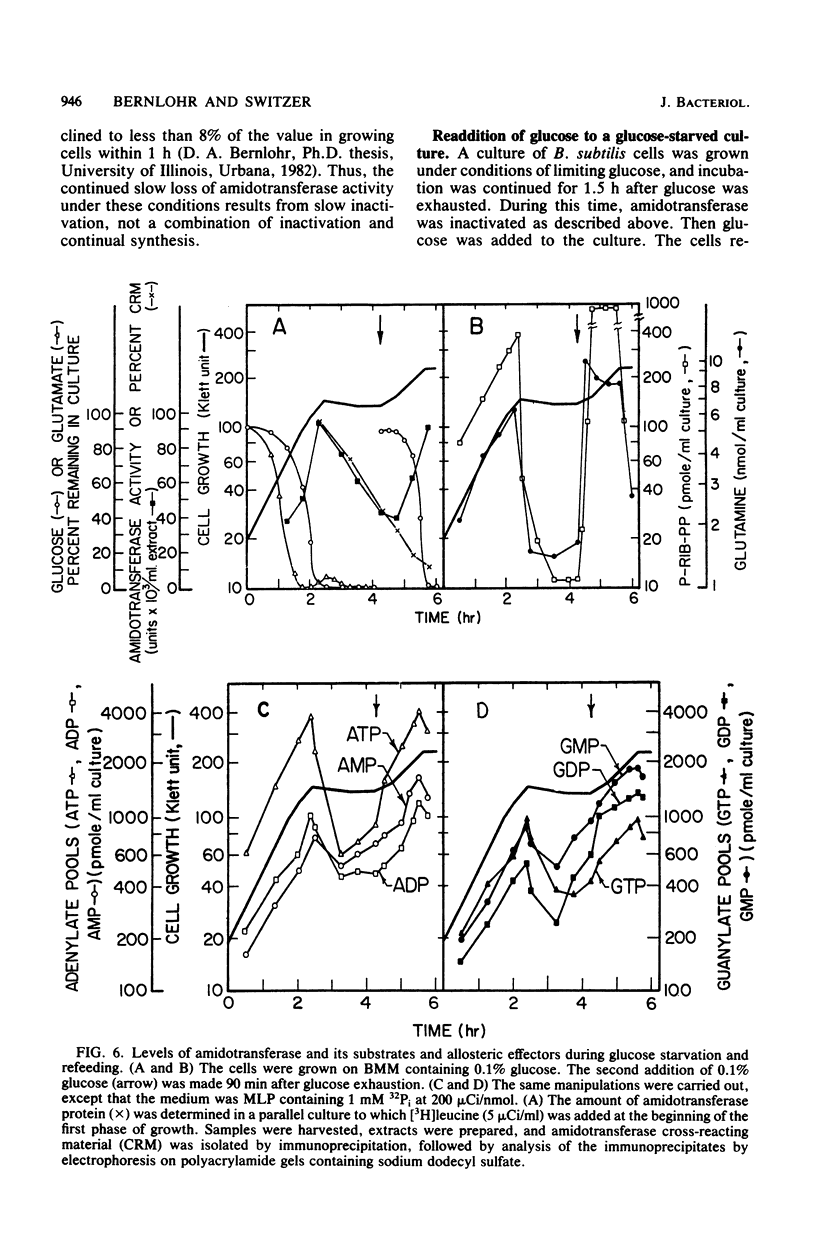



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Averill B. A., Dwivedi A., Debrunner P., Vollmer S. J., Wong J. Y., Switzer R. L. Evidence for a tetranuclear iron-sulfur center in glutamine phosphoribosylpyrophosphate amidotransferase from Bacillus subtilis. J Biol Chem. 1980 Jul 10;255(13):6007–6010. [PubMed] [Google Scholar]
- Bernlohr D. A., Switzer R. L. Reaction of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase with oxygen: chemistry and regulation by ligands. Biochemistry. 1981 Sep 29;20(20):5675–5681. doi: 10.1021/bi00523a006. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Selective precipitation orthophosphate from mixtures containing labile phosphorylated metabolites. Anal Biochem. 1982 May 1;122(1):100–107. doi: 10.1016/0003-2697(82)90257-3. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Kleinschmidt J. A. Selective inactivation of nitrogenase in Azotobacter vinelandii batch cultures. J Bacteriol. 1976 Oct;128(1):117–122. doi: 10.1128/jb.128.1.117-122.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. M., Marks C. L., Freese E. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim Biophys Acta. 1979 Oct 4;587(2):238–252. doi: 10.1016/0304-4165(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Meyer E., Switzer R. L. Regulation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase activity by end products. J Biol Chem. 1979 Jun 25;254(12):5397–5402. [PubMed] [Google Scholar]
- Sadler W. C., Switzer R. L. Regulation of Salmonella phosphoribosylpyrophosphate synthetase activity in vivo. Deductions from pool measurements. J Biol Chem. 1977 Dec 10;252(23):8504–8511. [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in stationary-phase cultures of Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):108–114. doi: 10.1128/jb.121.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in vitro inactivation. J Bacteriol. 1975 Jan;121(1):115–120. doi: 10.1128/jb.121.1.115-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. Y., Bernlohr D. A., Turnbough C. L., Switzer R. L. Purification and properties of glutamine phosphoribosylpyrophosphate amidotransferase from Bacillus subtilis. Biochemistry. 1981 Sep 29;20(20):5669–5674. doi: 10.1021/bi00523a005. [DOI] [PubMed] [Google Scholar]
- Wong J. Y., Meyer E., Switzer R. L. Glutamine phosphoribosylpyrophosphate amidotransferase from Bacillus subtilis. A novel iron-sulfur protein. J Biol Chem. 1977 Nov 10;252(21):7424–7426. [PubMed] [Google Scholar]



