Abstract
UVB-induced DNA damage is a crucial event in UVB-mediated apoptosis. On the other hand, UVB directly activates death receptors on the cell surface including CD95, implying that UVB-induced apoptosis can be initiated at the cell membrane through death receptor clustering. This study was performed to measure the relative contribution of nuclear and membrane effects in UVB-induced apoptosis of the human epithelial cell line HeLa. UVB-mediated DNA damage can be reduced by treating cells with liposomes containing the repair enzyme photolyase followed by exposure to photoreactivating light. Addition of photolyase followed by photoreactivation after UVB reduced the apoptosis rate significantly, whereas empty liposomes had no effect. Likewise, photoreactivating treatment did not affect apoptosis induced by the ligand of CD95, CD95L. UVB exposure at 4°C, which prevents CD95 clustering, also reduced the apoptosis rate, but to a lesser extent. When cells were exposed to UVB at 4°C and treated with photolyase plus photoreactivating light, UVB-induced apoptosis was almost completely prevented. Inhibition of caspase-3, a downstream protease in the CD95 signaling pathway, blocked both CD95L and UVB-induced apoptosis, whereas blockage of caspase-8, the most proximal caspase, inhibited CD95L-mediated apoptosis completely, but UVB-induced apoptosis only partially. Although according to these data nuclear effects seem to be slightly more effective in mediating UVB-induced apoptosis than membrane events, both are necessary for the complete apoptotic response. Thus, this study shows that nuclear and membrane effects are not mutually exclusive and that both components contribute independently to a complete response to UVB.
UV radiation in the middle-wavelength range between 290 and 320 nm (UVB) represents one of the most relevant environmental dangers because of its hazardous effects, including skin aging (1), induction of skin cancer (2), and exacerbation of infections (3). Like other adverse agents (alkylating chemicals, oxidants), UVB induces changes in mammalian cell gene expression (4–6). Elucidation of the underlying molecular mechanisms is of primary importance for the understanding of how UVB can damage cells and thus act as a pathogen. One of the most controversial issues in this context is whether the cellular UVB response is initiated at the cell membrane or in the nucleus (7). To exert its biological effects, UVB must be first absorbed by a cellular chromophore, which transfers the energy into a biochemical signal. Among a number of chromophores (porphyrins, aromatic amino acids, urocanic acid), DNA is regarded as the most important for several reasons. (i) The wavelength dependency of some UVB effects is similar to that for DNA absorption (8). (ii) Acceleration of DNA repair inhibits particular biological UVB effects (8–12). (iii) Lower UVB doses are necessary to achieve the same biological effects in DNA repair-deficient than -proficient cells (13). Thus, these data favor the concept that DNA is the most important molecular target for UVB and that DNA damage is crucially involved in mediating the biological effects of UVB.
On the other hand, there is accumulating evidence that UV can exert particular biological effects without a nuclear signal. Devary et al. (14) and Simon et al. (15) demonstrated that activation of the transcription factor nuclear factor-κB (NFκB) does not require a nuclear signal. In addition, growth factor receptors are involved in the UV response because UV triggers immediate tyrosine phosphorylation of the epidermal growth factor receptor (16). Even more importantly, Rosette and Karin (17) reported that UV can activate the Jun N-terminal kinase cascade via activation of multiple cytokine receptors. Exposure of HeLa cells to UVB induced clustering and internalization of cell-surface receptors for epidermal growth factor, tumor necrosis factor, and interleukin-1. These findings demonstrated that surface receptors can be activated by UVB without binding of any ligand in a DNA damage-independent way. Rosette and Karin predicted that any receptor whose activation mechanism involves multimerization should be activatable by UV (17). We and others (18, 19) recently confirmed this prediction by showing that UV also activated the apoptosis-related surface receptor CD95 through this mechanism (see below).
Recently, Bender et al. (20) demonstrated that NFκB becomes activated by UV sequentially in a DNA damage-independent and -dependent way. Using DNA repair-deficient fibroblasts, they showed that early degradation of the inhibitory protein IκB is not initiated by UV-induced DNA damage, whereas late activation of NFκB is mediated through DNA damage-induced cleavage of the interleukin-1 precursor.
A hallmark event of UV exposure is the induction of apoptotic cell death of keratinocytes, which within the epidermis appear as sunburn cells (21). The tumor-suppressor gene p53 appears to be critically involved in this process, because p53 knockout mice develop almost no sunburn cells compared with control mice (22). This is compatible with the current hypothesis that keratinocytes, which did not repair UV-induced DNA damage sufficiently, initiate apoptosis and die as sunburn cells (23). Furthermore, in vivo studies showed that enhancement of DNA repair by topical application of the repair enzyme T4 endonuclease V in liposomes reduces sunburn-cell formation (10). Similar observations were made in opossums on photoreactivation (24). Accordingly, photoreactivation of UV-irradiated fish cells that have endogenous photolyase (25, 26) reduces apoptosis (27). In addition, detection of sunburn cells in skin treated with other DNA-damaging irradiation procedures (e.g., psoralen and UVA) supports the view that DNA is the relevant chromophore (28). Consequently, it is generally accepted that the severity of DNA damage determines whether programmed cell death is initiated after UV exposure.
On the other hand, UV directly activates the apoptosis-related surface molecule CD95 (18, 19). CD95 (Fas/APO-1) is a death receptor that, on interaction with its ligand, CD95L, becomes activated and induces apoptosis (29). On trimerization of CD95, a cascade of cystein proteases, called caspases, becomes activated, which ultimately mediates apoptosis (30). UV is able to directly activate CD95 without need of the ligand (18, 19). Because prevention of UV-induced CD95 clustering was associated with a partial reduction of apoptosis, these data suggest that UV-induced apoptosis can also be initiated at the cell membrane (18). In addition, Sheikh et al. (31) recently showed that the tumor necrosis factor receptor-1 becomes aggregated by UV but not by drugs that damage DNA only. Taken together, these data challenge the concept that UV-induced apoptosis is exclusively dependent on DNA damage.
Because there are solid indications that UV-induced apoptosis can be initiated both at the cell membrane and in the nucleus, it was the aim of the present study to determine the relative contribution of nuclear and membrane effects in UVB-induced apoptosis. We used the approach of enhancing DNA repair by addition of exogenous DNA repair enzymes encapsulated in liposomes (32). We used the photoreactivating enzyme photolyase from Anacystis nidulans recombinantly produced in Escherichia coli. The photolyase binds to a UV-induced cyclobutane pyrimidine dimer (CPD) in DNA and catalyzes its splitting by electron transfer from absorbing wavelengths above 320 nm (photoreactivating light, PRL) (33). Here, we show that photoreactivation reduced both CPD- and UV-induced apoptosis remarkably but did not protect cells completely. Exposure of HeLa cells to UVB at 4°C, which prevents death receptor clustering, also reduced apoptosis but to a lesser extent than photoreactivation. In contrast, when cells were exposed to UVB at 4°C and subsequently photoreactivated, UVB-induced apoptosis was reduced in an additive way. These data indicate that nuclear and membrane effects are not mutually exclusive and that both components contribute independently to a complete UVB response.
MATERIALS AND METHODS
Cell Culture and UV Irradiation.
The human epithelial carcinoma cell line HeLa (American Tissue Culture Collection) was cultured in RPMI 1640 with 10% FCS. Cells were exposed to UVB through colorless medium without FCS. For UVB irradiation, FS20 bulbs (Westinghouse) were used, which emit most of their energy (65%) within the UVB range (290–320 nm) with an emission peak at 313 nm. Throughout this study a dose of 400 J/m2 was used. Control cells were subjected to the identical procedure without being UVB-exposed.
Reagents.
To induce CD95-mediated apoptosis, an agonistic mouse IgM Ab against human CD95 (CH-11, Immunotech, Luminy, France) or recombinant CD95L (Alexis, San Diego) were used. Activation of caspases was blocked by adding the following specific oligopeptides (20 μM each) to the medium: z-Val-Ala-Asp-CH2F (z-VAD) for ICE-proteases, z-Asp-Glu-Val-Asp-CH2F (z-DEVD) for caspase-3, and z-Ile-Glu-Thr-Asp-CH2F (z-IETD) for caspase-8 (Enzyme Systems Products, Livermore, CA).
Induction of DNA Repair Via Photoreactivation.
Photolyase was encaspulated at a concentration of 1.2 mg/ml into liposomes (Photosomes, AGI Dermatics, Freeport, NY) (32). Liposomes consisted of the lipids egg phosphatidylcholine, egg phosphatidyl trans-ethanolamine, and oleic acid and the membrane stabilizer cholesterol hemisuccinate. As negative controls, empty liposomes were used, referred to as liposomes. For photoreactivation, cells were first UVB-irradiated, and either Photosomes or liposomes (40 μl/ml each) were added. Cells were incubated at 37°C for 1 hr in the dark followed by illumination with PRL. As a source for PRL, UVA fluorescent bulbs (TL09, Philips) filtered through a 6-mm glass plate (short wavelength cutoff at 330 nm) with peak emission at 365 nm were used. Cells were exposed for 20 min, which corresponds to a PRL fluence of 12 kJ/m2. After photoreactivation, cells were supplemented with RPMI containing 10% FCS and incubated for 16 hr at 37°C.
Detection of Cell Death.
Cells were analyzed for apoptosis by a cell death detection ELISA (Boehringer Mannheim) 16 hr after treatment with UVB, recombinant CD95L (0.1 μg/ml), or agonistic anti-CD95-Ab (0.5 μg/ml). The enrichment of mono- and oligonucleosomes released into the cytoplasm of cell lysates was detected by biotinylated anti-histone- and peroxidase-coupled anti-DNA-Ab and is calculated by using the formula: absorbance of sample cells/absorbance of control cells. Enrichment factor was used as a parameter of apoptosis. Variation of the enrichment factor which was observed in some experiments, may be due to the fact that cells do not always undergo exactly the same rate of apoptosis. This appears to depend on the condition and also on the passage of the cells, but the differences between the groups within one experiment were always in the same range. Enrichment factor is given as the mean ± SD of three independently performed experiments, unless otherwise stated.
Western Blot Analysis.
Cells were lysed in lysis buffer (50 mM Hepes, pH 7.5/150 mM NaCl/10% glycerol/1% Triton-X-100/1.5 mM MgCl2/1 mM EGTA/100 mM NaF/10 mM pyrophosphate/0.01% NaN3/Complete protease inhibitor cocktail) for 15 min on ice. The protein samples were subjected to SDS/12% or 8% PAGE, blotted to nitrocellulose membranes, and incubated with Abs against caspase-3 (Dianova, Hamburg, Germany), poly(ADP ribose)-polymerase (PARP) (Boehringer Mannheim) or α-tubulin (Calbiochem).
Southwestern Dot-Blot Analysis.
After photoreactivation, genomic DNA was isolated from 1 × 106 cells according to the protocol from Biozym Diagnostik (Hessisch Oldendorf, Germany). Genomic DNA (2 μg, measured at 260 nm) was transferred to a nylon membrane by vacuum-dot-blotting and fixed at 80°C for 20 min. Because photolyase repairs all base compositions of CPD, with a preference for thymine–thymine dimers (34), a mAb against thymine dimers (Kamiya Biomedical, Thousand Oaks, CA) was used for Southwestern analysis, detected with a horseradish peroxidase-conjugated anti-mouse-Ab.
RESULTS
Exposure of HeLa Cells to UVB at Low Temperature Reduces UVB-Induced Apoptosis.
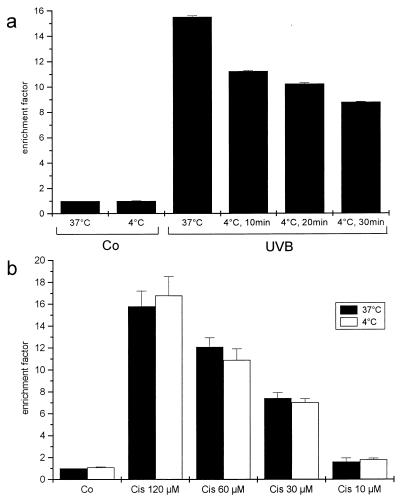
Exposure of HeLa cells to 400 J/m2 UVB resulted in apoptosis, as measured by a cell death detection ELISA (Fig. 1a). Exposing the spontaneously transformed human keratinocyte cell line HaCaT to UVB at low temperature during irradiation caused reduction of apoptosis (18). Thus, HeLa cells were kept at 4°C for various periods of time before and for 5 min after exposure to 400 J/m2 and then incubated at 37°C. Control cells were incubated at 4°C for 30 min but not UVB-exposed. The apoptosis rate was reduced 16 hr later in cells exposed to UVB at low temperature, and reduction increased proportionally with the period of time cells were kept at 4°C. Keeping cells at low temperature does not affect the apoptotic machinery in general, because the apoptotic response to the cytostatic drug cis-platin, which acts exclusively via damaging DNA (31), was the same irrespective of whether the cells were kept at 4°C for 30 minutes or at 37°C throughout the experiment (Fig. 1b). In contrast, UVB-induced apoptosis in HeLa cells was reduced when cells were UVB-exposed at 4°C (Fig. 1a). Maximum reduction was observed when cells were kept at 4°C for 30 min. In accordance with previous findings (18), confocal laser scanning microscopy indicated that keeping cells at such low temperature during UVB exposure prevents clustering of the CD95 receptor (data not shown). Although receptor aggregation was blocked by >90%, low-temperature irradiation blocked less than half of UVB-induced apoptosis. Because it is unlikely that the few receptors still clustered at 4°C are responsible for mediating the remaining apoptosis, other pathways, presumably not membrane-related ones, have to be involved as well.
Figure 1.
(a) Apoptosis is reduced on UVB exposure at low temperature. HeLa cells were UVB-irradiated (400 J/m2) at either 37°C or after being kept at 4°C for 10, 20, or 30 min. After irradiation, cells were kept on ice for 5 min and then incubated at 37°C for 16 hr. Control cells were not UVB-exposed but were incubated at 4°C for 30 min or kept at 37°C. (b) Cells were incubated with different concentrations of cis-platin either at 37°C or kept for 30 min before and 5 min after cis-platin stimulation. Apoptosis was evaluated 16 hr later with a cell death ELISA. Rate of apoptosis is reflected by the enrichment of nucleosomes in the cytoplasm shown on the y axis (mean ± SD of triplicate samples). Data presented show one representative of two independently performed experiments.
Enhancement of DNA Repair by Photoreactivation Reduces UVB-Induced Apoptosis.
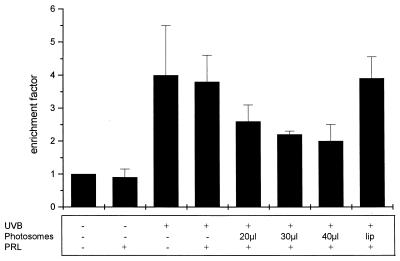
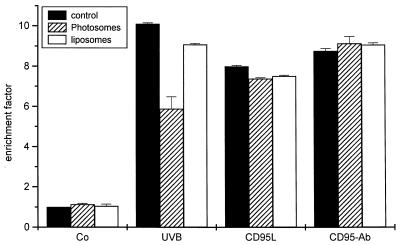
Because DNA is regarded as the major molecular target for UVB and UVC, it has been postulated for a long time that DNA damage is involved in UV-induced apoptosis (21, 23). Thus, an increase in DNA repair should result in reduction of the apoptotic response after UV exposure. Therefore, we determined whether treatment of HeLa cells with Photosomes and PRL inhibits UVB-induced apoptosis. HeLa cells were UVB-irradiated; immediately thereafter, increasing amounts of Photosomes were added and cells were incubated at 37°C in the dark. After 1 hr, cells were exposed to PRL. PRL by itself did not affect viability of HeLa cells; likewise, it had no effect on the apoptosis rate induced by UVB. In contrast, cells treated with Photosomes and PRL proportionally showed decreasing apoptosis rates (Fig. 2). As a negative control, empty liposomes not containing photolyase were used.
Figure 2.
Enhancement of DNA repair reduces UVB-induced apoptosis. After UVB irradiation, increasing amounts of Photosomes were applied to the medium and cells were incubated at 37°C for 1 hr. Subsequently, photoreactivation was carried out by irradiating cells with PRL. For control purposes, cells were either left untreated, irradiated with PRL or UVB alone, or with UVB and PRL in the absence of Photosomes. In addition, cells were incubated with empty liposomes (lip) followed by exposure to PRL. After 16 hr at 37°C, apoptosis was evaluated with a cell death ELISA. Enrichment factor was used as a parameter of apoptosis and is given as the mean ± SD of three independently performed experiments.
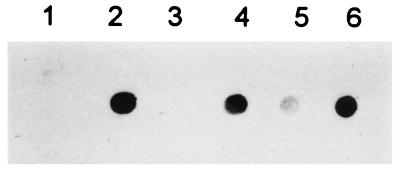
To determine whether the combination of Photosomes plus PRL effectively reduces DNA damage, Southwestern dot-blot analysis with an Ab directed against thymine–thymine CPD was performed. No thymine–thymine CPD were present in genomic DNA from untreated cells or cells exposed to PRL only (Fig. 3). In contrast, thymine–thymine CPD were detected in HeLa cells exposed to UVB or to UVB followed by PRL. The amount of thymine–thymine CPD was significantly reduced when UVB-irradiated cells were treated with the combination of Photosomes plus PRL. Densitometric analysis indicated a reduction from 50 to 70%. Empty liposomes had no effect on the amount UV-induced thymine dimers. Thus, these data show that the combination of Photosomes plus PRL enhances DNA repair by removing thymine–thymine CPD in UVB-exposed HeLa cells.
Figure 3.
Photoreactivation reduces UVB-induced DNA damage. Cells were left untreated (lane 1) or were exposed either to UVB (lane 2), to PRL (lane 3), or to UVB followed by PRL (lane 4). In lanes 5 and 6, UVB-exposed cells were treated with Photosomes (lane 5) or empty liposomes (lane 6) for 1 hr followed by exposure to PRL. Immediately after photoreactivation, genomic DNA was extracted and subjected to Southwestern dot-blot analysis by using an Ab against thymine–thymine CPD.
Enhanced DNA Repair Reduces UVB-Induced Cleavage of Caspase-3 and PARP.
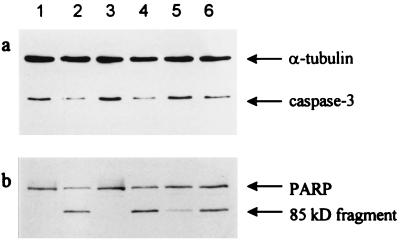
Caspase-3 is a member of the family of interleukin-1β-converting enzyme proteases that is crucially involved in apoptosis induced by various stimuli, including CD95 activation (35, 36). Caspase-3 becomes active when it is cleaved into its 17-kDa form. Recently, we showed that cleavage of caspase-3 is also induced by UVB (18). Thus, caspase-3 cleavage can be used as an intermediate milepost to evaluate UVB-mediated apoptosis. Therefore, HeLa cells were UVB-exposed and subsequently treated with Photosomes and the appropriate controls as described above. Cell lysates were prepared 16 hr later for Western blot analysis with an Ab against caspase-3. This Ab is directed against a domain of the caspase-3 proform and cannot recognize the processed 17-kDa form, resulting in loss of the immunoreactive band in samples in which caspase-3 has been activated. A significant reduction of caspase-3 was observed in samples of UVB-exposed HeLa cells (Fig. 4a). PRL alone did not affect caspase-3 activation; likewise, it had no effect on the UVB-mediated reduction. In contrast, the amount of the caspase-3 proform was much higher in extracts of UVB-exposed cells that were subsequently photoreactivated. Empty liposomes, however, had no effect on the UVB-mediated cleavage of caspase-3.
Figure 4.
Enhancement of DNA repair by photoreactivation reduces caspase-3 activation and PARP cleavage. Cells were left untreated (lane 1) or exposed either to UVB (lane 2), to PRL (lane 3), or to UVB followed by PRL (lane 4). In lanes 5 and 6, UVB-exposed cells were treated with Photosomes (lane 5) or empty liposomes (lane 6) for 1 hr followed by exposure to PRL. After 16 hr, proteins were subjected to Western blot analysis with a caspase-3 Ab (a) or a PARP Ab (b). Equal loading was monitored with an Ab directed against α-tubulin.
Because one of the consequences of caspase-3 activation is cleavage of the death substrate PARP (37), we determined whether photoreactivation prevents or reduces UVB-mediated PARP cleavage. PARP was found cleaved from its intact 116-kDa form into the inactive 85-kDa fragment in samples of UVB-exposed HeLa cells (Fig. 4b). In accordance with the caspase-3 data, PARP cleavage was significantly reduced in samples obtained from UVB-exposed cells that were subsequently subjected to photoreactivation.
Enhanced DNA Repair Does Not Influence CD95-Mediated Apoptosis.
Inhibition of UVB-mediated cytotoxicity by photoreactivation implies that DNA damage is an important mediator during UVB-induced apoptosis. If Photosomes in combination with PRL reduce apoptosis by affecting UVB-mediated DNA damage, Photosomes should not affect apoptosis induced in a DNA-independent way. Therefore, HeLa cells were treated either with recombinant CD95L or with an agonistic anti-CD95-Ab, both of which induce apoptosis via exclusive activation of CD95 (29). In contrast to UVB-induced apoptosis, photoreactivation had absolutely no effect on cell killing mediated either by CD95L or by the Ab (Fig. 5). These data indicate that Photosomes do not rescue cells in a nonspecific way but act exclusively in UV-damaged cells.
Figure 5.
Photoreactivation does not affect CD95-mediated apoptosis. Cells were exposed to UVB, CD95L or to an agonistic CD95-Ab. Subsequently, cells were treated with Photosomes or liposomes followed by PRL. Apoptosis was evaluated 16 hr later by using a cell death ELISA. Rate of apoptosis is reflected by the enrichment of nucleosomes in the cytoplasm, shown on the y axis (mean ± SD of triplicate samples). Data presented show one representative of two independently performed experiments.
Prevention of Receptor Clustering and Enhancement of DNA Repair Prevent UVB-Induced Apoptosis.
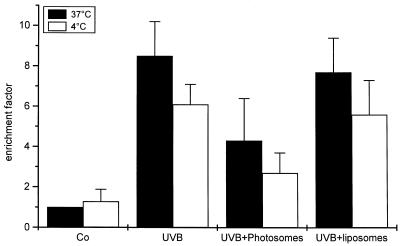
The data above indicate that UVB-induced apoptosis can be reduced either by exposing cells to UVB at low temperature or by enhancing DNA repair. The first observation indicates that membrane events, most likely death receptor activation, are involved, whereas the latter observation identifies DNA damage as an important mediator for UVB-induced apoptosis. However, although we tried to modify the conditions in several ways either by keeping cells for longer periods at 4°C or by increasing the amount of Photosomes, which still further enhances DNA repair, we were never successful in blocking UVB-induced apoptosis completely with either one of these strategies alone (data not shown). Because these findings indicate that both pathways are of functional relevance, however, we were interested to see whether both interventions act in an additive way and whether their combination results in a further inhibition of UV-induced apoptosis. Therefore, HeLa cells were kept on ice for 10 min, UVB-irradiated, and incubated on ice for a further 5 min. Photosomes were added, cells were put back to 37°C, and photoreactivation was induced. Identical experiments were performed with liposomes and compared with control cells that were only UVB-irradiated at either 37°C or 4°C. UV exposure of cells at low temperature had a moderate reducing effect on apoptosis compared with the pronounced effect of photoreactivation (Fig. 6). Most importantly, when cells were exposed at 4°C and photoreactivated, UVB-induced apoptosis was further reduced almost to baseline levels. Together, these data indicate that nuclear effects seem to be more effective in mediating UVB-induced apoptosis than membrane events, and they act independently to reach the complete apoptotic response.
Figure 6.
Enhancement of DNA repair and prevention of surface receptor aggregation most effectively block UVB-induced apoptosis. HeLa cells were UVB exposed at 37°C (open bars) or at 4°C (filled bars). Subsequently, cells were treated with Photosomes or liposomes followed by PRL, and 16 hr later, apoptosis was evaluated by using a cell death ELISA. Enrichment factor was used as a parameter of apoptosis and is given as the mean ± SD of three independently performed experiments.
Caspase Inhibitors Suppress CD95- and UVB-Mediated Apoptosis Differentially.
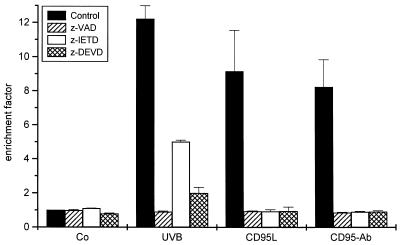
The present data confirm previous observations that activation of CD95 is of functional relevance in UVB-induced apoptosis, but they also demonstrate clear differences between the signal-transduction pathways involved in CD95L- and UVB-mediated apoptosis. Therefore, we addressed whether these different signaling pathways use the same effector mechanisms executing apoptosis. As shown in Fig. 7, pretreatment of HeLa cells with the broad-spectrum caspase inhibitor z-VAD completely blocked UVB- as well as CD95L- or CD95-Ab induced apoptosis, confirming previous reports (18, 38, 39). Similar findings were obtained when using z-DEVD, which specifically inhibits caspase-3 (Fig. 7). Because both UVB- and CD95L-induced apoptosis were reduced almost equally effectively, this indicates that caspase-3 controls both the receptor-ligand and DNA damage pathway of apoptosis. Accordingly, UVB-mediated activation of caspase-3 was effectively reduced by enhancement of DNA-repair (Fig. 4a). In contrast, z-IETD, which inhibits the more upstream-acting caspase-8, completely blocked apoptosis induced either by CD95L or CD95-Ab, whereas UVB-induced cell death was only partially inhibited. Because caspase-8 is crucially involved in CD95 signaling, activation of caspase-8 during UVB-induced apoptosis appears to be most likely caused by the direct triggering of CD95 by UVB. Because, as shown above, CD95 only partially contributes to UVB-induced apoptosis, it is not surprising that inhibition of caspase-8 does not cause complete prevention but only reduction of UVB-induced cell death. Thus, these data imply that caspase-8 is not, or at least is much less critically, involved in executing DNA-damage mediated apoptosis than in cell death, which is exclusively caused by activation of CD95. In addition, the data obtained with the caspase inhibitors are in accordance with the above findings indicating that both nuclear and membrane effects contribute to UVB-induced apoptosis, although nuclear events might be the more important ones.
Figure 7.
Caspase inhibitors suppress CD95- and UVB-mediated apoptosis differentially. Cells were treated with one of the caspase inhibitors z-VAD, z-DEVD, or z-IETD (20 μM each). Cells were UVB-irradiated or incubated with CD95L and an agonistic CD95-Ab, respectively, 1 hr later. Control cells were stimulated with the inhibitors only. Apoptosis was evaluated 16 hr later by using a cell death ELISA. Enrichment factor was used as a parameter of apoptosis and is given as the mean ± SD of three independently performed experiments.
DISCUSSION
One of the most important issues addressed in molecular photobiology refers to the identification of the molecular target(s) of UV. Although there is increasing evidence that UV can affect both nuclear and cell-membrane targets, it is not clear how these relate to each other and whether they are mutually exclusive. It is generally accepted that UV-induced apoptosis is a consequence of UV-mediated DNA damage. On the other hand, UV can directly activate death receptors on the cell surface, which contributes to the induction of apoptosis (18, 19, 31). Anticipating that both nuclear and membrane events are essentially involved, we surmised that blockage of both pathways should result in complete prevention or at least further reduction of UV-induced apoptosis.
Activation of death receptors on the cell surface is associated with trimerization. Receptor multimerization can be prevented by keeping cells at low temperature (17, 18). Although the mechanism underlying this observation is not clear, changes in membrane fluidity have been suggested. In any case, low temperature prevents receptor aggregation irrespective of whether it is induced by the respective receptor ligands, UVB, or osmotic shock (17). Accordingly, UVB-induced apoptosis could be partially reduced by exposing HeLa cells to UVB at 4°C. However, CD95 may not be the only death receptor activated by UV. The same was recently observed for the tumor necrosis factor receptor (31). Because all death receptors described so far require multimerization to become activated, it is the advantage of low temperature that it blocks all cell surface death receptors possibly involved. Reduction of UVB-induced cell death at low temperature is not caused by induction of stress proteins by cold shock (18). In addition, it does not appear to interfere with transcription because tumor necrosis factor-induced release of interleukin-6 is not affected when HeLa cells are kept at 4°C for 30 min (data not shown). It is also unlikely that low temperature interferes with UV-induced DNA damage, because generation of CPD is not prevented in the cold (40). Although as observed previously (18), receptor clustering was almost completely prevented, reduction of UV-induced cell death was only partial, implying that other independent pathways may be involved as well.
To examine the role of DNA damage in UVB-induced apoptosis, we enhanced DNA repair by addition of liposomes containing DNA repair enzymes. Treatment of UVB-exposed HeLa cells with Photosomes followed by PRL significantly reduced both thymine–thymine CPD formation and the rate of apoptosis. In addition, photolyase and PRL had no effect on apoptosis induced by DNA damage-independent pathways e.g., by triggering the CD95 receptor either by its natural ligand CD95L or an agonistic Ab. Although enhancement of DNA repair by photoreactivation rescued most of the cells and the rescuing effect appeared to be stronger than that caused by low temperature (Fig. 6), it did not completely prevent UVB-induced apoptosis. First, this may be because of the fact that not all damaged DNA is repaired, as shown by dot-blot analysis. Complete CPD removal has never been reached by using Photosomes, and the maximum achieved is between 50 and 70%. On the other hand, all CPDs do not appear to be equal, because it recently was shown that CPDs in transcribed regions are the important ones for signal transduction (41). Second, because DNA damage is a quick event, nuclear signals initiating the apoptosis program may go out before damage is removed. Third, other presumably extranuclear pathways like activation of the death receptors, e.g. CD95, may be involved.
Therefore, we investigated whether enhancement of DNA repair and prevention of surface receptor aggregation act additively in suppressing UVB-induced apoptosis. When HeLa cells were exposed to UVB at low temperature followed by photoreactivation, UVB-induced apoptosis was almost completely prevented. Although activation of death receptors and DNA damage are induced by the same stimulus, i.e., UVB, they are completely independent events that are not biochemically linked. Inhibition of both results in an additive reduction of apoptosis; therefore, death receptor activation and DNA damage contribute independently to UVB-induced apoptosis. Furthermore, these data show that both nuclear and membrane effects are not mutually exclusive and that both components are necessary to yield the complete UVB response.
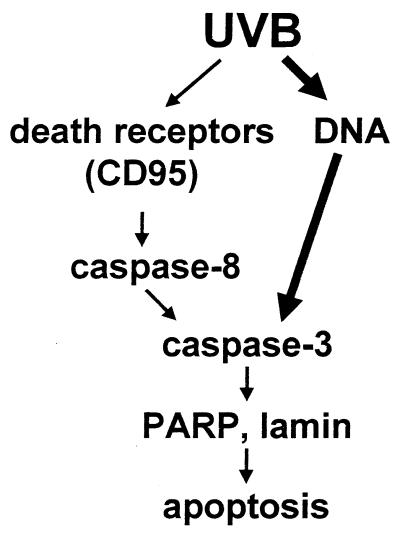
Activation of caspases appears to be crucial for executing apoptosis induced either by UVB or by CD95L because both UVB- and CD95L-induced apoptosis can be completely blocked by the broad spectrum caspase inhibitor z-VAD (18). Triggering of the CD95 receptor causes activation of caspase-8, which subsequently activates caspase-3 (reviewed in ref. 29). Caspase-3 by itself causes cleavage of several death substrates like PARP or lamin, which ultimately results in apoptosis (38). Hence, in the CD95 pathway, caspase-3 acts downstream of caspase-8. Activation of caspase-3 appears to be crucial in both UVB- and CD95L-mediated apoptosis, because both were found to be significantly reduced by specific inhibitory peptides. In contrast, addition of z-IETD, which selectively inhibits the upstream protease caspase-8, only completely blocked CD95L- and CD95-Ab-mediated apoptosis, whereas UVB-induced apoptosis was only partially inhibited. We assume that activation of caspase-8 during UVB-mediated apoptosis is caused by UVB-induced triggering of the CD95 receptor. Because UVB only partially exerts its apoptotic effect by activating death receptors, including CD95, it is not surprising that the inhibitory effect of the caspase-8 inhibitor is less pronounced in UVB- than in CD95L-induced cell death. The much stronger inhibition of UVB-induced apoptosis by inhibition of caspase-3 implies that caspase-3 is critically involved also in the initiation of the apoptosis program activated by DNA damage. Accordingly, we found that enhancement of DNA repair reduces cleavage of caspase-3 into its active form. Together, these data suggest that caspase-3 acts downstream of DNA damage (Fig. 8).
Figure 8.
Proposed signaling model for UVB-induced apoptosis.
Based on our findings, we propose the following scenario for UVB-induced apoptosis. UVB induces DNA damage, which initiates the apoptosis program via activation of caspase-3. In addition, UVB triggers death receptors like CD95, which initiates the apoptosis program via activation of caspase-8 followed by activation of caspase-3. Although nuclear effects seem to be slightly stronger than membrane events, both contribute to apoptosis because only inhibition of both results in prevention of UVB-induced cell death. Taken together, these data indicate that UVB-mediated nuclear and membrane effects are additive and therefore necessary for yielding the complete UVB response.
Acknowledgments
This work was supported by grants from the German Research Foundation (Schw625/1–2, SFB503/B2), the European Community (ENV4-CT97–0556, ENV4-CT95–0174), and the Federal Ministry of Education and Research.
ABBREVIATIONS
- CD95L
CD95 ligand
- CPD
cyclobutane pyrimidine dimers
- NFκB
nuclear factor κB
- PARP
poly(ADP ribose)-polymerase
- PRL
photoreactivating light
References
- 1.Chapman R S, Cooper K D, DeFabo E C, Frederich J E, Gelatt K N, Hammond S P, Hersey P, Koren H S, Ley R D, Noonan F, et al. Photochem Photobiol. 1995;61:223–247. doi: 10.1111/j.1751-1097.1995.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 2.De Gruijl F R, Sterenborg H J, Forbes P D, Davies R E, Cole C, Kelfkens G, van Weelden H, Slaper H, van der Leun J C. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- 3.Fisher G J, Datta S C, Talwar H S, Wang Z Q, Varani J, Kang S, Voorhees J J. Nature (London) 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 4.Herrlich P, Ponta H, Rahmsdorf H J. Rev Physiol Biochem Pharmacol. 1992;119:187–223. doi: 10.1007/3540551921_7. [DOI] [PubMed] [Google Scholar]
- 5.Herrlich P, Rahmsdorf H J. Curr Opin Cell Biol. 1994;6:425–431. doi: 10.1016/0955-0674(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 6.Schenk H, Klein M, Erdbrügger W, Dröge W, Schulze-Osthoff K. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz T. J Photochem Photobiol B. 1998;44:91–96. doi: 10.1016/S1011-1344(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 8.Petit-Frére C, Clingen P H, Grewe M, Krutmann J, Roza L, Arlett C F, Green M H L. J Invest Dermatol. 1998;111:354–359. doi: 10.1038/sj.jid.5602962. [DOI] [PubMed] [Google Scholar]
- 9.Kripke M L, Cox P A, Alas L G, Yarosh D B. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf P, Cox P, Yarosh D B, Kripke M L. J Invest Dermatol. 1995;104:287–292. doi: 10.1111/1523-1747.ep12612828. [DOI] [PubMed] [Google Scholar]
- 11.Kibitel J, Hejmadi V, Alas L, O’Connor A, Sutherland B M, Yarosh D. Photochem Photobiol. 1998;67:541–546. [PubMed] [Google Scholar]
- 12.Nishigori C, Yarosh S B, Ullrich S E, Vink A A, Bucana C B, Roza L, Kripke M L. Proc Natl Acad Sci USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krutmann J, Bohnert E, Jung E G. J Invest Dermatol. 1994;102:428–432. doi: 10.1111/1523-1747.ep12372947. [DOI] [PubMed] [Google Scholar]
- 14.Devary Y, Rosette C, DiDonato J A, Karin M. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 15.Simon M M, Aragane Y, Schwarz A, Luger T A, Schwarz T. J Invest Dermatol. 1994;120:422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- 16.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf H J. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 17.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 18.Aragane Y, Kulms D, Metze D, Kothny G, Pöppelmann B, Luger T A, Schwarz T. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehemtulla A, Hamilton C A, Chinnaiyan A M, Dixit V M. J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 20.Bender K, Göttlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young A R. Photodermatology. 1987;4:127–134. [Google Scholar]
- 22.Ziegler A, Jonason J S, Leffel D W, Simon J A, Sharma A W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 23.Brash D E, Ziegler A, Jonason A S, Simon J A, Kunala S, Lefell D J. J Invest Dermatol Symp Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- 24.Ley R D, Applegate L A. J Invest Dermatol. 1985;85:365–367. doi: 10.1111/1523-1747.ep12276992. [DOI] [PubMed] [Google Scholar]
- 25.Hart R W, Setlow R B, Woodhead A D. Proc Natl Acad Sci USA. 1977;74:5574–5578. doi: 10.1073/pnas.74.12.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achey P M, Woodhead A D, Setlow R B. Photochem Photobiol. 1979;29:305–310. doi: 10.1111/j.1751-1097.1979.tb07053.x. [DOI] [PubMed] [Google Scholar]
- 27.Nishigaki R, Mitani H, Shima A. Exp Cell Res. 1998;244:43–53. doi: 10.1006/excr.1998.4180. [DOI] [PubMed] [Google Scholar]
- 28.Woodcock A, Magnus I A. Br J Dermatol. 1976;95:459–468. doi: 10.1111/j.1365-2133.1976.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 29.Peter M E, Krammer P H. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 30.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 31.Sheikh M S, Antinore M J, Huang Y, Fornace A J., Jr Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- 32.Yarosh D, Klein J. Trends Photochem Photobiol. 1994;3:175–181. [Google Scholar]
- 33.Eker A P, Kooiman P, Hessels J K C, Yasui A. J Biol Chem. 1990;265:8009–8015. [PubMed] [Google Scholar]
- 34.Sancar A. Biochemistry. 1994;33:2–9. doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- 35.Enari M, Talanian R V, Wong W W, Nagata S. Nature (London) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 36.Schlegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T T, Nicholson D W. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 37.Lazebnik Y A, Kaufmann S H, Desnoyer S, Poirier G G, Earnshaw W C. Nature (London) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 38.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 39.Los M, van de Craen M, Penning L, Schenk H, Westendorp M, Baeuerle P, Dröge W, Krammer P H, Fiers W, Schulze-Osthoff K. Nature (London) 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 40.Beukers R, Berends W. Biochim Biophys Acta. 1960;41:550–554. doi: 10.1016/0006-3002(60)90063-9. [DOI] [PubMed] [Google Scholar]
- 41.Blattner C, Bender K, Herrlich P, Rahmsdorf H J. Oncogene. 1998;16:2827–2834. doi: 10.1038/sj.onc.1201827. [DOI] [PubMed] [Google Scholar]