Abstract
The inositol 1,4,5-trisphosphate receptor (InsP3R) is an intracellular Ca2+-release channel localized in endoplasmic reticulum (ER) with a central role in complex Ca2+ signaling in most cell types. A family of InsP3Rs encoded by several genes has been identified with different primary sequences, subcellular locations, variable ratios of expression, and heteromultimer formation. This diversity suggests that cells require distinct InsP3Rs, but the functional correlates of this diversity are largely unknown. Lacking are single-channel recordings of the recombinant type 3 receptor (InsP3R-3), a widely expressed isoform also implicated in plasma membrane Ca2+ influx and apoptosis. Here, we describe functional expression and single-channel recording of recombinant rat InsP3R-3 in its native membrane environment. The approach we describe suggests a novel strategy for expression and recording of recombinant ER-localized ion channels in the ER membrane. Ion permeation and channel gating properties of the rat InsP3R-3 are strikingly similar to those of Xenopus type 1 InsP3R in the same membrane. Using two different two-electrode voltage clamp protocols to examine calcium store-operated calcium influx, no difference in the magnitude of calcium influx was observed in oocytes injected with rat InsP3R-3 cRNA compared with control oocytes. Our results suggest that if cellular expression of multiple InsP3R isoforms is a mechanism to modify the temporal and spatial features of [Ca2+]i signals, then it must be achieved by isoform-specific regulation or localization of various types of InsP3Rs that have relatively similar Ca2+ permeation properties.
Keywords: calcium, calcium release channel, electrophysiology, expression, Xenopus
INTRODUCTION
Modulation of cytoplasmic free Ca2+ concentration ([Ca2+]i) is a ubiquitous signaling system involved in the regulation of numerous cell physiological processes, in which the second messenger inositol 1,4,5-trisphosphate (InsP3) plays a central role in most cell types. Analysis of InsP3-mediated [Ca2+]i signals in single cells has revealed an unexpected complexity in numerous cell types. In the temporal domain, this complexity is manifested as repetitive spikes or oscillations, the frequencies of which are often tuned to the degree of stimulation. Observations of [Ca2+]i spiking have suggested that [Ca2+]i signals may be transduced not only by amplitude detection, but by frequency encoding as well (Lechleiter and Clapham 1992; Berridge 1993; Bootman and Berridge 1995; Clapham 1995; Toescu 1995). In the spatial domain, [Ca2+]i signals may initiate at specific locations in the cell and propagate as waves, at velocities ranging from 10 to 50 μm/s (Boitano et al. 1992; Amundson and Clapham 1993; Atri et al. 1993; Rooney and Thomas 1993). Thus, [Ca2+]i signals are often organized to provide different signals to discrete parts of the cell.
InsP3-mediated [Ca2+]i signals are therefore precisely controlled in both time and space, resulting in a signaling system at once ubiquitous, versatile, and specific. A family of InsP3 receptors (InsP3Rs) with different primary sequences derived from different genes has been identified (Furuichi et al. 1989; Sudhof et al. 1991; Blondel et al. 1993; De Smedt et al. 1994; Maranto 1994; Mignery et al. 1989) with alternatively spliced isoforms (Danoff et al. 1991; Nakagawa et al. 1991; Ferris and Snyder 1992). The InsP3Rs are ∼2,700–2,800 amino acid integral membrane proteins (Furuichi et al. 1994) that exist as tetramers (Supattapone et al. 1988; Maeda et al. 1991) in the endoplasmic reticulum (ER). The type 1 InsP3R (InsP3R-1) was originally cloned from rat (Mignery et al. 1989) and mouse (Furuichi et al. 1989) cerebellum where it is expressed at high levels. Full-length InsP3R-1 cDNAs have also been cloned from Caenorhabditis elegans (Dal Santo et al. 1999), Drosophila (Yoshikawa et al. 1992), Xenopus (Kume et al. 1993), and human (Yamada et al. 1994). The predicted sequences are 50–70% homologous. Full-length sequences of cDNAs for two distinct isoforms, type 2 (InsP3R-2) and type 3 (InsP3R-3), have also been determined (Sudhof et al. 1991; De Smedt et al. 1994; Maranto 1994). The three full-length sequences are 60–80% homologous (Joseph 1995). The different isoforms have distinct and overlapping patterns of expression in different tissues (Maranto 1994; Fujino et al. 1995; Furuichi and Mikoshiba 1995). Most, if not all, mammalian cells examined outside the central nervous system express more than one isoform (Bush et al. 1994; De Smedt et al. 1994; Newton et al. 1994; Sugiyama et al. 1994; Fujino et al. 1995; Joseph et al. 1995; Nucifora et al. 1996), and expression levels, both absolute and relative to other isoforms, can be modified during cell differentiation (Nakagawa et al. 1991; Kume et al. 1993) and by use-dependent degradation (Magnusson et al. 1993; Wojcikiewicz et al. 1994; Honda et al. 1995; Wojcikiewicz 1995). In tissues that express more than one InsP3R isoform, isoform-specific antibodies immunoprecipitate others, suggesting that receptors may associate in heteroligomeric complexes (Joseph et al. 1995; Monkawa et al. 1995; Wojcikiewicz and He 1995; Nucifora et al. 1996).
This diversity of InsP3R expression is impressive, and suggests that cells require distinct InsP3Rs to regulate specific functions. Nevertheless, the functional correlates and physiological implications of this diversity are virtually unknown. That the InsP3R is a ligand-gated ion channel was originally demonstrated by tracer Ca2+ fluxes after reconstitution of purified cerebellar type 1 receptors into liposomes (Ferris et al. 1989). The intracellular location of the InsP3Rs has restricted studies of their single-channel properties (Bezprozvanny et al. 1991; Bezprozvanny and Ehrlich 1995; Kaftan et al. 1997), largely necessitating the use of indirect measurements to infer their channel activities; e.g., studies involving populations of InsP3Rs using measurements of [Ca2+] or fluxes in intact or permeabilized cells or membrane vesicles (Taylor and Richardson 1991; Putney and Bird 1993; Berridge 1995). The single-channel properties of the type 1 InsP3R have been examined by reconstitution of mammalian channels in lipid bilayer membranes (Bezprozvanny et al. 1991; Watras et al. 1991; Bezprozvanny et al. 1994; Bezprozvanny and Ehrlich 1994) and more recently by patch clamp of the outer nuclear membrane of Xenopus oocytes (Stehno-Bittel et al. 1995; Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). The type 2 receptor was recently examined by bilayer reconstitution (Perez et al. 1997; Ramos-Franco et al. 1998b). To date, there has been only one report of putative type 3 channel activity, using bilayer reconstitution of membranes from a cell type that expressed more type 3 relative to other isoforms (Hagar et al. 1998). Notably, there have been no single-channel recordings of the recombinant type 3 receptor. The InsP3R-3 is a widely expressed InsP3R isoform (De Smedt et al. 1997; Newton et al. 1994) and it has been implicated in roles in addition to Ca2+ release, including plasma membrane Ca2+ influx in response to depletion of intracellular Ca2+ stores (DeLisle et al. 1996; Putney 1997) and in apoptosis (Khan et al. 1996). Here, we describe the first functional expression and single-channel recording of recombinant rat InsP3R-3 (r-InsP3R-3) in its native membrane environment. The approach we describe suggests a novel general strategy for successful expression and recording of recombinant ER-localized ion channels in the ER membrane. Use of conditions that were similar to those previously employed in studies of the endogenous type 1 InsP3R channel in the outer nuclear membrane has now enabled the first comparison of the permeation and gating properties of different InsP3R isoforms in the same native membrane environment. Our results demonstrate that the ion permeation and channel gating properties of the r-InsP3R-3 channel in the ER membrane are remarkably similar in most respects to those of the Xenopus InsP3R-1 (X-InsP3R-1) in the same membrane. The similarities of permeation and gating properties of InsP3Rs between isoforms and species suggest that if cellular expression of multiple InsP3R isoforms is a mechanism to modify the temporal and spatial features of [Ca2+]i signals, then it must be achieved by isoform-specific regulation and/or localization of various types of InsP3Rs that have relatively similar channel gating and permeation properties.
MATERIALS AND METHODS
Synthesis of r-InsP3R-3 cRNA for Expression in Xenopus Oocytes
Rat InsP3R-3 cDNA was introduced into a modified pSP64 vector by digesting pSP-CFTR (provided by Dr. B. Skach, Oregon State University of the Health Sciences, Corvallis, OR) with Ava1 and Xba1 to remove the CFTR cDNA. The vector was blunt-ended with T4 DNA polymerase, religated with T4 ligase, linearized with EcoR1, and ligated by T4 DNA ligase to a Not1/EcoR1 adapter sequence. The new vector was linearized by Not1 and dephosphorylated by calf intestinal alkaline phosphatase. InsP3R-3 cDNA was excised from pCB6-InsP3R-3 (provided by Dr. G. Bell, University of Chicago, Chicago, IL) by Not1, purified (Geneclean III kit; Bio 101, Inc.), and introduced into pSP64 to create pSP-InsP3R-3. Plasmid DNA of selected clones of transfected Escherichia coli was digested to verify the orientation and sequence of subcloned cDNAs. The final selected clones were amplified, and plasmid DNA was extracted and purified (plasmid purification kit; QIAGEN Inc.). In vitro transcribed cRNA was synthesized using the RiboMAX Large Scale RNA Production System (Promega Corp.).
Selection and Microinjection of Xenopus Oocytes
Maintenance of Xenopus laevis and surgical extraction of ovaries were carried out as described previously (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). Because oocytes have an endogenous InsP3R-1 (X-InsP3R-1), it was necessary to distinguish them from expressed channels in our patch-clamp experiments using isolated oocyte nuclei. Endogenous channel activity detected in patch-clamp experiments using oocyte nuclei is highly variable from batch to batch of oocytes, although the activity level among oocytes from the same batch is very consistent (Mak and Foskett 1994). It has been common to find batches of oocytes that fail to exhibit any channel activity when their nuclei are patched. Although this has been a significant problem in our studies of the X-InsP3R-1, we reasoned that it would enable the use of this system for expression studies by providing a null background. Therefore, for each new batch of oocytes, a day of patch clamping of isolated nuclei (at least six nuclei, 6–10 patches from each) was performed to determine the endogenous channel expression. Only batches with extremely low channel activity (<1 of 15 patches exhibited InsP3R channel activity) were used for subsequent cRNA injections.
Oocytes selected for microinjection were defolliculated (Jiang et al. 1998). Xenopus ovaries were mechanically separated into pieces containing five or fewer oocytes each, and treated with 2 mg/ml solution of collagenase type IV (Sigma Chemical Co.) in Ca2+-free SOS (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH adjusted to 7.6 with NaOH) at room temperature for 1 h. Defolliculated oocytes were washed four to five times and kept in SOS (same as Ca2+-free SOS except with 1.8 mM CaCl2), and then used for injection <24 h after defolliculation.
23 nl of cRNA (1 μg/μl) was injected into the cytoplasm of defolliculated oocytes using a nanoliter injector (World Precision Instruments) (Goldin 1992). cRNA-injected and uninjected but defolliculated control oocytes were placed in individual wells in 96-well plates containing 200 μl of ASOS [SOS with 3 mM Na pyruvate, 100 μg/ml gentamycin, and 100 μM ALLN (N-acetyl-Leu-Leu-Norleucinal; Sigma Chemical Co.)]. 80 μl of ASOS in each well was changed daily. Patch clamp and two-electrode voltage clamp studies using injected oocytes were performed 4–5 d after cRNA microinjection.
Western Analysis
For each sample, 10 oocytes were placed in 200 μl of ice-cold oocyte extraction buffer [OEB, containing 150 mM NaCl, 100 mM TrisHCl, 10 mM EDTA, 1% Triton X100 (wt/vol), pH 8.0] with protease inhibitor mix (1 mM PMSF, 1 μM pepstatin A,1 μM leupeptin, 10 μM E-64; Sigma Chemical Co.) and homogenized. The homogenate was placed on ice for 30 min, and then spun at top speed for 15 min on a bench-top centrifuge (Eppendorf). 150 μl of the clear supernatant, excluding the top emulsion layer from yolk, was extracted. 15 μl of the clear extract (approximately equivalent to one oocyte) was separated by 5% SDS-PAGE, transferred to nitrocellulose, and analyzed by blotting with an InsP3R-1–specific antibody (Ab-1; Joseph and Samanta 1993; Joseph et al. 1995), or an InsP3R-3–specific antibody (Ab-3; Transduction Laboratories) and detected by enhanced chemiluminescence (Amersham Pharmacia Biotechnology).
Immunoprecipitation
The protocol used was modified from that used for immunoprecipitation of InsP3Rs from WB cell extracts (Joseph et al. 1995). For each sample, 15 oocytes were placed in 300 μl of ice-cold OEB with protease inhibitor mix and homogenized. 225 μl of clear oocyte extract was obtained as described for Western analyses. The extract was precleared for 2 h with 40 μl of protein A agarose [50% slurry (vol/vol); GIBCO BRL], and then treated overnight with Ab-1. Immune complexes of Ab-1 and X-InsP3R-1 were precipitated with protein A agarose pretreated with OEB containing 1% bovine serum albumin. The immunoprecipitated complexes were washed once using ice-cold OEB with an additional 350 mM NaCl and 1% SDS and twice using ice-cold OEB, separated by 5% SDS-PAGE, transferred to nitrocellulose, immunoblotted with Ab-3, and detected by enhanced chemiluminescence. Similar oocyte samples were precleared with protein G agarose [50% slurry (vol/vol); GIBCO BRL], treated overnight with Ab-3, immunoprecipitated with BSA-pretreated protein G agarose, washed, and immunoblotted with Ab-1.
Patch Clamping the Oocyte Nucleus
Patch-clamp experiments were performed as described previously (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). In brief, stage V or VI oocytes were opened mechanically just before use. The nucleus was separated from the cytoplasm and transferred to a dish on the stage of a microscope for patch clamping. Due to channel inactivation (Mak and Foskett 1994, Mak and Foskett 1997), unless stated otherwise, experiments were done in “on-nucleus” configuration, with the solution in the perinuclear lumen between the outer and inner nuclear membranes in apparent equilibrium with the bath solution (Mak and Foskett 1994). Following standard conventions, the applied potential is that of the pipette electrode minus the reference bath electrode (positive current flows from pipette outward). Experiments were performed at room temperature.
Single-channel currents were amplified with an Axopatch-1D amplifier (Axon Instruments) with anti-aliasing filtering at 1 kHz, transferred to a Power Macintosh 8100 via an ITC-16 interface (Instrutech Corp.), digitized at 5 kHz, and written directly onto hard disk by Pulse+PulseFit software (HEKA Elektronik). Data were analyzed and fitted with theoretical curves using MacTac 3.0 (Bruxton), and Igor Pro 3 (WaveMetrics).
Solutions for Patch-clamp Experiments
Unless stated otherwise, the pipette solutions used in patch-clamp experiments contained (mM): 140 KCl, 10 HEPES, 0.5 Na2ATP, pH adjusted to 7.1 with KOH with 0 or 3 MgCl2. Because of chelation of Mg2+ by ATP, the actual free Mg2+ in the solutions was 0 or 2.5 mM. By using K+ as the current carrier and appropriate quantities of the high-affinity Ca2+ chelator, BAPTA [100–500 μM 1,2-bis(O-aminophenoxy) ethane-N,N,N ′,N ′-tetraacetic acid; Molecular Probes, Inc.], or the low-affinity Ca2+ chelator, 5,5′-dibromo BAPTA (100–400 μM; Molecular Probes, Inc.), or just ATP (0.5 mM) to buffer [Ca2+] in the experimental solutions, [Ca2+] was tightly controlled in our experiments. Total Ca2+ content in the solutions (5–350 μM) was determined by induction-coupled plasma mass spectrometry (Mayo Medical Laboratory, Rochester, MN). Free [Ca2+] were calculated using the Maxchelator software (C. Patton, Stanford University, Stanford, CA). Pipette solutions contained various concentrations of InsP3 (Molecular Probes, Inc.) as stated. Unless stated otherwise, all patch-clamp experiments used a bath solution with the same composition as the pipette solutions except with no Na2ATP or MgCl2, and calculated free Ca2+ concentration of 250 nM.
In ion selectivity determinations, the low K+ solution contained 110 mM N-methyl-d-glucamine Cl, 30 mM KCl, 10 mM HEPES, 1.27 mM BAPTA, pH adjusted to 7.1 by KOH. [Ca2+] was calculated to be 2 μM. The high Ca2+ solution contained 50 mM CaCl2, 45 mM N-methyl-d-glucamine Cl, 30 mM KCl, 10 mM HEPES, pH adjusted to 7.1 by KOH.
Oocyte Two-Electrode Voltage-clamp Experiments
Conventional two-electrode voltage-clamp (TEV) methods (Stühmer 1992) were used to measure membrane currents in either InsP3R-3–expressing oocytes (InsP3R cRNA injected, with functional expression confirmed by patch clamping of isolated nuclei), or uninjected control oocytes, using an OC-725C oocyte clamp amplifier (Warner Instrument Corp.) connected to a PowerMac 7100 via an ITC-16 interface. Single oocytes were placed in a chamber (0.6 ml vol) containing a low-Ca2+ solution LCa96 (96 mM NaCl, 1 mM KCl, 0.2 mM CaCl2, 5.8 mM MgCl2, 10 mM HEPES, pH 7.5 by NaOH). Pulse+PulseFit software was used to ramp the applied transmembrane potential (Vm) at regular intervals. Vm was clamped at the prestimulation reversal potential in between the voltage ramps. Transmembrane current (Im) and Vm were digitized at 1 kHz during the voltage ramps and written directly onto hard disk. The whole-oocyte membrane conductance was evaluated as the slope (dIm/dVm) of a fifth-order polynomial (Jiang et al. 1998) fitted to the raw, monotonically increasing Im − Vm curve from each voltage ramp using Igor Pro 3 software.
In experiments that monitored changes in [Ca2+]i by measuring the plasma membrane Ca2+-activated Cl− current (ICl-1 described by Hartzell 1996), Vm was ramped from −60 to 20 mV in 550 ms, repeated at 1-s intervals. The membrane conductance was evaluated at Vm = 10 mV. To provide sustained stimulation to activate the InsP3R maximally, 23 nl of 10 μM InsP3 was repeatedly (90-s intervals) microinjected into the voltage-clamped oocytes using a nanoliter injector (Hartzell 1996). After relaxation of the InsP3-induced membrane current to a steady level (usually 250–300 s after first injection of InsP3), 6 ml of high-Ca2+ solution HCa96 (same composition as LCa96 except with 5.8 mM CaCl2 and 0.2 mM MgCl2) was perfused into the oocyte chamber, and the Cl− current induced by Ca2+ influx through the store-operated Ca2+ channel, ISOC described in Hartzell 1996, was recorded.
In experiments designed to measure ISOC directly, protocols previously described in Yao and Tsien 1997 were followed. Ca2+ store depletion was accomplished by extracellular application of fetal bovine serum (1:100) in LCa96 (Miledi and Parker 1989). Vm was ramped from −100 to 50 mV in 700 ms, repeated at 3-s intervals. Contributions to the membrane current by the Ca2+-activated Cl− channels were minimized by microinjection of Ca2+ chelator—41.4 nl of 100 mM BAPTA for a final concentration of ∼4 mM (Yao and Tsien 1997); and evaluating the conductance at Vm = −80 mV, where the Ca2+-activated Cl− channels (ICl-1 and ICl-2) were least active (Hartzell 1996). The bathing LCa96 solution was replaced with HCa96 by perfusion of 6 ml of HCa96 into the oocyte chamber to observed ISOC directly.
RESULTS
Expression of r-InsP3R-3 in Xenopus Oocytes
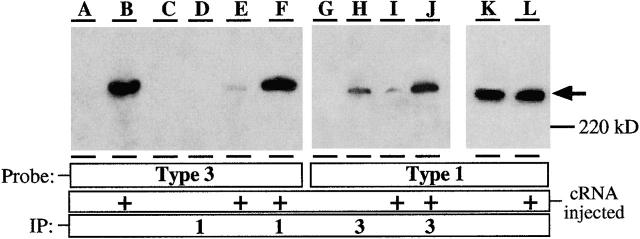
Multiple isoforms of InsP3R exist in mammalian cells, coded by different genes and expressed in distinct and overlapping patterns in various tissues. To determine whether different isoforms exhibit distinct single-channel properties, we used Xenopus oocytes because we have demonstrated that it is possible to record InsP3R channel activity in their isolated nuclei (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998), and oocytes can efficiently express mammalian ion channel proteins from injected cRNAs and cDNAs (Soreq and Seidman 1992). Oocytes microinjected with the cRNA of r-InsP3R-3 were sampled at various times after microinjection for biochemical determinations of InsP3R-3 expression. Western blot analysis using a specific InsP3R-3 antibody (Ab-3) revealed a band at the appropriate molecular weight (∼250 kD) in cRNA-injected oocytes (Fig. 1 B) that was not present in uninjected control oocytes (A), confirming expression of r-InsP3R-3 in oocytes by cRNA injection. Expression was detectable as early as 7 h after injection, and increased to a steady level by 48 h after injection. Expression level then remained constant up to 5 d after injection, before declining (not shown).
Figure 1.
Expression of endogenous type 1 and recombinant type 3 InsP3 receptors in Xenopus oocytes. (A–F) Immunoblotted with Ab-3; (G–K) immunoblotted with Ab-1. (A) Western blot of lysate from an equivalent of one control oocyte, demonstrating lack of endogenous type 3 InsP3R expression; (B) Western blot of lysate from an equivalent of one r-InsP3R-3 cRNA-injected oocyte, demonstrating expression of the recombinant r-InsP3R-3 protein; (C) Western blot of type 3 InsP3R adsorbed nonspecifically to agarose beads, from lysate from fifteen control oocytes; (D) Western blot of type 3 InsP3R in InsP3R-1 immunoprecipitate, from lysate from fifteen control oocytes; (E) Western blot of type 3 InsP3R adsorbed nonspecifically to agarose beads, from lysate from fifteen cRNA-injected oocytes; (F) Western blot of type 3 InsP3R in InsP3R-1 immunoprecipitate from lysate, from fifteen cRNA-injected oocytes. Difference between F and E represents the amount of recombinant r-InsP3R-3 specifically immunoprecipitated with X-InsP3R-1. (G) Western blot of X-InsP3R-1 adsorbed nonspecifically to agarose beads, from lysate from fifteen control oocytes; (H) Western blot of X-InsP3R-1 in InsP3R-3 immunoprecipitate, from lysate from fifteen control oocytes. The InsP3R detected is most likely caused by cross-interaction of the immunoprecipitating Ab-3 with the large amount of endogenous X-InsP3R-1 present in the lysate from fifteen oocytes. (I) Western blot of X-InsP3R-1 adsorbed nonspecifically to agarose beads, from lysate from fifteen cRNA-injected oocytes; (J) Western blot X-InsP3R-1 in InsP3R-3 immunoprecipitate, from lysate from fifteen cRNA-injected oocytes. Difference in protein amounts between H/I and J represents the amount of X-InsP3R-1 specifically immunoprecipitated with recombinant r-InsP3R-3. (K) Western blot of X-InsP3R-1 in lysate from an equivalent of one control oocyte; (L) Western blot of X-InsP3R-1 in lysate from an equivalent of one cRNA-injected oocyte. Comparisons of K and L indicate that r-InsP3R-3 expression does not induce changes in the expression levels of the endogenous X-InsP3R-1. Western blots of type 1 InsP3R in InsP3R-1 immunoprecipitates or type 3 InsP3R in InsP3R-3 immunoprecipitates are not shown because too much protein was present in those lanes for the protein bands to be properly detected with the same exposure as the other lanes.
Functional Detection of r-InsP3R-3 Expressed in Oocytes
To reduce the likelihood of detecting endogenous X-InsP3R-1 channels in patch-clamp experiments using r-InsP3R-3 cRNA-injected oocytes, we determined the level of functional expression of the endogenous X-Ins-P3R-1 channel in the outer nuclear envelope by patch-clamp experiments on isolated nuclei from freshly procured oocytes (uninjected). As described in materials and methods, batch-to-batch variability among oocytes in the functional expression of endogenous X-InsP3R-1 enabled suitable oocytes with sufficiently low endogenous channel activity to be chosen for expression studies of recombinant r-InsP3R-3. We selected for cRNA microinjection only batches of oocytes in which the probability of detecting X-InsP3R-1 activity in a nuclear patch-clamp experiment (with 10 μM InsP3 in the pipette) was extremely low: <1 of 15 patches exhibited endogenous InsP3R channel activity. Of 336 nuclear patches from uninjected oocytes in such selected batches, only 17 channels were detected in nine patches. The mean number of InsP3R channels per nuclear patch ≪n≫was 0.051. The probability of detection of endogenous InsP3R in nuclear envelopes of uninjected control oocytes maintained under identical conditions as cRNA-injected oocytes never increased over time. On the other hand, the probability of detecting InsP3R-like channel activities increased dramatically in injected oocytes measured 4–5 d after cRNA microinjection. In 767 experiments, 1,546 channels were detected in 381 patches, with 286 patches exhibiting multiple InsP3R channels. ≪n≫for the cRNA-injected oocytes increased to 2.02. Thus, the r-InsP3R-3 cRNA injection into appropriately selected batches of oocytes resulted in a >40-fold increase in the mean number of InsP3R channels detected by nuclear patch-clamp experiments.
The channel activities detected in the nuclei from the cRNA-injected oocytes (Fig. 2 A) were highly reminiscent of those displayed by the X-InsP3R-1 (B) (Mak and Foskett 1994, Mak and Foskett 1997). Two sets of experiments were conducted to confirm their identities as InsP3-sensitive channels. First, in multiple nuclear patches with the pipette solutions alternately containing either 10 μM or no InsP3, the channels were only detected in the presence of InsP3 (59 channels in 13 patches from three nuclei), but not in its absence (zero channels in seven patches). Second, the channel activities were completely suppressed by the competitive InsP3 inhibitor heparin (100 μg/ml) in all nine patches obtained from five nuclei, which showed 55 channels in 22 patches without heparin. These pharmacological results, together with the statistical data above, strongly suggested that the channel activities observed in nuclei from cRNA-injected oocytes were recombinant r-Ins-P3R-3 expressed due to the cRNA injection.
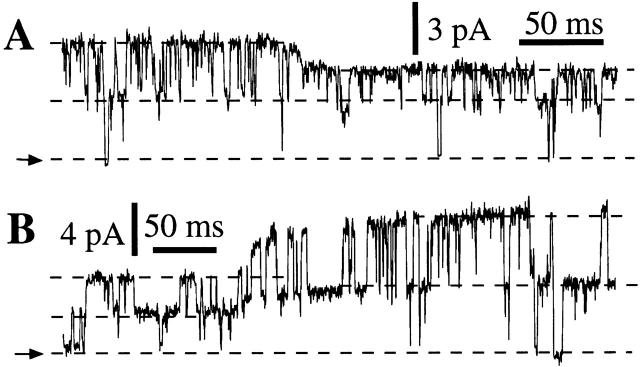
Figure 2.
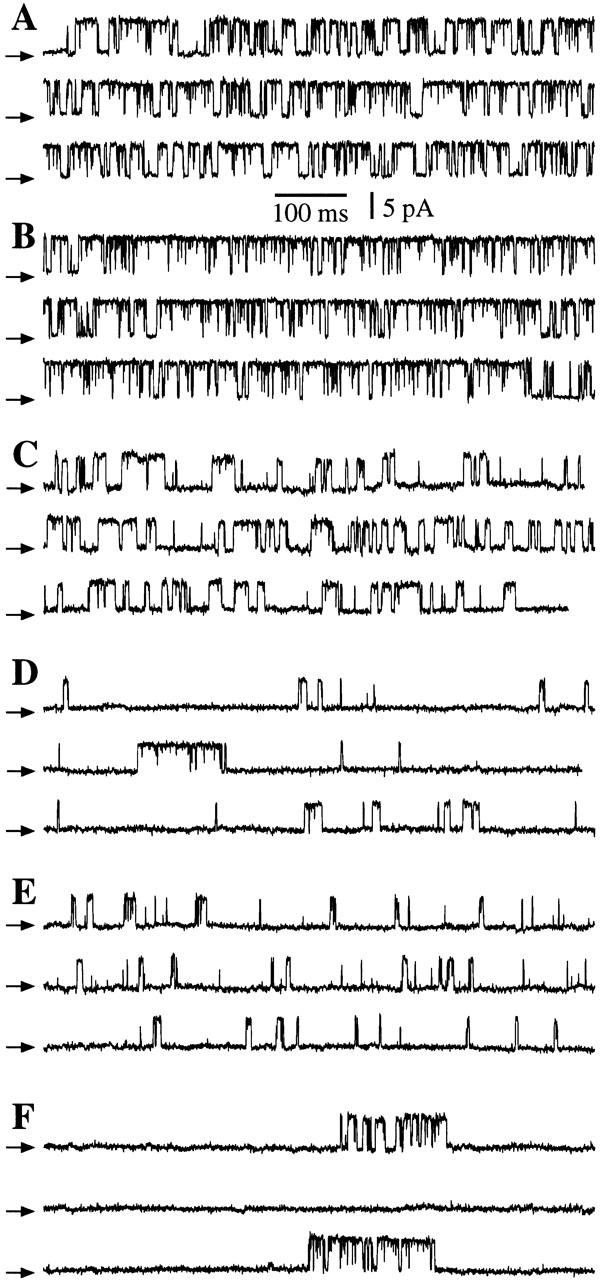
Current traces obtained by patch clamping the outer membrane of isolated oocyte nuclei in symmetric 0-mM Mg2+ solutions. All current records shown were obtained with Vapp = 20 mV, and with 0.5 mM free ATP and 10 μM InsP3 in the pipette solution. The arrows indicate the closed channel current level. (A, C, and E) Nuclei from r-InsP3R-3 cRNA-injected oocytes. (B, D, and F) Nuclei from uninjected oocytes. (A and B) [Ca2+]i = 1,150 nM. (C and D) [Ca2+]i = 80 nM. (E and F) [Ca2+]i = 57.5 μM. The average P o (±SEM) evaluated for n experiments under the shown experimental conditions are: (A) 0.75 ± 0.03 (n = 16); (B) 0.80 ± 0.02 (n = 8); (C) 0.45 ± 0.07 (n = 4); (D) 0.14 ± 0.03 (n = 7); (E) 0.23 ± 0.07 (n = 4); (F) 0.27 ± 0.11 (n = 6).
Two additional sets of evidence further indicated that the InsP3R detected in patch-clamp experiments using nuclei from cRNA-injected oocytes were not contributed by the endogenous X-InsP3R-1. First, a series of Western blot analyses of cRNA-injected oocytes conducted using a type 1 isoform-specific antibody (Ab-1) demonstrated that there was no detectable increase in the expression levels of the endogenous X-InsP3R-1 in the oocytes injected with r-InsP3R-3 cRNA compared with the uninjected ones (Fig. 1K and Fig. L). Thus, the 40-fold greater number of InsP3R channels observed in cRNA-injected oocyte nuclei was not caused by upregulation of X-InsP3R-1 expression. Second, although the InsP3R channel activities observed in cRNA-injected oocyte nuclei when [Ca2+]i > 1 μM were similar to those previously observed in uninjected oocyte nuclei (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998) (Fig. 2A and Fig. B), and the channel activities in both injected and uninjected oocyte nuclei exhibited dramatic inhibition by [Ca2+]i > 50 μM (Fig. 2E and Fig. F), when [Ca2+]i was lowered to <500 nM, InsP3R channels from r-InsP3R-3 cRNA-injected oocytes gated with a significantly higher open probability (P o) than those from uninjected oocytes (Fig. 2C and Fig. D). Thus, the channels observed upon expression of recombinant r-InsP3R-3 were distinguishable from the endogenous channels. Taken together, these results strongly suggest that the channel activities we recorded in the present work were those of recombinant r-InsP3R-3.
Heteroligomeric Interactions of r-InsP3R-3 and X-InsP3R-1
Since types 1 and 3 InsP3R isoforms can form heterotetrameric complexes in cells that coexpress them both (Joseph et al. 1995; Monkawa et al. 1995; Wojcikiewicz and He 1995; Nucifora et al. 1996), we also considered whether some of the r-InsP3R-3 channel activities we recorded might be contributed by heterotetramers formed with the X-InsP3R-1. As indicated above, the mean number of InsP3R channels detected by nuclear patch clamping increased 40-fold after r-InsP3R-3 cRNA injection. Assuming that InsP3R channels are tetramers and that they are formed by random, binomial associations of X-InsP3R-1 and r-InsP3R-3, the large majority (90.3%) of the channels detected in cRNA-injected oocytes are predicted to be homotetrameric r-InsP3R-3 channels, and most of the rest of the channels (9.4%) are predicted to be composed of one X-InsP3R-1 monomer associated with three r-InsP3R-3 monomers. To more directly investigate the extent of heterotetramer formation in our studies, we undertook a series of coimmunoprecipitation experiments. Using lysates from oocytes microinjected with r-InsP3R-3 cRNA, immunoprecipitation of X-InsP3R-1 with Ab-1 coimmunoprecipitated the expressed r-InsP3R-3, as evidenced by its mobility in SDS-PAGE and detection by immunoblotting with Ab-3 (Fig. 1 F). Two sets of control experiments demonstrated the specificity of the coimmunoprecipitation. First, r-InsP3R-3 was not detected in similar protocols using uninjected oocytes (Fig. 1C and Fig. D). Second, for both the control and injected oocytes, nonspecific binding to the agarose beads was also evaluated (Fig. 1C and Fig. E). Converse experiments were similarly performed: immunoprecipitation by Ab-3 of r-InsP3R-3 from cRNA-injected oocyte lysates coimmunoprecipitated X-InsP3R-1 (Fig. 1 J). Again, similar controls, for nonspecific, antibody-independent binding to beads (Fig. 1G and Fig. I), and using uninjected oocyte lysates (Fig. 1G and Fig. H), were performed. These experimental results suggest that the Xenopus type 1 and rat type 3 InsP3R isoforms may form heterotetrameric complexes in oocytes. The amount of r-InsP3R-3 that coimmunoprecipitated with the X-InsP3R-1 from the pooled lysates of 15 cRNA-injected oocytes (Fig. 1 F) was roughly equivalent to the amount of r-InsP3R-3 protein present in the lysate of one cRNA-injected oocyte (Fig. 1 B). In control experiments, the amount of X-InsP3R-1 immunoprecipitated by Ab-1 from the pooled lysates of 15 uninjected oocytes and detected by immunoblotting with Ab-1 was over an order of magnitude greater than the amount of X-InsP3R-1 detected by Ab-1 in the lysate of one uninjected oocyte (not shown), indicating an efficiency of immunoprecipitation of X-InsP3R-1 by Ab-1 of >67%. Therefore, the coimmunoprecipitation result indicates that ∼7–10% of the r-InsP3R-3 expressed in the cRNA-injected oocytes was involved in heteroligomer formation with the endogenous X-InsP3R-1. This biochemical result compares well with the ∼9% predicted from the binomial statistics. The good agreement between these completely independent determinations suggests that >90% of the channel activities observed in r-InsP3R-3–injected oocyte nuclei in the present studies were contributed by homotetramers of r-InsP3R-3 monomers, and <10% of them were contributed by tetramers that contained one X-InsP3R-1 monomer.
Characterization of the Single-channel Conductance and Gating Properties of r-InsP3R-3 in Xenopus Oocytes
Because these results strongly suggested that the channel activities we measured in oocytes expressing r-InsP3R-3 were overwhelmingly contributed by type 3 homotetramers, we proceeded to use this expression system to characterize the properties of the r-InsP3R-3 channel. The major rationale for developing this expression system was to determine whether different InsP3R isoforms have similar or distinct permeation and gating properties when their channel activities are measured in the same physiological membrane system. To facilitate comparisons of the permeation and gating behaviors of the two isoforms, we used conditions that were previously employed to characterize the X-InsP3R-1 in this same nuclear membrane (Mak and Foskett 1994, Mak and Foskett 1998). In symmetric KCl solution containing 2.5 mM Mg2+, the r-InsP3R-3 channel has a stable conductance (Fig. 3 A), with a channel current–voltage relation that is nonlinear and asymmetric with respect to the origin, with I(−Vapp) = −0.7 × (Vapp), where Vapp is the applied voltage (Vapp > 0 mV in this equation) (Fig. 3 B). The slope conductance near 0 mV was ∼119 pS, increasing to ∼360 pS at +50 mV. We previously demonstrated that nonlinearity of the X-InsP3R-1 I-V relation was caused by Mg2+ permeation in the channel (Mak and Foskett 1998). We therefore investigated the I-V relation in the absence of free Mg2+. In symmetric KCl solution with 0 mM Mg2+, the InsP3R-3 I-V relation became linear with a conductance of 358 ± 8 pS (see Fig. 5 B). Thus, Mg2+ is a permeant ion blocker, reducing channel conductance and causing rectification of the I-V relation in the r-InsP3R-3, as it does in the X-InsP3R-1 (Mak and Foskett 1998). Mg2+ also plays an important role in stabilizing the conductance of X-InsP3R-1 channel. A similar role for Mg2+ was observed for the r-InsP3R-3 channel. Thus, in the absence of Mg2+ on both (Fig. 4 A) or just one (B) side of the r-InsP3R-3 channel, the channel conductance occasionally fluctuated under constant Vapp during the course of a recording (∼2 min), or even during single-channel openings (∼10 ms). In patches with multiple channels, their conductances fluctuated independently (Fig. 4 A) or in concert (Fig. 4 B). However, most records showed channels with stable conductances (Fig. 2). To avoid possible effects of channel conductance fluctuations, a voltage ramp protocol (Fig. 5) was used to determine the channel conductance of the r-InsP3R-3.
Figure 3.
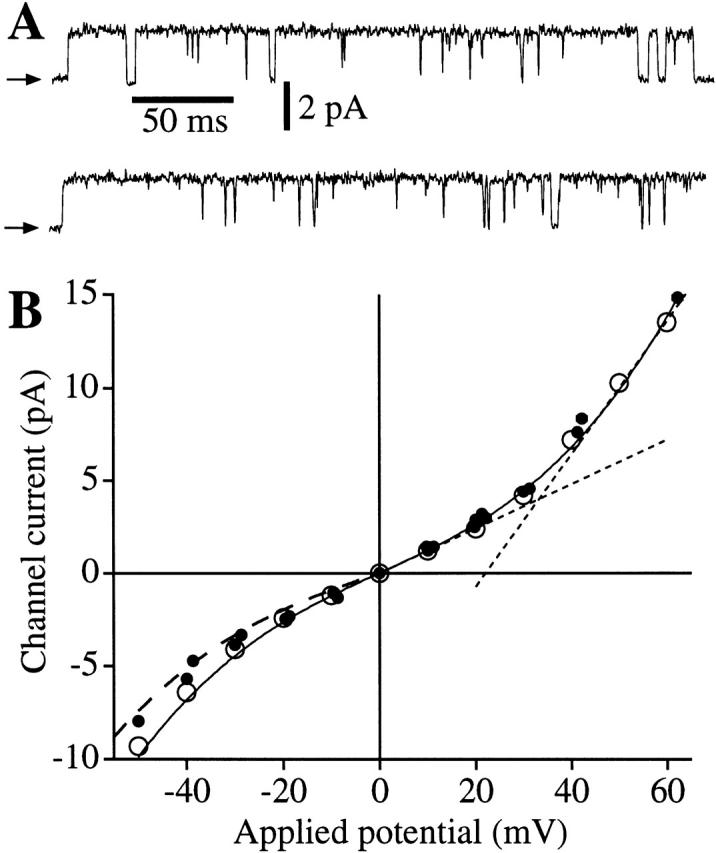
Conductance of the recombinant r-InsP3R-3 in the nuclear membrane. (A) Typical patch clamp current traces for r-InsP3R-3 channels in symmetric solutions containing 2.5 mM Mg2+ and [Ca2+]i = 940 nM. (B) I-V relation for the r-InsP3R-3 (•) in symmetric 2.5 mM Mg2+ solutions. Channel currents were averaged from all channel opening and closing events from five current records (over 100 events in each record). SEM bars are smaller than the symbols. I-V relation for the X-InsP3R-1 channels in the same ionic conditions (○) obtained previously (Mak and Foskett 1998) is also plotted for comparison. Solid curve is a fifth-order odd polynomial [I = a 1Vapp + a 3(Vapp)3 + a 5(Vapp)5] fitted to the r-InsP3R-3 data under positive Vapp. (Dotted line) The slope conductances (dI/dV) of 119 pS at 0 mV and 360 pS at 50 mV. Dashed curve: 0.7× the value of the solid curve.
Figure 5.
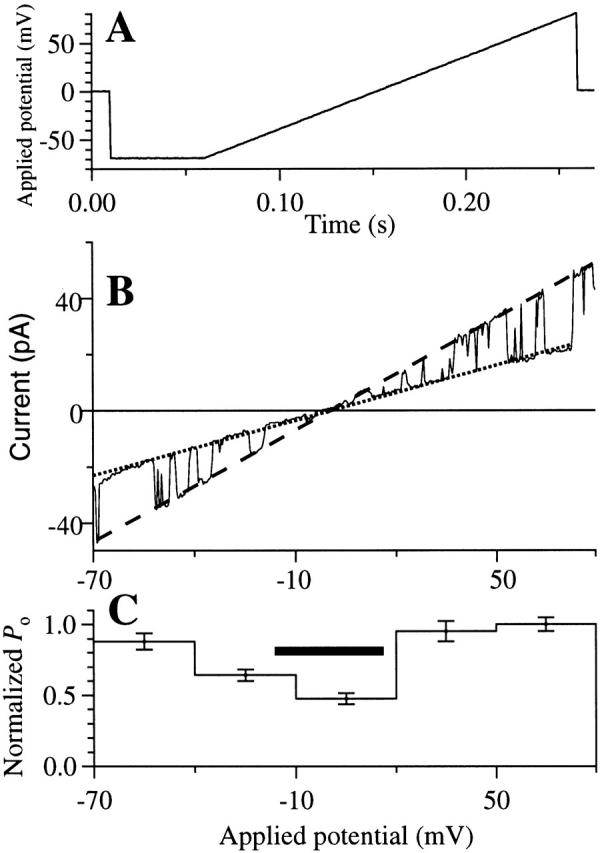
Channel properties of the r-InsP3R-3 in symmetric 0 mM Mg2+ solutions ([Ca2+]i = 80 nM) under a voltage ramp. (A) The applied voltage ramp used to obtain the current traces. (B) I-V relation obtained from a typical current trace using the voltage ramp. Dotted and dashed lines are the fitted closed and open channel I-V relations, respectively. The channel conductance was evaluated as the difference between the slopes of the two fitted lines. (C) Voltage dependence of P o of the r-InsP3R-3 channel. The P o of the channel observed at various Vapp during the voltage ramp was binned and averaged over 28 traces to generate the histogram. SEM of the P o is also plotted. The horizontal bar indicates the voltage range −15 to 15 mV in which channel openings and closings were difficult to discern, so P o was underestimated.
Figure 4.
r-InsP3R-3 channel conductance fluctuates in the absence of Mg2+. (A) Current trace from a patch with two InsP3R-3 channels, one of which had fluctuating conductance, in symmetric solutions containing 0 mM Mg2+; [Ca2+]i = 221 nM. (B) Current trace showing two InsP3R-3 channels with conductance fluctuating in concert, with 2.5 mM Mg2+ on the pipette side and 0 mM Mg2+ on the bath side of the channel; [Ca2+]i = 1,260 nM.
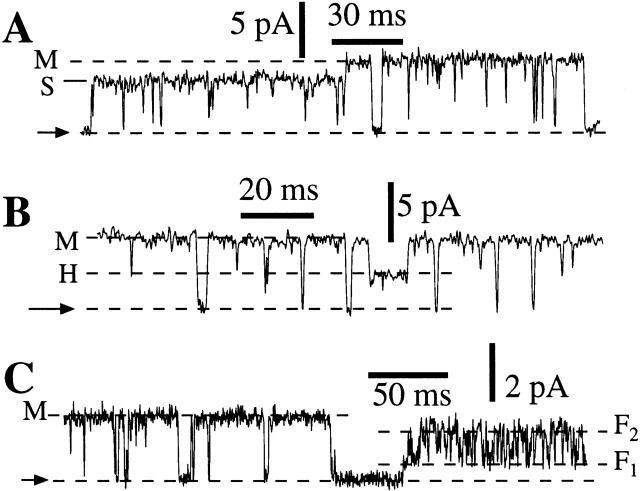
Conductance states other than the predominant main (M) state were also observed in either the presence or absence of Mg2+. Most noticeably, a small conductance state (S) with conductance ∼0.7 that of the M state (Fig. 6 A) was frequently observed (>20% of channel open time). A half (H) conductance state with conductance 0.5 that of state M was observed occasionally (<1% of channel open time) (Fig. 6 B). Besides the normal kinetic mode, the InsP3R-3 channel was sometimes (<5% of the patches) observed in a “flicker” kinetic mode, in which the channel rapidly alternated between two conductance states (F1 and F2), with conductances ∼0.25 and 0.75 that of the M state. Similar flicker kinetics have been observed in the X-InsP3R-1 channel (Mak and Foskett 1997). The conductance substates (S and H) and flicker kinetic mode were observed in both the presence and absence of Mg2+. From statistical considerations based on the frequency of observance of the substate and the probability of detecting heteroligomeric InsP3R channels, the more frequently observed substate (S) must be a substate of the r-InsP3R-3 homotetramer, but we cannot ascertain whether the less frequently observed substates (H, F1, and F2) occurred in r-InsP3R-3 homotetramers or in heterotetramers of X-InsP3R-1 and r-InsP3R-3.
Figure 6.
Conductance substates of the InsP3R channels observed in nuclear patches obtained from r-InsP3R-3 cRNA-injected oocytes. (A) Current trace showing a transition of an InsP3R channel from the small (S) conductance state to the main (M) conductance state in symmetric solutions containing 0 mM Mg2+; [Ca2+]i = 1,150 nM. (B) Current trace showing an InsP3R channel in the rare half (H) conductance state in symmetric solutions containing 0 mM Mg2+; [Ca2+]i = 255 nM. (C) Current trace showing an InsP3R channel in the normal and flicker kinetic modes in symmetric 2.5 mM Mg2+; [Ca2+]i = 940 nM. F1 and F2 denote the conductance states in the flicker kinetic mode.
To determine the ion selectivity of the r-InsP3R-3 channel, asymmetric ionic conditions were used. In the presence of a [K+] gradient, in the absence of Mg2+, the channel I-V relation is linear (Fig. 7 A) with a reversal potential (Vrev) of 25.2 mV. Using the Goldman-Hodgkin-Katz (GHK) equations (Hille 1992), the relative permeabilities of the channel to K+ and Cl− (P K/P Cl) were calculated to be 4.6. In the absence of Mg2+, with a [Ca2+] gradient, the channel I-V relation is nonlinear with Vrev = 20.6 mV (Fig. 7 B). Using the GHK equations and the value of P K/P Cl, the relative permeabilities of Ca2+ to K+ (P Ca/P K) were determined to be 11. Thus, the channel is a Ca2+-selective cation channel.
Figure 7.
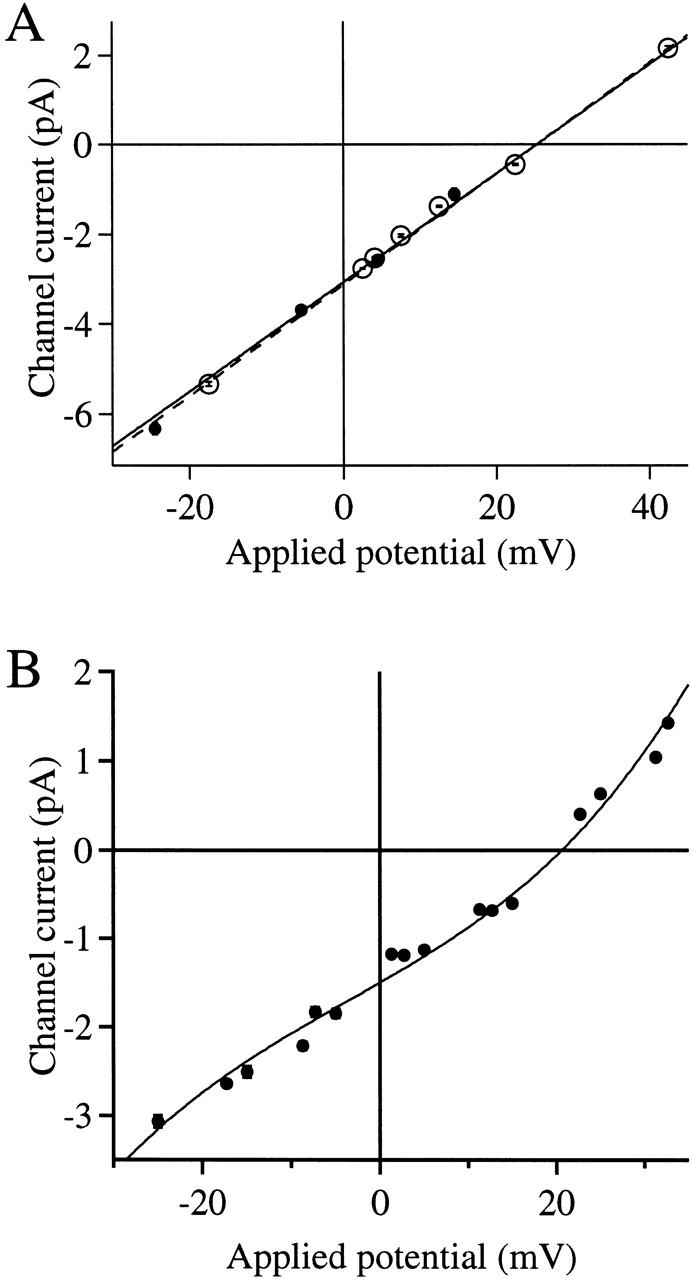
Ion selectivity of the r-InsP3R-3. (A) The I-V curve of the InsP3R-3 in the presence of solutions containing asymmetric K+ concentrations. The pipette contained the low K+ solution. The bath solution was the standard 140 mM KCl solution with 0 mM Mg2+. Vapp values were corrected for the liquid junction potential (Neher, 1992) between the asymmetric solutions across the membrane patch. Channel currents were obtained as in Fig. 3. • are data points for r-InsP3R-3 fitted with the dashed line, giving Vrev = 25.2 ± 0.7 mV. ○ are data points for X-InsP3R-1 fitted with the solid line, giving Vrev = 25.3 ± 0.2 mV. (B) The I-V curve of InsP3R-3 in the presence of a [Ca2+] gradient. The pipette contained the standard 140 mM KCl solution with 0 mM Mg2+ and [Ca2+]i = 24.7 nM. To avoid exposing the oocyte nucleus to high [Ca2+], which interferes with formation of giga-ohm seals (Mak and Foskett 1994), an excised patch with InsP3R activities was first obtained from a nucleus in the standard 140 mM KCl solution (0 mM Mg2+). The bath solution was then replaced with the high Ca2+ solution by perfusion. Vapp values were corrected for liquid junction potential (Neher, 1992) between the asymmetric solutions across the membrane patch. Channel currents were obtained as in Fig. 3. The solid curve is a fifth order polynomial fitted to the data points, giving Vrev = 20.6 ± 0.5 mV.
In all experimental conditions, inactivation of the r-InsP3R-3 channel was consistently observed. In >90% of the experiments, channel activities terminated within 2 min of formation of the seal. In some of the patches (∼10%), the inactivation could be reversed by an increase of 20–40 mV in Vapp. In some patches, the channels could be repeatedly reactivated by jumps in Vapp before they inactivated irreversibly. These results indicate that the r-InsP3R-3 channel, like the X-InsP3R-1 (Mak and Foskett 1997), has two inactivated states: one (IVdep) from which it can be reactivated by a jump in Vapp, and one (Iirr) from which it cannot exit in the presence of the steady [InsP3] and [Ca2+]i conditions used in our experiments.
Analysis of the InsP3R-3 P o in experiments in which Vapp was altered by ramps indicates that P o between −70 and −40 mV was not significantly different from that between 20 and 80 mV (Fig. 5). The decrease in P o observed between −40 and 20 mV is likely an artifact caused by the difficulty in discerning channel openings due to the small channel current under those potentials.
Clustering of the Expressed r-InsP3R-3
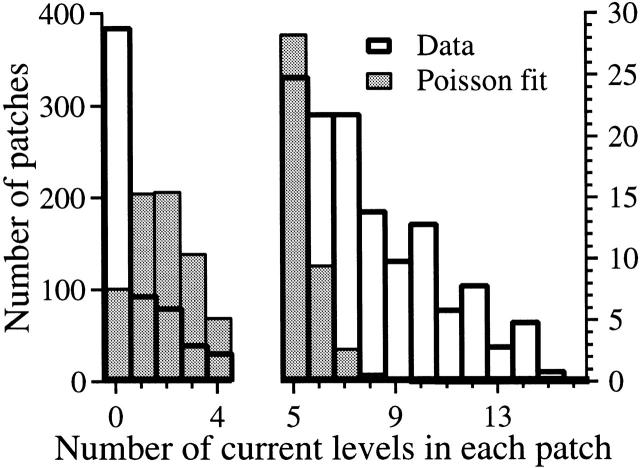
We previously reported that the X-InsP3R-1 exists in clusters in the outer membrane of the nuclear envelope (Mak and Foskett 1997). To determine whether channel clustering was also a property of the expressed r-InsP3R-3, we analyzed the distribution of channel densities among the nuclear patches from cRNA-injected oocytes. The number of channels in each patch was estimated by determining the number of InsP3R channel current levels observed. Histogram analysis of the distribution of patches exhibiting various numbers of InsP3R channel current levels was undertaken using the results from 767 nuclear patches (Fig. 8). Analysis of these results and comparisons with the Poisson distribution (Fig. 8) suggests that the expressed r-InsP3R-3 are not evenly distributed over the surface of the outer nuclear membrane, but are localized in clusters. The similar clustering observed for both the endogenous X-InsP3R-1 and recombinant expressed X-InsP3R-3 suggests that the ability of the InsP3R tetramers to associate into clusters is a general property of InsP3Rs.
Figure 8.
Histogram analysis of number of current levels (abscissa) observed in 767 nuclear patch clamp experiments using isolated nuclei from r-InsP3R-3 cRNA-injected oocytes (unshaded histogram with thick outline). Also plotted is the Poisson distribution (shaded histogram with thin outline) with the same mean current level number ≪n≫=2.016per patch. Note the different y-axis scales used in the histogram. The number of patches containing a large number of channels (6–15) exceeds that predicted from Poisson statistics, suggesting that the recombinant type 3 InsP3R channel localizes in clusters.
Role of the r-InsP3R-3 in Ca2+ Release-activated Plasma Membrane Ca2+ Influx
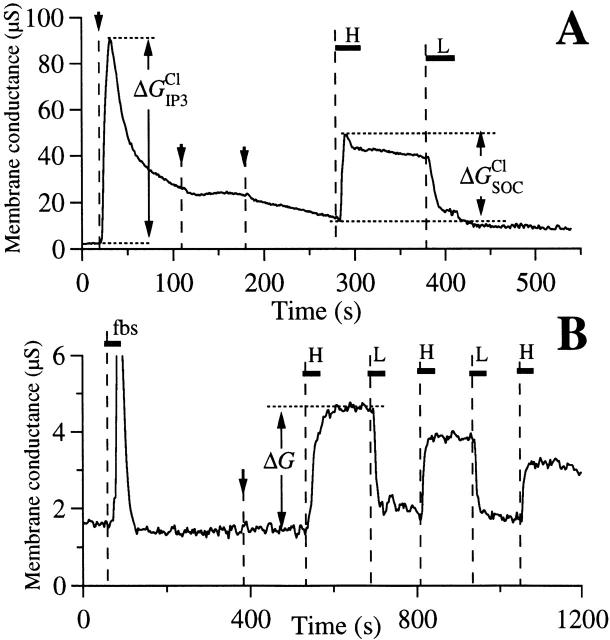
Two studies have previously concluded that the r-InsP3R-3 plays a role in mediating Ca2+ flux into the cell across the plasma membrane. In lymphocytes, the r-InsP3R-3 was localized at or near the plasma membrane in apoptotic cells, and antisense inhibition of its expression diminished apoptosis, presumably by diminishing Ca2+ influx (Khan et al. 1996). In Xenopus oocytes, expression of r-InsP3R-3 was without effect on InsP3-mediated Ca2+ release, but enhanced the extent and duration of Ca2+ influx activated by InsP3-induced Ca2+ release from intracellular stores (DeLisle et al. 1996). Ca2+ influx across the plasma membrane in response to depletion of intracellular Ca2+ stores, Ca2+-release-activated Ca2+ influx (DeLisle et al. 1996) or store-operated Ca2+ (SOC) influx (Parekh et al. 1997), has been observed in numerous cell types, but the mechanisms that couple the state of Ca2+ filling of the internal stores with the plasma membrane influx pathway has remained obscure. In a recent review, Putney 1997 suggested, based in part on the above studies, that the InsP3R-3 may contribute to, or in fact be, a plasma membrane Ca2+ influx pathway involved in this process. The InsP3R-3 has been localized near or at the plasma membrane in several cell types (Maranto 1994; Nathanson et al. 1994; Lee et al. 1997; Yule et al. 1997). Our patch-clamp results suggest that the InsP3R-3 expressed in oocytes localizes to the outer membrane of the nuclear envelope, and presumably to the ER as well, although they do not exclude a plasma membrane localization. We therefore examined whether expression of r-InsP3R-3 in Xenopus oocytes conferred an enhanced SOC influx in this cell type. We employed two different published TEV protocols to examine SOC influx in oocytes injected with r-InsP3R-3 cRNA. In one (Hartzell 1996), [Ca2+]i was monitored by recording the magnitude of the Ca2+-activated Cl− current using TEV (Fig. 9 A). InsP3 was injected into the cytoplasm to activate the InsP3R, as evidenced by a rapid increase of the Ca2+-activated Cl− current ΔG IP3 Cl.After depletion of the internal Ca2+ stores, as evidenced by the lack of Cl− current responses to repeated subsequent InsP3 injections (Fig. 9 A), the magnitude of Ca2+ influx was monitored indirectly by the Cl− current response ΔG SOC Clto an acute step increase in [Ca2+] in the bathing solution. Comparison of responses of control (n = 14 oocytes from five different batches) and r-InsP3R-3–expressing (n = 21 oocytes from the same five batches) oocytes revealed no significant difference in the magnitudes of ΔG IP3 Cl(24.2 ± 2.6 μS, mean ± SEM, for controls oocytes; 22.9 ± 3.8 μS for r-InsP3R-3 expressing oocytes; P = 0.77) or ΔG SOC Cl(26.6 ± 5.0 μS for control oocytes; 20.2 ± 3.9 μS for expressing oocytes; P = 0.32). Functional expression of the r-InsP3R-3 in these experiments was verified by high probability of detecting InsP3R channel activities during subsequent patch clamping of nuclei from the oocytes used in the TEV experiments. Thus, we were unable to reproduce the results of DeLisle et al. 1996. A second protocol (Yao and Tsien 1997) was also employed (Fig. 9 B). Membrane conductance (ΔG) due to the SOC influx pathway was not different (P = 0.98) in control (n = 3, mean = 2.4 ± 0.5 μS) compared with InsP3R-3–expressing oocytes (n = 4, mean = 2.4 ± 0.3 μS). These studies also do not support the hypothesis that InsP3R-3 plays a rate-limiting role in mediating SOC influx.
Figure 9.
Store-operated calcium influx across the oocyte plasma membrane. Whole-cell transmembrane conductance of r-InsP3R-3 cRNA-injected Xenopus oocytes was recorded in two types of TEV experiments. (A) Ca2+-activated plasma membrane Cl− channel current after microinjection of InsP3 in various bathing solutions. Arrows indicate injections of InsP3 into the oocyte. Horizontal bars indicate exchange of bathing solution by perfusion with a high Ca2+ solution HCa96 (H) or a low Ca2+ solution LCa96 (L). The magnitudes of the changes in membrane conductance due to plasma membrane Ca2+-activated Cl− current response to InsP3 injection ΔG IP3 Cland due to Ca2+ influx through store-operated Ca2+ channels ΔG SOC Clare marked. (B) Store-operated Ca2+ currents after activation of InsP3R in various bathing solutions. Horizontal bars indicate exchange of bathing solution with high Ca2+ solution HCa96 (H), low Ca2+ solution LCa96 (L), and low Ca2+ LCa96 containing 1:100 fetal bovine serum (fbs). Arrow indicates microinjection of BAPTA. Magnitude of changes in membrane conductance due to Ca2+ influx through store-operated Ca2+ channels (ΔG) is indicated.
DISCUSSION
In this report, we have demonstrated the functional expression of the type 3 isoform of the InsP3R, a widely expressed isoform in mammalian tissues, in a system which enabled recording of its single-channel activity in its normal ER membrane environment. Patch-clamp electrophysiology of the r-InsP3R-3 in the Xenopus nuclear envelope represents the first description of the permeation and gating properties of the recombinant type 3 InsP3R.
Use of Xenopus oocytes for Expression of Recombinant ER-localized Ion Channels
A major impediment to understanding [Ca2+]i signaling has been the lack of robust systems to study the gating and permeation properties of the InsP3Rs. As intracellular ion channels, they have been considered inaccessible to patch-clamp electrophysiological approaches. Most studies have therefore employed indirect measurements that can only infer channel activity, and which report the responses from populations of channels containing multiple receptor isoforms. Some recent studies have employed cultured cells that express predominantly one isoform (Kaznacheyeva et al. 1998), but these studies continue to suffer from the problems of receptor heterogeneity as well as the inability to understand Ca2+ release properties within the context of relevant ion channel parameters, including gating and permeation measures. As with other intracellular ion channels, reconstitution of the InsP3R into planar lipid membranes has been used to record the single-channel properties (Bezprozvanny et al. 1991, Bezprozvanny et al. 1994; Watras et al. 1991; Bezprozvanny and Ehrlich 1994; Perez et al. 1997; Ramos-Franco et al. 1998b). Two strategies have been employed. In the first, membranes from cerebellum (Bezprozvanny et al. 1991; Watras et al. 1991), heart (Perez et al. 1997; Ramos-Franco et al. 1998b) and RIN (Hagar et al. 1998) cells have been used, because of their high expression levels of InsP3R-1, InsP3R-2, and InsP3R-3, respectively. In the second, membranes were obtained from cells engineered to over-express recombinant InsP3R: InsP3R-1 in HEK-293 cells (Kaznacheyeva et al. 1998), and wild-type and mutant InsP3R-1 in COS-1 cells (Ramos-Franco et al. 1998a, Ramos-Franco et al. 1999). The latter approach has the potential advantage of enabling structure–function studies of the protein. However, the relevance of channel behavior in vitro in artificial bilayers for in vivo behavior in ER membrane remains unknown.
Since the ER is continuous with the outer membrane of the nuclear envelope (Dingwall and Laskey 1992), we reasoned that ER-localized ion channels would also be present in the nuclear envelope outer membrane, and that single-channel properties of the InsP3R in its native membrane environment could be examined by patch clamping isolated nuclei. Formation of seals with gigaohm resistance on the outer nuclear membrane, a prerequisite for studying single ion channel activity, was achieved with high frequency despite the high density of nuclear pores in the Xenopus oocyte nuclear envelope (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Stehno-Bittel et al. 1995). We have used this system to study the endogenous InsP3R channel (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). The oocyte expresses only a single InsP3R isoform (type 1) and lacks other (e.g., ryanodine receptor) Ca2+ release channels (Parys et al. 1992). Because the Xenopus oocyte has been used extensively to express functional recombinant mammalian plasma membrane ion channels, we reasoned that this system, combined with nuclear patch clamping, would enable the recording of recombinant InsP3R channel activities in their native ER membrane. The results presented here demonstrate that this is the case. The major potential complication in this approach is that the oocyte nucleus may not present a null background for expression of recombinant channels, due to the presence of endogenous InsP3R channels. Nevertheless, we have been able to minimize this problem by using batches of oocytes in which endogenous channel activity could not be detected by nuclear patch clamping. The basis for frog-to-frog variability in our ability to record the endogenous InsP3R-1 in oocyte nuclei is not clear, although we believe there is a seasonal component, in addition to others. It has not been uncommon in our experience to examine oocytes from many different frogs over the course of a couple of months and not record the endogenous channel in any of hundreds of nuclear patches. In preliminary experiments, we determined that the lack of detectable endogenous InsP3R channel activities immediately after oocyte isolation did not reverse over time in culture. Therefore, in the present study, we selected “null backgrounds” for expression of the type 3 channel by performing a day of screening patch-clamp experiments to ascertain that the endogenous channel was minimally present in the nuclei of the oocytes used for injection. In contrast, in oocytes injected to express the r-InsP3R-3, the number of InsP3R channels observed in nuclear patches was dramatically increased by nearly 40-fold. Based purely on statistical probabilities, there is therefore little likelihood of contamination of the data by InsP3R-1 homotetramers in our studies. Nevertheless, it remained possible that expression of the recombinant r-InsP3R-3 might have upregulated the endogenous Xenopus type 1 channel. However, Western blot analysis revealed that the amount of X-InsP3R-1 remained constant in both control and injected oocytes. These results strongly indicate that channel activities recorded in the r-InsP3R-3–expressing oocytes were contributed by the recombinant channels. Assuming random association of monomers to form tetrameric functional channels, binomial statistics indicates that >90% of the recorded channels in these studies are contributed by r-InsP3R-3 homotetramers, whereas <10% of recorded channels represent tetramers containing three type 3 monomers with one type 1 monomer. The validity of these statistically based conclusions was evaluated in coimmunoprecipitation experiments using control and injected oocytes. We determined that the endogenous type 1 InsP3R coimmunoprecipitated with the expressed recombinant type 3 InsP3R. Coimmunoprecipitation of different InsP3R isoforms has previously been interpreted to indicate heterotetramer formation (Joseph et al. 1995; Monkawa et al. 1995; Wojcikiewicz and He 1995; Nucifora et al. 1996). Of note, quantification of the amounts of coimmunoprecipitated protein suggests that <10% of the expressed recombinant type 3 channels were associated with the endogenous Xenopus channels. This biochemical result is in good agreement with the extent of isoform associations predicted from patch clamp statistics. Thus, the combined patch-clamp results and their statistical analysis, strongly corroborated by biochemical evidence, indicates that a large majority (>90%) of the patch-clamp data in the present experiments were contributed by r-InsP3R-3 homotetramers.
The similar permeation properties of the expressed and endogenous channels, as we have now defined in the present work, necessitated this exhaustive approach to ensure the identities of the recorded channels. Nevertheless, we discovered that the gating properties of the two channels can be used to distinguish them, under conditions of low cytoplasmic Ca2+ concentration. Thus, whereas the P o of both channels is equivalent (∼0.8) when cytoplasmic Ca2+ concentration is 1 μM (Mak et al. 1998, and this study), the P o of the type 3 channel is considerably higher than that of the endogenous channel when cytoplasmic Ca2+ concentration is ∼80 nM. Although more detailed studies are required to define the Ca2+ concentration dependence of InsP3R-3 gating, this ability to distinguish the expressed channels from the endogenous ones provides additional strong support for the validity of this approach. Parenthetically, our results suggest that high [Ca2+]i (>50 μM) inhibits the type 3 channel similarly to the endogenous type 1 channel. The conclusion that high [Ca2+]i inhibition does not distinguish the types 1 and 3 channels contrasts with that reached in a bilayer study using microsomes isolated from a cell type expressing predominately the type 3 isoform (Hagar et al. 1998). The bases for the discrepant results in the two studies are not known. In the present study, homotetramers of the type 3 isoform were recorded, as discussed above, in a native membrane environment, whereas the bilayer study recorded channels of unknown isoform composition in artificial membranes after reconstitution. Further, more detailed studies are in progress to determine the complete [Ca2+]i dependence of InsP3R-3 gating, which may help to resolve these discrepancies. Our success in the approach described in the current work suggests that it will now be possible to express and record the properties of mutant InsP3Rs in the ER in future studies of structure–function relationships, and that it will be useful for studying other ER-localized ion channels, whether localized there normally or as a result of natural or engineered mutations. Of note, however, it may be necessary to perform a similar series of control experiments for each mutant expressed to ensure that the system behaves as we have described for the wild-type r-InsP3R-3.
Permeation and Gating Properties of the r-InsP3R-3 and X-InsP3R-1 from Nuclear Patch Clamp Studies
We examined the basic channel properties of the r-InsP3R-3 and found that they are remarkably similar, particularly in view of both the isoform and species differences, to those of the X-InsP3R-1 in the same membrane system.
In terms of permeation, both channels are relatively nonselective cation channels: P K/P Cl is 4.6 for both X-InsP3R-1 and r-InsP3R-3 (Fig. 3). Our previously reported value of P K/P Cl for X-InsP3R-1 (Mak and Foskett 1994) was overestimated because Mg2+ present in the pipette and bath solutions in those experiments caused nonlinearity in the I-V relation of the channel (Mak and Foskett 1998) and the use of the Goldman-Hodgkin-Katz equation to fit the experimental I-V relation and determine Vrev was inappropriate. Applying this revised value of P K/P Cl, P Ca:P Ba:P Mg:P K for X-InsP3R-1 is calculated to be 12.7:8.6:6.4:1 based on Vrev measured in Mak and Foskett 1998. Thus, the values for P Ca/P K for the two channels are very similar (12.7 for X-IP3R-1; 11.0 for r-IP3R-3). In both channels, the I-V relation is linear in the absence of Mg2+, and the absence of Mg2+ is associated with occasional conductance fluctuations under constant Vapp during the course of a recording (∼2 min), or even during single-channel openings (∼10 ms). The conductances of both channels are stabilized and become rectified as [Mg2+] is increased on either side of the membrane. Mg2+ decreases single-channel conductance of both channels to a similar extent (Fig. 3 B), due to permeant ion block (Mak and Foskett 1998).
Both channels display conductance states of S and H (Mak and Foskett 1994, Mak and Foskett 1998), while both gate primarily in the main M state, though the frequency of finding the channel in state S was much higher in the r-InsP3R-3. Both channels undergo two forms of InsP3-dependent inactivation: one (relatively rare) that can be reversed by momentarily jumping the applied potential to 60 mV, and one (all channels) that is apparently irreversible under our steady state experimental conditions. Although we have not quantified the kinetics of irreversible inactivation for the InsP3R-3, it is our general impression that they are similar to those of the X-InsP3R-1 (time constant ∼25 s) (Mak and Foskett 1997). Both channels displayed flicker kinetics, although this was observed less frequently for the type 3 channel, and the relative values of the two flicker state conductances is the same for both receptors (Mak and Foskett 1997).
A difference between the two types of InsP3R is their voltage dependencies. Whereas P o of the X-InsP3R-1 was reduced by an increase in Vapp (Mak and Foskett 1997), the P o of r-InsP3R-3 showed little voltage dependence, or was even slightly activated by high magnitude Vapp regardless of polarity (this study).
Comparison of the Permeation and Gating Properties of Different InsP3R Isoforms
Our results suggest that the permeation pathways and gating mechanisms are remarkably similar between the types 1 and 3 InsP3R channels. Including the present data, single-channel recordings have now been obtained for all three known InsP3R isoforms. A side-by-side comparison of the bovine type 1 and ferret type 2 receptors in proteoliposome-fused bilayers revealed that their conductance properties were nearly identical (Ramos-Franco et al. 1998b). Taken together with our results, these data indicate that the permeation pathways in all three InsP3R channels are very similar. This result has implications for the structural properties of the conduction pores in the three isoforms. Of note, there is conservation among the isoforms of amino acid sequences in regions that are speculated to be involved in pore formation (Yamamoto-Hino et al. 1994).
Nevertheless, some differences have been reported in the gating behaviors of the three channel isoforms, although it is not clear whether they reflect isoform differences or are due to unknown variables associated with different experimental protocols. The type 1 channel has been studied in different systems, including reconstitution of cerebellar membranes (Bezprozvanny et al. 1991, Bezprozvanny et al. 1994; Watras et al. 1991; Bezprozvanny and Ehrlich 1994) and proteoliposomes (Kaznacheyeva et al. 1998) and patch clamp of the Xenopus nuclear envelope (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Stehno-Bittel et al. 1995), whereas the type 2 channel has been studied using reconstituted proteoliposomes (Perez et al. 1997; Ramos-Franco et al. 1998b), and the type 3 channel has been studied by reconstitution (Hagar et al. 1998) and nuclear patch clamping (this study). Furthermore, the channels have come from different species (type 1: canine, bovine, Xenopus, rat; type 2: ferret; type 3: rat). Reconstituted receptors in bilayers are distinguished by a maximum P o of ∼0.7 for the type 2 channel vs. ∼0.2 for the type 1 isoform (Ramos-Franco et al. 1998b) and ∼0.05 for the type 3 channel (Hagar et al. 1998). These differences were interpreted as reflecting isoform differences (Ramos-Franco et al. 1998b), but the fact that the maximum P o observed for both the X-InsP3R-1 (Mak et al. 1998) and r-InsP3R-3 (Fig. 2 and Fig. 3 A) was nearly identical (∼0.8) suggests that isoform differences may not be particularly relevant. Indeed, other factors may be more important since the gating properties reported for the type 1 receptor from Xenopus in the nuclear membrane and rat/canine in artificial bilayers are quite distinct, and those for the rat type 3 channel in endoplasmic reticulum (this study) versus bilayers (Hagar et al. 1998) are similarly distinct. The X-InsP3R-1 studied by nuclear patch clamping (Mak and Foskett 1997) has a much higher maximum P o, longer open time constant, and prominence of a different conductance state compared with the r-InsP3R-1 studied by reconstitution into lipid bilayers (Watras et al. 1991), and demonstrates inactivation and voltage-dependent and flicker kinetics that have not been observed in the r-InsP3R-1 studies. Similarly, the r-InsP3R-3 studied by nuclear patch clamping (this study) has a maximum P o of 80% vs. 5% for the reconstituted channel in bilayers (Hagar et al. 1998), and also displays the same rich repertoire of gating behaviors not observed in the bilayer studies. It is possible that the InsP3R channel properties are sensitive to the membrane isolation, solubilization, and reconstitution protocols employed in bilayer studies. This explanation cannot, however, account for the differences observed between the types 1 and 2 receptors using similar reconstitution protocols (Ramos-Franco et al. 1998b) unless there is a differential sensitivity among isoforms or tissue sources to these procedures. In preliminary studies, we expressed the r-InsP3R-1 in Xenopus oocytes and patch clamped the oocyte nuclear membrane, and have observed gating properties that are similar to those of the X-InsP3R-1 and r-InsP3R-3 in the same membrane (our unpublished data). This result suggests that the channels in the same native membrane environment gate similarly, and that the channel properties are sensitive to the membrane environment or to the protocols involved in reconstitution. Although further single-channel permeation and gating determinations are necessary to resolve these issues, the data available to date suggest that the three receptor isoforms share quite similar permeation properties. Taken together, these results therefore suggest that if cellular expression of multiple InsP3R isoforms is a mechanism to modify the temporal and spatial features of [Ca2+]i signals, then it must be achieved by isoform-specific regulation or localization of various types of InsP3Rs that have relatively similar Ca2+ permeation properties.
Acknowledgments
We thank Dr. Graeme I. Bell and Dr. Bill Skach for providing expression vectors.
This work was supported by grants to J.K. Foskett from the National Institutes of Health (NIH) (GM56328) and the Department of Defense, and to S.K. Joseph from the NIH (DK34804).
Footnotes
Abbreviations used in this paper: Ab-1, InsP3R-1–specific antibody; ER, endoplasmic reticulum; InsP3, inositol 1,4,5-trisphosphate; InsP3R, InsP3 receptor; SOC, store-operated Ca2+; TEV, two-electrode voltage-clamp.
References
- Amundson J., Clapham D. Calcium waves. Curr. Opin. Neurobiol. 1993;3:375–382. doi: 10.1016/0959-4388(93)90131-h. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys. J. 1993;65:1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signaling. Ann. NY Acad. Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Bezprozvannaya S., Ehrlich B.E. Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate–gated calcium channels from cerebellum. Mol. Biol. Cell. 1994;5:97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. Inositol (1,4,5)-trisphosphate (InsP3)–gated Ca channels from cerebellumconduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G.I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 1993;268:11356–11363. [PubMed] [Google Scholar]
- Boitano S., Dirksen E.R., Sanderson M.J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Bush K.T., Stuart R.O., Li S.-H., Moura L.A., Sharp A.H., Ross C.A., Nigam S.K. Epithelial inositol 1,4,5-trisphosphate receptors. Multiplicity of localization, solubility, and isoforms. J. Biol. Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Dal Santo P., Logan M.A., Chisholm A.D., Jorgensen E.M. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C-elegans . Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- Danoff S.K., Ferris C.D., Donath C., Fischer G., Munemitsu S., Ullrich S., Snyder S.H., Ross C.A. Inositol 1,4,5-trisphosphate receptorsdistinct neuronal and nonneuronal forms generated by alternative splicing differ in phosphorylation. Proc. Natl. Acad. Sci. USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt H., Missiaen L., Parys J.B., Bootman M.D., Mertens L., Van den Bosch L., Casteels R. Determination of relative amounts of inositol trisphosphate receptor mRNA isoforms by ratio polymerase chain reaction. J. Biol. Chem. 1994;269:21691–21698. [PubMed] [Google Scholar]
- De Smedt H., Missiaen L., Parys J.B., Henning R.H., Sienaert I., Vanlingen S., Gijsens A., Himpens B., Casteels R. Isoform diversity of the inositol trisphosphate receptor in cell types of mouse origin. Biochem. J. 1997;322:575–583. doi: 10.1042/bj3220575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Blondel O., Longo F.J., Schnabel W.E., Bell G.I., Welsh M.J. Expression of inositol 1,4,5-trisphosphate receptors changes the Ca2+ signal of Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1996;270:C1255–C1261. doi: 10.1152/ajpcell.1996.270.4.C1255. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. The nuclear membrane. Science. 1992;258:942–947. doi: 10.1126/science.1439805. [DOI] [PubMed] [Google Scholar]
- Ferris C.D., Huganir R.L., Supattapone S., Snyder S.H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Ferris C.D., Snyder S.H. Inositol phosphate receptors and calcium disposition in the brain. J. Neurosci. 1992;12:1567–1574. doi: 10.1523/JNEUROSCI.12-05-01567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino I., Yamada N., Miyawaki A., Hasegawa M., Furuichi T., Mikoshiba K. Differential expression of type 2 and type 3 inositol 1,4,5-trisphosphate receptor mRNAs in various mouse tissuesin situ hybridization study. Cell Tissue Res. 1995;280:201–210. doi: 10.1007/BF00307790. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400 . Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Kohda K., Miyawaki A., Mikoshiba K. Intracellular channels. Curr. Opin. Neurobiol. 1994;4:294–303. doi: 10.1016/0959-4388(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- Goldin A.L. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–278. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- Hagar R.E., Burgstahler A.D., Nathanson M.H., Ehrlich B.E. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H.C. Activation of different Cl currents in Xenopus oocytes by Ca liberated from stores and by capacitative Ca influx. J. Gen. Physiol. 1996;108:157–175. doi: 10.1085/jgp.108.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes 1992. Sinauer Associates, Inc; Sunderland, MA: pp. 341–348 [Google Scholar]
- Honda Z., Takano T., Hirose N., Suzuki T., Muto A., Kume S., Mikoshiba K., Itoh K., Shimizu T. Gq pathway desensitizes chemotactic receptor-induced calcium signaling via inositol trisphosphate receptor down-regulation. J. Biol. Chem. 1995;270:4840–4844. doi: 10.1074/jbc.270.9.4840. [DOI] [PubMed] [Google Scholar]
- Jiang Q.S., Mak D., Devidas S., Schwiebert E.M., Bragin A., Zhang Y., Skach W.R., Guggino W.B., Foskett J.K., Engelhardt F. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J. Cell Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K., Samanta S. Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J. Biol. Chem. 1993;268:6477–6486. [PubMed] [Google Scholar]
- Joseph S.K. The inositol trisphosphate receptor family. Cell Signal. 1995;8:1–7. doi: 10.1016/0898-6568(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Lin C., Pierson S., Thomas A.P., Maranto A.R. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J. Biol. Chem. 1995;270:23310–23316. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- Kaftan E.J., Ehrlich B.E., Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J. Gen. Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva E., Lupu V.D., Bezprozvanny I. Single-channel properties of inositol (1,4,5)-trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol. 1998;111:847–856. doi: 10.1085/jgp.111.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.A., Soloski M.J., Sharp A.H., Schilling G., Sabatini D.M., Li S.H., Ross C.A., Snyder S.H. Lymphocyte apoptosismediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptorstructure, function, and localization in oocytes and eggs. Cell. 1993;73:555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Lechleiter J.D., Clapham D.E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu X., Zeng W.Z., Diaz J., Wojcikiewicz R.J.H., Kuo T.H., Wuytack F., Racymaekers L., Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells—correlation with initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J. Biol. Chem. 1991;266:1109–1116. [PubMed] [Google Scholar]
- Magnusson A., Haug L.S., Walaas S.I., Ostvold A.C. Calcium-induced degradation of the inositol (1,4,5)-trisphosphate receptor/Ca2+-channel. FEBS Lett. 1993;323:229–232. doi: 10.1016/0014-5793(93)81345-z. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto A.R. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J. Biol. Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- Mignery G.A., Sudhof T.C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J. Physiol. 1989;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkawa T., Miyawaki A., Sugiyama T., Yoneshima H., Yamamoto-Hino M., Furuichi T., Saruta T., Hasegawa M., Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J. Biol. Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Okano H., Furuichi T., Aruga J., Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally-specific manner. Proc. Natl. Acad. Sci. USA. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M.H., Fallon M.B., Padfield P.J., Maranto A.R. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J. Biol. Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- Newton C.L., Mignery G.A., Südhof T.C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3 . J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- Nucifora F.C., Jr., Sharp A.H., Milgram S.L., Ross C.A. Inositol 1,4,5-trisphosphate receptors in endocrine cellslocalization and association in hetero- and homotetramers. Mol. Biol. Cell. 1996;7:949–960. doi: 10.1091/mbc.7.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A.B., Fleig A., Penner R. The store-operated calcium current ICRACnonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Parys J., Sernett S., DeLisle S., Snyder P., Welsh M., Campbell K. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J. Biol. Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- Perez P.J., Ramos-Franco J., Fill M., Mignery G.A. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- Putney J.W., Jr., Bird G.S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr. Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Putney J.W., Jr. Type 3 inositol 1,4,5-trisphosphate receptor and capacitative calcium entry. Cell Calc. 1997;21:257–261. doi: 10.1016/s0143-4160(97)90050-6. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J., Caenepeel S., Fill M., Mignery G. Single channel function of recombinant type-1 inositol 1,4,5-trisphosphate receptor ligand binding domain splice variants Biophys. J. 75 1998. 2783 2793a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco J., Fill M., Mignery G.A. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels Biophys. J. 75 1998. 834 839b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco J., Galvan D., Mignery G.A., Fill M. Location of the permeation pathway in the recombinant type I inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol. 1999;114:243–250. doi: 10.1085/jgp.114.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T.A., Thomas A.P. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calc. 1993;14:674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- Soreq H., Seidman S. Xenopus oocyte microinjectionfrom gene to protein. Methods Enzymol. 1992;207:225–265. doi: 10.1016/0076-6879(92)07016-h. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Lückhoff A., Clapham D.E. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Stühmer W. Electrophysiological recording from Xenopus oocytes. Methods Enzymol. 1992;207:319–338. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C., Newton C.L., Archer B.T.I., Ushkaryov Y.A., Mignery G.A. Structure of a novel InsP3-receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Furuya A., Monkawa T., Yamamoto-Hino M., Satoh S., Ohmori K., Miyawaki A., Hanai N., Mikoshiba K., Hasegawa M. Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptorapplication to the studies on inflammatory cells. FEBS Lett. 1994;354:149–154. doi: 10.1016/0014-5793(94)01099-4. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P.F., Baraban J.M., Snyder S.H. Solubilization, purification and isolation of an inositol trisphosphate receptor. J. Biol. Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- Taylor C.W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol. Ther. 1991;51:97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Toescu E.C. Temporal and spatial heterogeneities of Ca2+ signalingmechanisms and physiological roles. Am. J. Physiol. 1995;269:G173–G185. doi: 10.1152/ajpgi.1995.269.2.G173. [DOI] [PubMed] [Google Scholar]
- Watras J., Bezprozvanny I., Ehrlich B.E. Inositol 1,4,5-trisphosphate-gated channels in cerebellumpresence of multiple conductance states. J. Neurosci. 1991;11:3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H., Furuichi T., Nakade S., Mikoshiba K., Nahorski S.R. Muscarinic receptor activation down-regulates the type I inositol 1,4,5-trisphosphate receptor by accelerating its degradation. J. Biol. Chem. 1994;269:7963–7969. [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H., He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor coimmunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem. Biophys. Res. Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- Yamada N., Makino Y., Clark R.A., Pearson D.W., Mattei M.-G., Guénet J.-L., Ohama E., Fujino I., Miyawaki A., Furuichi T., Mikoshiba K. Human inositol 1,4,5-trisphosphate type-1 receptor, InsP 3R1structure, function, regulation of expression and chromosomal localization. Biochem. J. 1994;302:781–790. doi: 10.1042/bj3020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Sugiyama T., Hikichi K., Mattei M.G., Hasegawa K., Sekine S., Sakurada K., Miyawaki A., Furuichi T., Hasegawa M., Mikoshiba K. Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Receptors Channels. 1994;2:9–22. [PubMed] [Google Scholar]
- Yao Y., Tsien R.Y. Calcium current activated by depletion of calcium stores in Xenopus oocytes. J. Gen. Physiol. 1997;109:703–715. doi: 10.1085/jgp.109.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Tanimura T., Miyawaki A., Nakamura M., Yuzaki M., Furuichi T., Mikoshiba K. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J. Biol. Chem. 1992;267:16613–16619. [PubMed] [Google Scholar]
- Yule D.I., Ernst S.A., Ohnishi H., Wojcikiewicz R.J.H. Evidence that zymogen granules are not a physiologically relevant calcium pool—defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J. Biol. Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]