Abstract
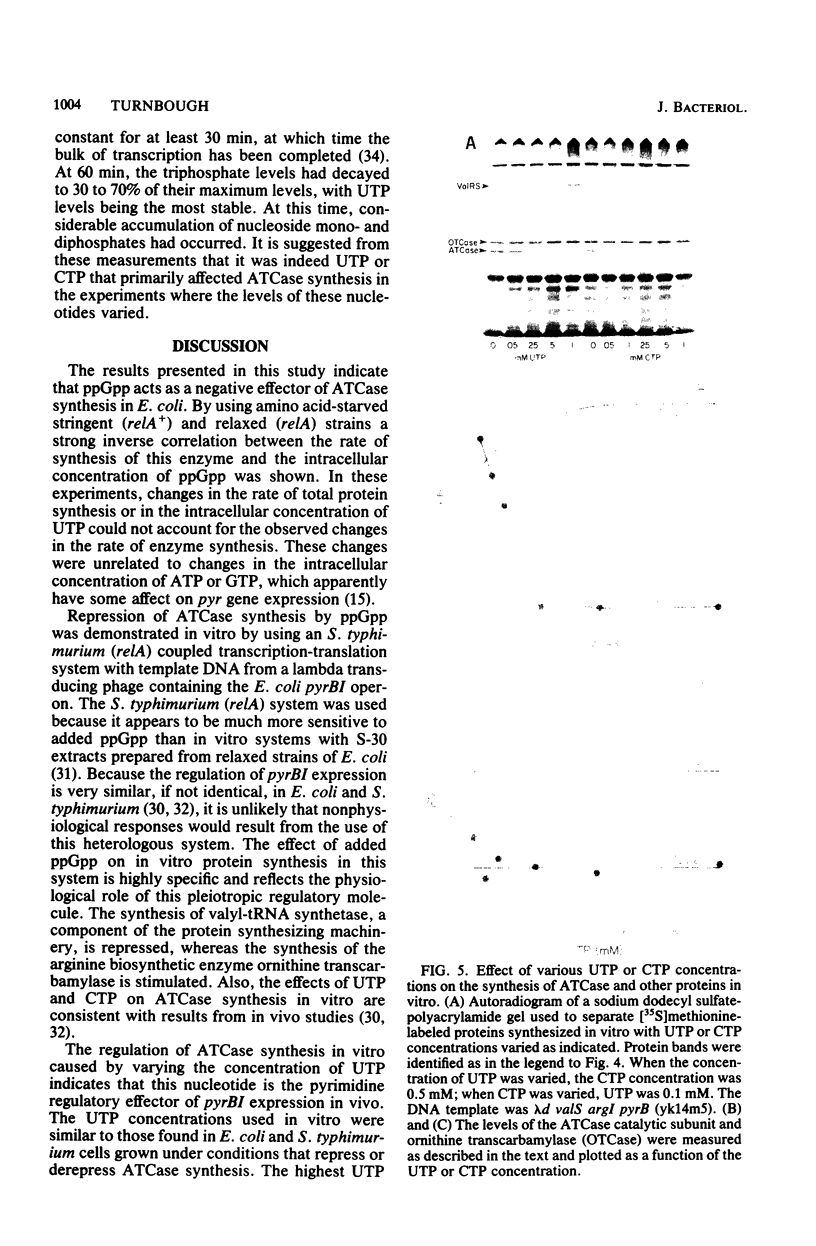
The effects of guanosine tetraphosphate (ppGpp) and pyrimidine ribonucleoside triphosphates on Escherichia coli aspartate transcarbamylase (ATCase) synthesis were examined. To determine the effect of ppGpp, a stringent (relA+) and relaxed (relA) isogenic pair of E. coli K-12 strains was starved for isoleucine, and the residual rate of synthesis of this enzyme was measured. It was necessary to starve the strains for uracil before the isoleucine limitation to maintain similar, low levels of UTP, the putative pyrimidine effector of ATCase synthesis. The isoleucine starvation of the stringent strain caused an immediate 10-fold increase in the intracellular concentration of ppGpp, which was coincident with the cessation of the synthesis of the enzyme. The elevated level of ppGpp then decayed until it reached an intracellular concentration similar to that found in unstarved cells. Enzyme synthesis resumed at this time. In the relaxed strain, the intracellular concentration of ppGpp did not increase upon isoleucine starvation and synthesis of the enzyme was not repressed. These experiments strongly indicated that ppGpp acts as a negative effector of ATCase synthesis. The repression of ATCase synthesis by ppGpp was demonstrated directly by using a Salmonella typhimurium (relA) in vitro coupled transcription-translation system with a lambda specialized transducing phage carrying the E. coli K-12 operon encoding the subunits of this enzyme (pyrBI) as a source of DNA. This in vitro system was also used to measure the effects of UTP and CTP on ATCase synthesis. Increasing the concentration of UTP in the in vitro reaction mixture resulted in strong repression of this synthesis, whereas increasing the CTP concentration did not affect synthesis significantly. Possible mechanisms for the regulation of pyr gene expression, including attenuation control, are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnara A. S., Finch L. R. Ribonucleoside triphosphate accumulation on amino acid starvation of "stringent" Escherichia coli. Biochem Biophys Res Commun. 1968 Oct 10;33(1):15–21. doi: 10.1016/0006-291x(68)90247-7. [DOI] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F. Apparent involvement of purines in the control of expression of Salmonella typhimurium pyr genes: analysis of a leaky guaB mutant resistant to pyrimidine analogs. J Bacteriol. 1979 Jun;138(3):731–738. doi: 10.1128/jb.138.3.731-738.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., Kinahan J. J., Foltermann K. F., O'Donovan G. A. Pyrimidine biosynthetic enzymes of Salmonella typhimurium, repressed specifically by growth in the presence of cytidine. J Bacteriol. 1975 Nov;124(2):764–774. doi: 10.1128/jb.124.2.764-774.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Gorini L. Similarity of genes argF and argI. Nature. 1975 Aug 21;256(5519):621–624. doi: 10.1038/256621a0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Nakata K., Winslow R. M. Coordinate control of ribonucleic acid synthesis during uracil deprivation. J Biol Chem. 1969 Jun 10;244(11):3092–3100. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978 Jul;14(3):545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Karels M. J., Navre M., Schachman H. K. Genes encoding Escherichia coli aspartate transcarbamoylase: the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4020–4024. doi: 10.1073/pnas.79.13.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol. 1975 Mar;121(3):814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- Travers A. A. Promoter sequence for stringent control of bacterial ribonucleic acid synthesis. J Bacteriol. 1980 Feb;141(2):973–976. doi: 10.1128/jb.141.2.973-976.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Nielsen L. D., Johnsen K., Molin S., Svenningsen B., Miozzari G. Reevaluation of the mode of action of streptolydigin in Escherichia coli: induction of transcription termination in vivo. Antimicrob Agents Chemother. 1978 Feb;13(2):234–243. doi: 10.1128/aac.13.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]