Abstract
The inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) is a ligand-gated intracellular Ca2+ release channel that plays a central role in modulating cytoplasmic free Ca2+ concentration ([Ca2+]i). The fungal metabolite adenophostin A (AdA) is a potent agonist of the InsP3R that is structurally different from InsP3 and elicits distinct calcium signals in cells. We have investigated the effects of AdA and its analogues on single-channel activities of the InsP3R in the outer membrane of isolated Xenopus laevis oocyte nuclei. InsP3R activated by either AdA or InsP3 have identical channel conductance properties. Furthermore, AdA, like InsP3, activates the channel by tuning Ca2+ inhibition of gating. However, gating of the AdA-liganded InsP3R has a critical dependence on cytoplasmic ATP free acid concentration not observed for InsP3-liganded channels. Channel gating activated by AdA is indistinguishable from that elicited by InsP3 in the presence of 0.5 mM ATP, although the functional affinity of the channel is 60-fold higher for AdA. However, in the absence of ATP, gating kinetics of AdA-liganded InsP3R were very different. Channel open time was reduced by 50%, resulting in substantially lower maximum open probability than channels activated by AdA in the presence of ATP, or by InsP3 in the presence or absence of ATP. Also, the higher functional affinity of InsP3R for AdA than for InsP3 is nearly abolished in the absence of ATP. Low affinity AdA analogues furanophostin and ribophostin activated InsP3R channels with gating properties similar to those of AdA. These results provide novel insights for interpretations of observed effects of AdA on calcium signaling, including the mechanisms that determine the durations of elementary Ca2+ release events in cells. Comparisons of single-channel gating kinetics of the InsP3R activated by InsP3, AdA, and its analogues also identify molecular elements in InsP3R ligands that contribute to binding and activation of channel gating.
Keywords: patch-clamp, Xenopus oocyte, single-channel electrophysiology, intracellular calcium signaling, calcium release channel
INTRODUCTION
The inositol 1,4,5-trisphosphate receptor (InsP3R) is an intracellular Ca2+ release channel that is localized to the endoplasmic reticulum. It plays a central role in the modulation of free cytoplasmic Ca2+ concentration ([Ca2+]i) by a ubiquitous cellular signaling system involving activation of phospholipase C. Binding of extracellular ligands to plasma membrane receptors generates InsP3, which diffuses through the cytoplasm to bind and activate the InsP3R, releasing Ca2+ from the endoplasmic reticulum lumen into the cytoplasm to raise [Ca2+]i. Complex InsP3-mediated calcium signals in the form of repetitive spikes, oscillations, and propagating waves initiated from specific locations in the cell have been observed in many cell types (Bootman and Berridge 1995; Toescu 1995). The molecular bases of these spatially and temporally complex calcium signals include cytoplasmic and organellar Ca2+ buffering systems, location of intracellular Ca2+ stores and, most importantly, the properties of the InsP3R. The InsP3R Ca2+ release channel is highly regulated by complex mechanisms that are still only poorly understood, including cooperative activation by InsP3 (Meyer et al. 1988; Finch et al. 1991; Mak et al. 1998) and biphasic concentration-dependent feedback from the permeant Ca2+ ion (Iino 1990; Bezprozvanny et al. 1991; Finch et al. 1991; Mak et al. 1998). Three isoforms of InsP3R (types 1, 2, and 3) as products of different genes with alternatively spliced isoforms have been identified and sequenced (Mignery et al. 1989; Mikoshiba 1993). The InsP3R isoforms all have ∼2,700 amino acid residues contained in three (InsP3-binding, regulatory [modulatory], and transmembrane channel-forming) domains (Mignery et al. 1989; Mikoshiba 1993). The sequences of the regulatory domains of all InsP3R isoforms include putative ATP-binding site(s) (Mikoshiba 1993). ATP has been shown to bind to the InsP3R (Maeda et al. 1991) and regulate InsP3R-mediated Ca2+ release in permeabilized cells (Ferris et al. 1990; Iino 1991; Bezprozvanny and Ehrlich 1993; Missiaen et al. 1997; Landolfi et al. 1998; Mak et al. 1999; Meas et al. 2000). At the single-channel level, ATP activates InsP3-dependent InsP3R gating (Bezprozvanny and Ehrlich 1993; Mak et al. 1999; Hagar and Ehrlich 2000). Activation of the Xenopus type 1 InsP3R channel by ATP is accomplished by allosteric tuning of the affinity of the Ca2+ activation sites, enabling InsP3-dependent channel gating to be more sensitive to activation by cytoplasmic Ca2+ (Mak et al. 1999).
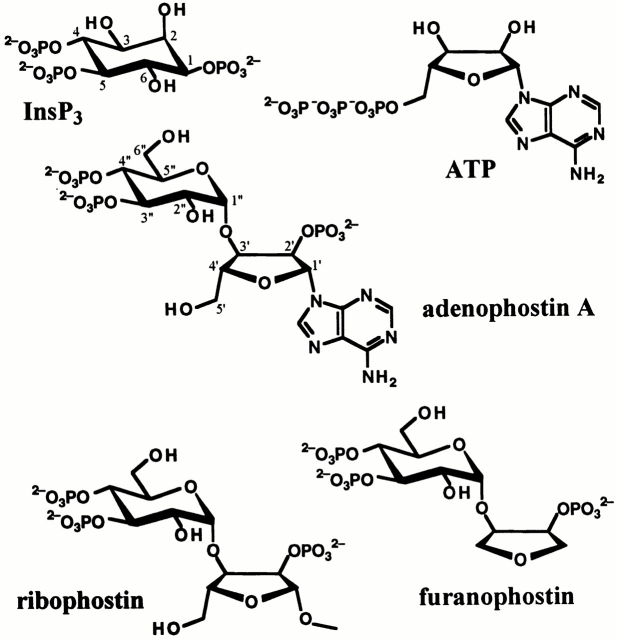
Adenophostin A (AdA), a fungal glyconucleotide metabolite (Takahashi et al. 1994), and its analogues (Marchant et al. 1997; Shuto et al. 1998; Beecroft et al. 1999) were recently discovered as potent agonists of the InsP3R. Although their molecular structures are significantly different from those of InsP3 and its analogues (Irvine et al. 1984; Fig. 1), they activate the channel by interactions with the InsP3 binding site (Glouchankova et al. 2000). AdA is 10–80-fold more potent than InsP3 in binding to the InsP3R and stimulating InsP3R-mediated Ca2+ release, and it is metabolically stable (Takahashi et al. 1994; Hirota et al. 1995; Murphy et al. 1997). AdA has been applied in studies of the InsP3R and its regulation (Missiaen et al. 1998; He et al. 1999; Adkins et al. 2000; Jellerette et al. 2000; Kashiwayanagi et al. 2000; Vanlingen et al. 2000), Ca2+ release mediated by InsP3R (Marchant and Parker 1998; Bird et al. 1999), and Ca2+ entry due to depletion of intracellular Ca2+ stores (DeLisle et al. 1997; Hartzell et al. 1997; Huang et al. 1998; Broad et al. 1999; Gregory et al. 1999; Machaca and Hartzell 1999). Compared with InsP3, AdA induced temporally and spatially different calcium signals (Marchant and Parker 1998; Bird et al. 1999) and Ca2+-dependent Cl− currents (Hartzell et al. 1997; Machaca and Hartzell 1999) in Xenopus oocytes. Furthermore, AdA, but not InsP3, activated Ca2+ entry with an apparent lack of Ca2+ release from stores (DeLisle et al. 1997). These observations suggested that the effects of AdA on calcium signaling were different from those expected if it was simply a more potent equivalent of InsP3. However, there have been no direct examinations of the single-channel properties of InsP3R activated by AdA.
Figure 1.
Molecular structures of various InsP3R-binding ligands.
Whereas AdA has a significantly higher affinity for binding to the InsP3R and a higher potency to activate the channel than InsP3, ribophostin (Rib) and furanophostin (Fur) (Marchant et al. 1997; Shuto et al. 1998), structural analogues of AdA (Fig. 1), have binding affinities and activating potencies that are comparable to that of InsP3. The relationships between the distinct binding affinities of these various ligands and the detailed gating properties of the InsP3R channel they elicit are unknown. The molecular structural feature that is unique to AdA and not shared by its less potent analogues is the adenine moiety linked to the ribose ring, which has structural resemblance to that of ATP (Fig. 1). Nevertheless, it has been reported that AdA does not bind to glutathione-S-transferase fusion polypeptides containing the putative ATP binding sequences of the type 1 InsP3R (Maes et al. 1999). Thus, the molecular determinants involved in the high affinity interaction of AdA with the InsP3R are still unclear.
The unique cytoplasmic calcium signals elicited by activation of the InsP3R with different ligands suggest that a detailed understanding of the mechanisms of action of AdA and its analogues could provide important novel insights into the molecular basis for the linkage between ligand binding and activation of the InsP3R channel. Here, we have performed a systematic investigation of the effects of AdA and its analogues on the single-channel activities of the InsP3R. We have previously applied the patch-clamp technique to the outer membrane of isolated Xenopus laevis oocyte nuclei to study extensively the single-channel properties of the endogenous type 1 InsP3R in its native membrane environment under rigorously controlled experimental conditions (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Mak et al. 1998, Mak et al. 1999). Here we characterize the conduction and channel gating properties of single InsP3R channels activated by AdA and its analogues, Rib and Fur, in the presence of a wide range of cytoplasmic Ca2+, ligand, and ATP concentrations. Our studies demonstrate that the channel conductance properties are identical for InsP3R channels activated by either AdA or InsP3. However, gating of the InsP3R activated by AdA or its analogues has a critical dependence on cytoplasmic ATP free acid concentration that is not observed for InsP3-liganded channels. Channel gating activated by AdA is indistinguishable from that elicited by InsP3 in the presence of 0.5 mM cytoplasmic free ATP, although the functional affinity of the channel is ∼60-fold higher for AdA. However, the AdA-liganded channel exhibits very different channel gating kinetics in the absence of cytoplasmic free ATP. The channel open time is reduced by nearly 50% when the channel is activated by AdA in the absence of ATP, resulting in a substantially lower maximum open probability than channels activated by AdA in the presence of ATP, or by InsP3 in the presence or absence of ATP. Furthermore, the higher functional affinity of AdA compared with InsP3 is nearly abolished in the absence of ATP. The low affinity AdA analogues Fur and Rib activated channels with gating properties similar to those of AdA in either the presence or absence of ATP. Our study reveals a prominent role of ATP as an allosteric regulator of the InsP3R channel, and it provides novel insights for interpretations of observed effects of AdA on intracellular calcium signaling. In particular, the effects of AdA on the kinetics of channel gating suggest novel mechanisms that determine the durations of elementary Ca2+ release events in cells. Comparisons of the single-channel gating kinetics of the InsP3R activated by InsP3, AdA, and its analogues have also enabled identification of molecular structural elements in InsP3R ligands that contribute to their ability to bind and activate channel gating.
MATERIALS AND METHODS
Patch-clamping the Oocyte Nucleus
Patch-clamp experiments were performed using isolated Xenopus oocyte nuclei as described previously (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Mak et al. 1998). In brief, stage V or VI oocytes, which express only a single InsP3R isoform (type 1) and lacks other (e.g., ryanodine receptor) Ca2+ release channels (Kume et al. 1993), were opened mechanically just before use. The nucleus was separated from the cytoplasm and transferred to a dish on the stage of a microscope for patch-clamping. Experiments were performed in the “on-nucleus” configuration, with the solution in the perinuclear lumen between the outer and inner nuclear membranes in apparent equilibrium with the bath solution (Mak and Foskett 1994), and the cytoplasmic aspect of the InsP3R channel facing into the patch pipet. Experiments were performed at room temperature with the pipet electrode at +20 mV relative to the reference bath electrode.
Data Acquisition and Analysis
Single-channel currents were amplified by an Axopatch-1D amplifier (Axon Instruments, Inc.) with antialiasing filtering at 1 kHz, digitized at 5 kHz, and recorded by Pulse+PulseFit software (HEKA Elektronik). The patch-clamped Xenopus InsP3R inactivates with a time constant of ∼30 s after its activation by InsP3 (Mak and Foskett 1997). Similar inactivation was observed in this study when the channels were activated by AdA. As inactivation was generally abrupt with no detectable change in channel kinetics up to the disappearance of channel activity (Mak and Foskett 1997), current traces obtained during the entire period the channels were active were analyzed (Mak and Foskett 1998; Mak et al. 1998). Channel opening and closing events were identified with a 50% threshold, and channel open probability P o and dwell time distribution evaluated using TAC software (Bruxton). Current traces exhibiting one InsP3R channel, or two InsP3R channels determined to be identical and independently gated (Mak and Foskett 1997), were used for P o evaluation, whereas only current traces with a single InsP3R channel were used for dwell time analyses. Each set of open and closed dwell time histograms was derived from one patch-clamp current record of a single active InsP3R channel. The probability density function (pdf) was fitted to the histograms by the maximum likelihood method (Sigworth and Sine 1987).
The number of channels in the membrane patch was assumed to be the maximum number of open channel current levels observed throughout the current record. Assuming there are n identical and independent channels in the membrane patch, and each channel is Markovian with open probability of P o and open duration distribution characterized by a single exponential component of time constant τo, the mean dwell time of highest channel current level is τo/n. If T is the minimum duration of an open event that is detectable in the experimental system, i.e., only events with duration >T will have amplitudes greater than the 50% threshold after filtering, then the rate of detection of the highest current level:
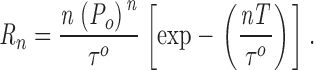 |
1 |
In our patch-clamp set up, T was empirically determined to be ∼0.2 ms using test pulses of variable duration. τo of InsP3R channels is ∼3–15 ms over the range of experimental conditions used (Mak et al. 1998). In experimental conditions with P o > 0.1, only current records with longer than 10 s of InsP3R channel activities were used. Because 10 s ≫ 1/R 3, there is little uncertainty in the number of channels in the current traces used. In experimental conditions with P o < 0.1, only current records exhibiting one open channel current level with record duration > 5/R 2 were used, to ensure that they were truly single-channel records.
Each data point shown is the mean of results from at least four separate patch-clamp experiments performed under the same conditions. Error bars indicate the SEM. Theoretical Hill equation curves were fitted to experimental P o data using IgorPro (WaveMetrics).
Solutions for Patch-clamp Experiments
All patch-clamp experiments were performed with solutions containing 140 mM KCl and 10 mM HEPES, with pH adjusted to 7.1 using KOH. By using K+ as the current carrier and appropriate quantities of the high affinity Ca2+ chelator, BAPTA (1,2-bis[O-aminophenoxy] ethane-N,N,N′,N′-tetraacetic acid; 100–1,000 μM; from Molecular Probes), or the low affinity Ca2+ chelator, 5,5′-dibromo BAPTA (100–400 μM; Molecular Probes), or ATP (0.5 mM) alone to buffer Ca2+ in the experimental solutions, free Ca2+ concentrations in our experimental solutions were tightly controlled. Total Ca2+ content (5–330 μM) in the solutions was determined by induction-coupled plasma mass spectrometry (Mayo Medical Laboratory). Free [Ca2+] was calculated using the Maxchelator software (C. Patton, Stanford University, Stanford, CA). The free [Ca2+] of the solutions was verified by measurements using Ca2+-selective minielectrodes (Baudet et al. 1994) and found to agree with the calculated [Ca2+] to within the accuracy of the electrode measurement (10%). The bath solutions used in all experiments had 140 mM KCl, 10 mM Hepes, 380 μM CaCl2, 500 μM BAPTA ([Ca2+] = 500 nM), and pH 7.1. Pipet solutions, to which the cytoplasmic aspects of the channels were exposed, contained either 0 or 0.5 mM of Na2ATP (Sigma-Aldrich), various concentration of InsP3 (Molecular Probes), AdA, Rib, or Fur (Calbiochem), as stated. All reagents were used without further purification. Because Mg2+ was absent from the experimental solutions, ATP mostly existed in free acid forms (ATP4−, ATP3−). We previously demonstrated that ATP free acid, not MgATP complex, was responsible for ATP regulation of InsP3R gating (Mak et al. 1999).
RESULTS
Properties of AdA-liganded InsP3R Channels in the Presence of 0.5 mM Cytoplasmic Free ATP
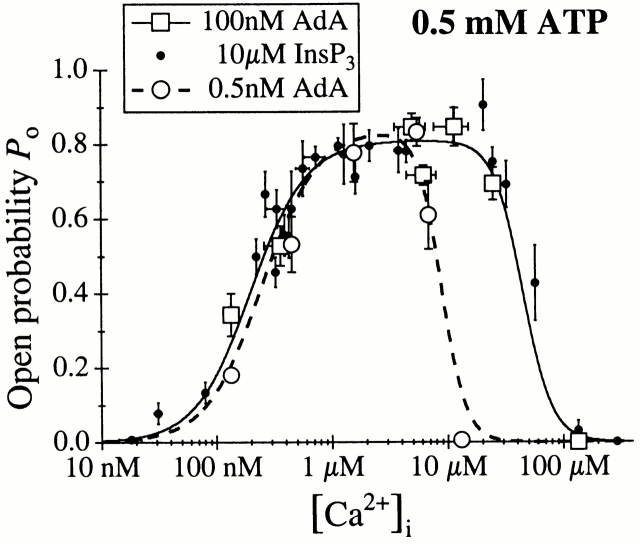
To compare the single-channel conductance and gating properties of the Xenopus type 1 InsP3R (X-InsP3R-1) activated by AdA with those activated by InsP3, we performed patch-clamp experiments on the outer membrane of nuclei isolated from Xenopus oocytes using the same experimental conditions employed in our previous studies (Mak and Foskett 1994, Mak and Foskett 1997; Mak et al. 1998), but with AdA instead of InsP3 as the agonist. With cytoplasmic ATP free acid concentration ([ATP]) of 0.5 mM, the conductance properties and gating kinetics of the X-InsP3R-1 channel activated by saturating concentrations of either AdA (100 nM) or InsP3 (10 μM) were indistinguishable in all cytoplasmic free Ca2+ concentrations ([Ca2+]i) examined (Fig. 2 A). In addition, all AdA-liganded X-InsP3R-1 channels observed in our experiments inactivated despite the continuous presence of AdA in the pipet solution, with durations of channel activity that were comparable to those observed for InsP3-liganded channels (Mak and Foskett 1997).
Figure 2.
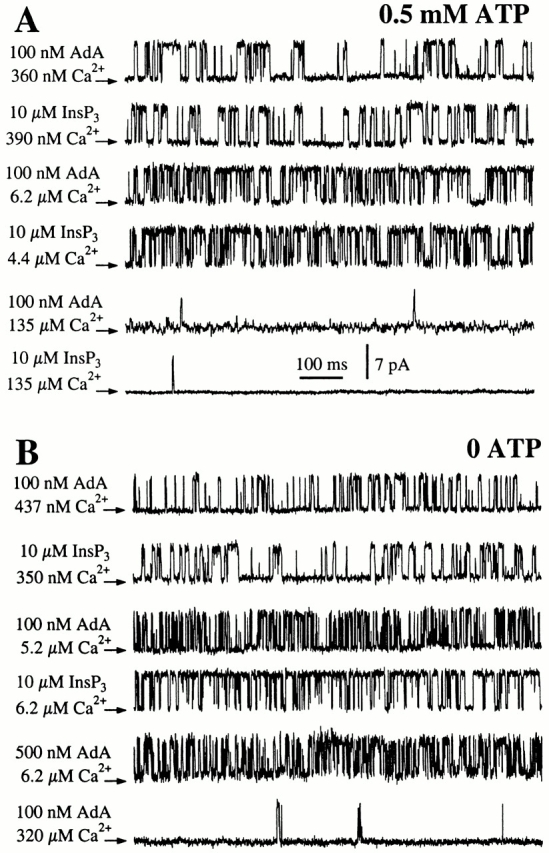
Typical single-channel current traces of X-InsP3R-1 in various [Ca2+]i, activated by InsP3 or AdA as indicated. Arrows indicate the closed channel current levels. (A) In the presence of 0.5 mM ATP. (B) In the absence of ATP.
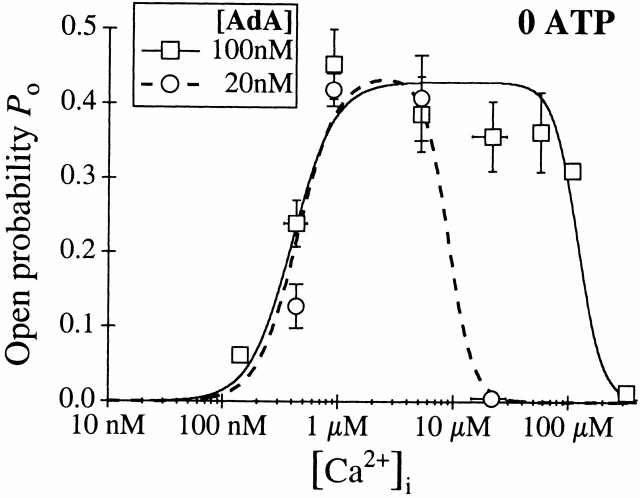
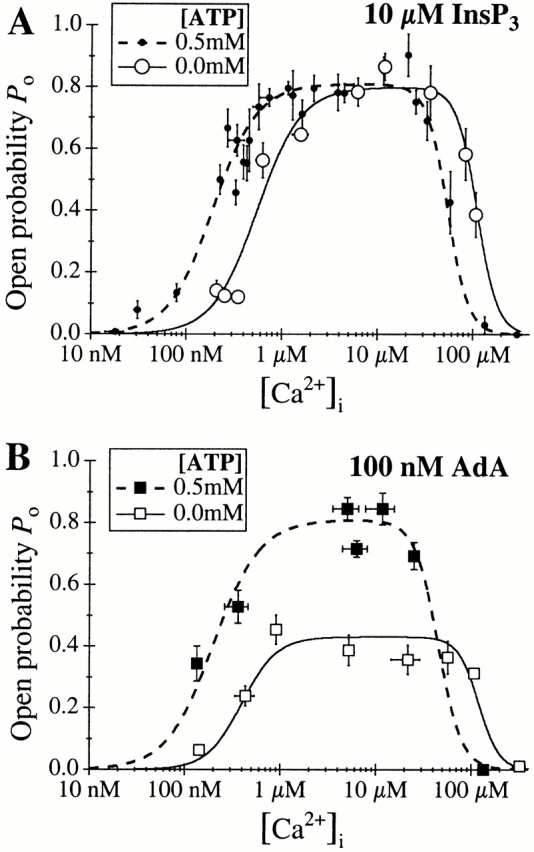
The open probability (P o) of the InsP3-liganded channel varies with [Ca2+]i in a biphasic manner (Mak et al. 1998). To determine the [Ca2+]i dependence of the gating of AdA-liganded channels, a saturating concentration (100 nM) of AdA was used as the ligand in the presence of various [Ca2+]i. The P o of the channel activated by 100 nM AdA also varied with [Ca2+]i in a biphasic manner (Fig. 3). At [Ca2+]i < 1 μM, increases in [Ca2+]i enhanced the channel P o. Between 1 and 20 μM [Ca2+]i, the channel P o remained high (∼0.8). As [Ca2+]i increased beyond 20 μM, P o decreased precipitously. This [Ca2+]i dependence of the AdA-liganded X-InsP3R-1 was essentially identical to that of the channel activated by saturating concentrations of InsP3 (Mak et al. 1998). The results were well fitted by a biphasic Hill equation:
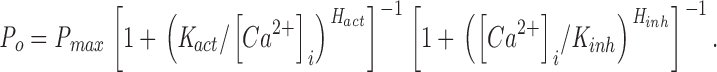 |
2 |
Figure 3.
[Ca2+]i dependence of the P o of the X-InsP3R-1 channel in the presence of 0.5 mM ATP, activated by AdA or InsP3 (Mak and Foskett 1998). The solid and dashed curves are theoretical fits by the Hill equation () of the P o data from [AdA] = 100 nM and 0.5 nM, respectively.
The Hill equation parameters—maximum P o (P max), half-maximal activating [Ca2+]i (K act), activation Hill coefficient (H act), half-maximal inhibitory [Ca2+]i (K inh), and inhibition Hill coefficient (H inh)—for the AdA-liganded channel were all very similar to those for the InsP3-liganded channel (Table , A and B). The identical P max indicates that InsP3 and AdA have similar efficacy in gating the channel in the presence of 0.5 mM free ATP.
Table 1.
Hill Equation Parameters of X-InsP3R-1
| Ligand concentration | P max | K act | H act | K inh | H inh | |
|---|---|---|---|---|---|---|
| nM | μM | |||||
| A | 100 nM AdA | 0.81 ± 0.03 | 200 ± 50 | 1.8 ± 0.3 | 45 ± 5 | 3.5 ± 0.4 |
| B | 10 μM InsP3 | 0.81 ± 0.03 | 190 ± 20 | 1.9 ± 0.3 | 54 ± 6 | 3.9 ± 0.7 |
| C | 0.5 nM AdA | 0.84 ± 0.03 | 250 ± 50 | 1.8 ± 0.3 | 8.9 ± 0.5 | 4.3 ± 0.7 |
| D | 33 nM InsP3 | 0.81 ± 0.03 | 190 ± 20 | 1.9 ± 0.3 | 11.0 ± 1.5 | 3.9 ± 0.7 |
| E | 20 nM InsP3 | 0.81 ± 0.03 | 190 ± 20 | 1.9 ± 0.3 | 0.21 ± 0.04 | 3.9 ± 0.7 |
| F | 10 μM InsP3 | 0.79 ± 0.02 | 420 ± 40 | 2.2 ± 0.3 | 110 ± 10 | 4.0 ± 0.7 |
| G | 33 nM InsP3 | 0.80 ± 0.05 | 540 ± 70 | 2.0 ± 0.3 | 1.4 ± 0.2 | 3.5 ± 0.7 |
| H | 100 nM AdA | 0.43 ± 0.03 | 400 ± 50 | 2.4 ± 0.3 | 130 ± 10 | 4.0 ± 0.7 |
| I | 20 nM AdA | 0.44 ± 0.03 | 440 ± 40 | 2.6 ± 0.6 | 9 ± 2 | 4.0 ± 0.7 |
Parameters for the biphasic Hill equations () that fit the [Ca2+]i dependence of the P o of X-InsP3R-1 channel under various experimental conditions.
The broad biphasic P o versus [Ca2+]i curve of the AdA-liganded X-InsP3R-1 channel remained the same when the concentration of AdA was reduced from 100 to 5 nM (data not shown). However, when the concentration of AdA was further decreased to 0.5 nM, the channel exhibited a higher sensitivity to Ca2+ inhibition, with K inh reduced, but H inh unaltered (Table C). The [Ca2+]i dependence of the activation of the channel and the P max were not significantly affected by the concentration of AdA (Fig. 3). AdA appears to activate the InsP3R channel by reducing the affinity of the Ca2+ inhibition site, which is reminiscent of the tuning of Ca2+ inhibition of the channel by InsP3 (Mak et al. 1998). Thus, the mechanism by which ligand binding activates the channel (elevation of K inh) is similar for both AdA and InsP3. The value of K inh of the channel activated by 0.5 nM AdA lies between those activated by 20 and 33 nM InsP3 (Table , D–E; Mak et al. 1998). Thus, in the presence of 0.5 mM ATP, AdA activates the X-InsP3R-1 channel in the same manner with a similar efficacy as InsP3, but AdA is ∼60 times more potent than InsP3.
InsP3-liganded X-InsP3R-1 Channel Gating in the Absence of ATP
Part of the molecular structure of AdA is analogous to that of InsP3: AdA has a glucose moiety with a 3′′,4′′-bisphosphate/2′′-hydroxyl motif that is structurally similar to the 4,5-bisphosphate/6-hydroxyl motif of InsP3 (Hotoda et al. 1999), and the 2′-phosphoryl group in the ribose ring of AdA is probably in an analogous position as the 1-phosphoryl group in InsP3 (Wilcox et al. 1995). However, the rest of the AdA molecular structure is very different from that of InsP3 (Fig. 1). In particular, AdA has an adenosine 2′-phosphate moiety not present in InsP3. An interaction between the adenine structure in AdA and unknown site(s) in the InsP3R has been suggested to contribute to the high potency of AdA as an agonist of the InsP3R (Hotoda et al. 1999).
The sequences of the regulatory domains of all InsP3R isoforms include putative ATP-binding site(s) (Mikoshiba 1993). ATP was shown to bind to the InsP3R and regulate InsP3R-mediated Ca2+ release and InsP3R single-channel gating (see introduction). Because ATP and AdA share a common adenine moiety (Fig. 1), we reasoned that an ATP binding site(s) in the InsP3R structure might interact with the adenine moiety in AdA to promote high affinity binding of AdA to the channel. Such a mechanism suggests that ATP might function as an antagonist, competing with AdA for the same binding site in the InsP3R. A prediction from this model is that the affinity of the channel for AdA would be increased in the absence of cytoplasmic free ATP. To test this hypothesis, we examined the activities of the channel in the absence of ATP, using either AdA or InsP3 to stimulate gating.
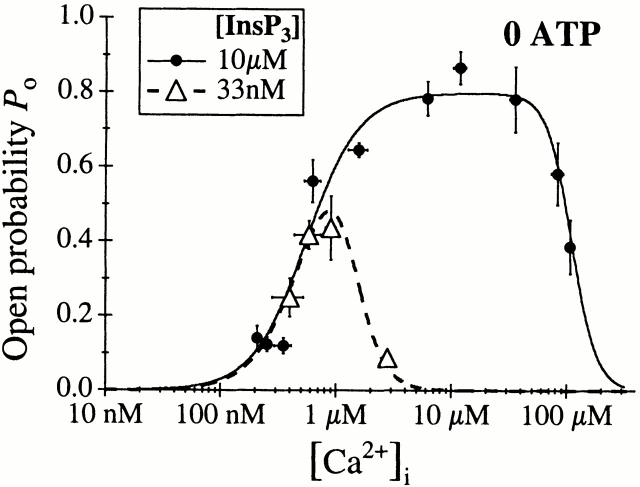
We first examined the effects of InsP3. In the absence of cytoplasmic free ATP, the channel conductance and gating properties activated by a saturating concentration of InsP3 (10 μM) were identical to those of the channel activated in the presence of 0.5 mM ATP (Fig. 2 B). The [Ca2+]i dependence of the channel P o (Fig. 4) remained well characterized by a biphasic Hill equation (). The channel was fully activated in 2 μM < [Ca2+]i < 50 μM with a P max of 0.8. Whereas H act and H inh were similar in either the presence or absence of free ATP, the InsP3-liganded channel in 0 ATP was less sensitive to Ca2+ activation and to Ca2+ inhibition (Fig. 5 A), with twofold increases in both K act and K inh (comparing Table , F and B).
Figure 4.
[Ca2+]i dependence of the P o of the X-InsP3R-1 channel in the absence of ATP, activated by saturating (10 μM) or subsaturating (33 nM) concentrations of InsP3. The solid and dashed curves are theoretical fits by the Hill equation () of the P o data.
Figure 5.
[Ca2+]i dependence of the P o of the X-InsP3R-1 channel activated by saturating concentrations of ligands in the presence or absence of ATP. (A) 10 μM InsP3; (B) 100 nM AdA. The solid and dashed curves are theoretical fits by the Hill equation () of the data in 0 or 0.5 mM of ATP, respectively.
The biphasic [Ca2+]i dependence of InsP3-liganded channel gating in the absence of ATP remained unchanged when the concentration of InsP3 was decreased from 10 μM to 100 nM (data not shown). A further reduction of the concentration of InsP3 to 33 nM caused the channel to exhibit a higher sensitivity to Ca2+ inhibition (Fig. 4). The [Ca2+]i dependence of the channel P o activated by 33 nM InsP3 in the absence of ATP was fitted by the biphasic Hill equation () with K inh reduced from 110 to 1.4 μM, while the other parameters H inh, K act, H act, and P max remained essentially unchanged (Table G). Thus, InsP3 regulation of X-InsP3R-1 channel gating was similar in the presence or absence of ATP, with comparable efficacy and functional affinity in both cases.
AdA-liganded X-InsP3R-1 Channel Gating in the Absence of ATP
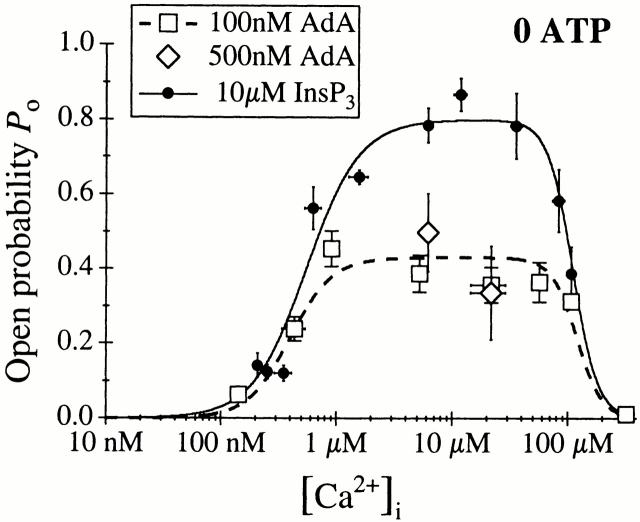
We next examined the effects of AdA. The conductance properties of the X-InsP3R-1 channel activated by a saturating concentration (100 nM) of AdA were indistinguishable in either the presence or absence of ATP (Fig. 2). In contrast, gating of the AdA-liganded channel in the absence of ATP was dramatically different from that of the InsP3-liganded channel. Whereas the InsP3-liganded channel exhibited P max ≈ 0.8 at [Ca2+]i > 2 μM, the P max of the AdA-liganded channel was only 0.4 (Fig. 5 B and 6). Instead of staying open most of the time with only brief closings like the InsP3-liganded channel, the AdA-liganded channel had substantially shorter channel openings (Fig. 2 B). Similar channel gating characterized by short openings (Fig. 2 B) and P max of ∼0.4 (Fig. 6) was also observed in suprasaturating concentrations (500 nM) of AdA. Therefore, the altered gating kinetics of the AdA-liganded channel observed in the absence of ATP was not due to insufficient channel activation by subsaturating concentrations of AdA.
Figure 6.
[Ca2+]i dependence of the P o of the X-InsP3R-1 channel activated by AdA or InsP3 in the absence of ATP. The solid and dashed curves are theoretical fits by the Hill equation () of the P o data from [InsP3] = 10 μM and [AdA] = 100 nM, respectively.
The [Ca2+]i dependence of the AdA-liganded channel P o in the absence of ATP (Fig. 5 A and 6) was well fitted by the biphasic Hill equation () with K act, H act, K inh, and H inh comparable with those for the channel activated by InsP3, but with a P max decreased to ∼0.4 (Table H). Therefore, in the absence of ATP, the affinities (K act and K inh) of the activating and inhibitory Ca2+-binding sites and their levels of cooperativity (H act and H inh) of the AdA and InsP3-liganded X-InsP3R-1 channels were similar, but the maximal level of channel activity induced by AdA in the absence of ATP was only about half that activated by InsP3 at all [Ca2+]i. In other words, in the absence of free ATP, AdA was less efficacious than InsP3 in activating channel gating, acting instead as a partial agonist.
When the concentration of AdA was reduced from 100 to 20 nM in the absence of ATP, the channel became more sensitive to Ca2+ inhibition (Fig. 7), with only K inh reduced while the other Hill equation parameters remained similar to those observed in 100 nM AdA (Table ). Thus, despite the lower P max value observed for the channel activated by AdA in the absence of ATP, the channel was still activated by ligand tuning of its sensitivity to Ca2+ inhibition, as it was when it was activated by InsP3 (Mak et al. 1998; Fig. 4), or by AdA in 0.5 mM ATP (Fig. 3).
Figure 7.
[Ca2+]i dependence of the P o of the X-InsP3R-1 channel in the absence of ATP, activated by saturating (100 nM) or subsaturating (20 nM) concentrations of AdA. The solid and dashed curves are theoretical fits by the Hill equation () of the P o data. Note that the scale of the P o axis is different from that in the previous P o versus [Ca2+]i graphs.
Of note, the value of K inh for the channel activated by 20 nM AdA in the absence of ATP lay between those values for channels activated by 33 and 100 nM InsP3. Thus, in the absence of ATP, AdA was only 1.5–5 times more potent than InsP3 in activating the channel, whereas it was ∼60 times more potent in the presence of 0.5 mM ATP.
In summary, the affinities of the activating and inhibitory Ca2+-binding sites (K act and K inh) of the InsP3-liganded channels were the only parameters affected by the presence or absence of ATP (Fig. 5 A). In contrast, ATP regulates not only the affinities of the Ca2+-binding sites, but also the level of maximum activity of the AdA-liganded channel (Fig. 5 B) as well as the potency of AdA to activate the channel. Thus, the presence or absence of ATP affects all regulation parameters of the AdA-liganded channel except the level of cooperativity of the Ca2+-binding sites. In addition, these results demonstrate that the high affinity of AdA is not conferred by its interaction with ATP-binding sites in the channel sequence, in contrast to our working hypothesis.
Properties of the X-InsP3R-1 Channel Activated by Rib and Fur
Because AdA and InsP3 had distinct effects on the gating properties of the InsP3R when the channel was stimulated in the absence of ATP, we speculated that the distinct molecular structures of the two ligands conferred unique ATP-dependent gating properties. To determine the molecular structural determinants in the activating ligand that influence the gating properties of the channel, we investigated the effects of Rib and Fur, structural analogues of AdA that lack the adenine moiety found in AdA (Fig. 1). In previous studies, these analogues of AdA were found to stimulate InsP3-mediated Ca2+ release with an apparent affinity that was significantly lower than that of AdA but similar to that of InsP3 (Marchant et al. 1997; Shuto et al. 1998).
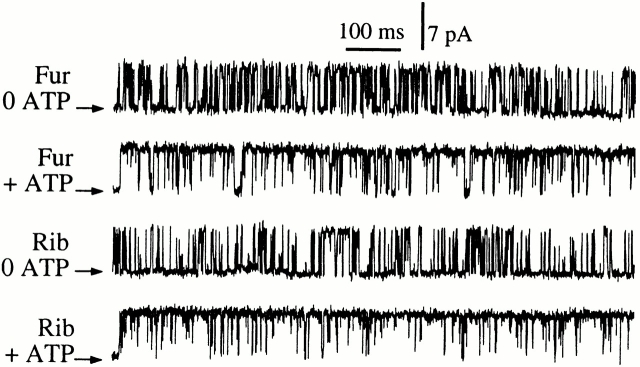
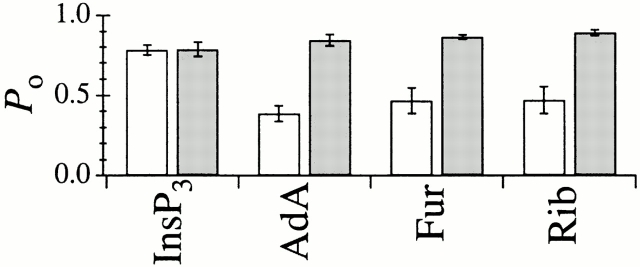
In optimal conditions, with [Ca2+]i between 4.4 and 6.2 μM and in the presence of saturating concentrations (10 μM) of Rib or Fur, the channels exhibited inactivation kinetics and conductance and gating properties (Fig. 8) that were indistinguishable from those observed when the channels were activated by AdA, in either the absence or presence (0.5 mM) of ATP (Fig. 2). Whereas InsP3-liganded channels exhibited P max of ∼0.8 in both 0 and 0.5 mM ATP, channels activated by AdA, Fur, or Rib only exhibited this high P max in the presence of 0.5 mM ATP. In the absence of ATP, the X-InsP3R-1 channel activated by AdA, Fur, or Rib had a significantly (P < 0.01) lower P max ≈ 0.4 (Fig. 9). Thus, the responses of the channel to saturating concentrations of Fur or Rib were clearly similar to that for AdA and different from those for InsP3.
Figure 8.
Typical single-channel current traces of the X-InsP3R-1 activated by 10 μM Fur or Rib. In 0.5 mM ATP (+ ATP), [Ca2+]i = 5.0 μM. In the absence of ATP (0 ATP), [Ca2+]i = 6.2 μM. The arrows indicate the closed channel current levels.
Figure 9.
P o of the X-InsP3R-1 channel in optimal [Ca2+]i (4.4–6.2 μM) and saturating concentrations of various ligands in 0 (white bars) and 0.5 mM (shaded bars) ATP.
Closed Channel Dwell Time Distributions of X-InsP3R-1 Channel Activated by Various Ligands
To elucidate the kinetic features associated with the regulation of X-InsP3R-1 channel gating, we studied in detail the mean open and closed channel durations (〈τo〉 and 〈τc〉, respectively) under various experimental conditions in the presence of different ligands. Furthermore, dwell time histogram analyses were performed on single-channel current records of channels activated by saturating concentrations of AdA (100 nM) or InsP3 (10 μM), in the presence or absence of ATP, and in various [Ca2+]i, except when such analyses were precluded by Ca2+ inhibition at high [Ca2+]i and channel inactivation (Mak and Foskett 1997).
In general, under all conditions examined (activation by AdA or InsP3, in the presence or absence of ATP), the [Ca2+]i dependence of the channel P o mainly resided in a [Ca2+]i dependence of 〈τc〉 (Mak et al. 1998; Fig. 10). The increase in P o due to Ca2+ activation in the low [Ca2+]i range (<1 or 2 μM, in the presence or absence of ATP, respectively) was mostly caused by a decrease in 〈τc〉 with increases in [Ca2+]i. 〈τc〉 stayed within a narrow range (1 to 5 ms) when P o remained at maximum level in higher, optimal [Ca2+]i. The precipitous decrease in P o at higher [Ca2+]i due to Ca2+ inhibition was mostly the result of a dramatic rise in 〈τc〉 as [Ca2+]i increased. The increase in the sensitivity of the channel to Ca2+ inhibition observed in the presence of subsaturating concentrations of either ligand (AdA or InsP3) was reflected in an onset of the rise in 〈τc〉 at lower [Ca2+]i.
Figure 10.
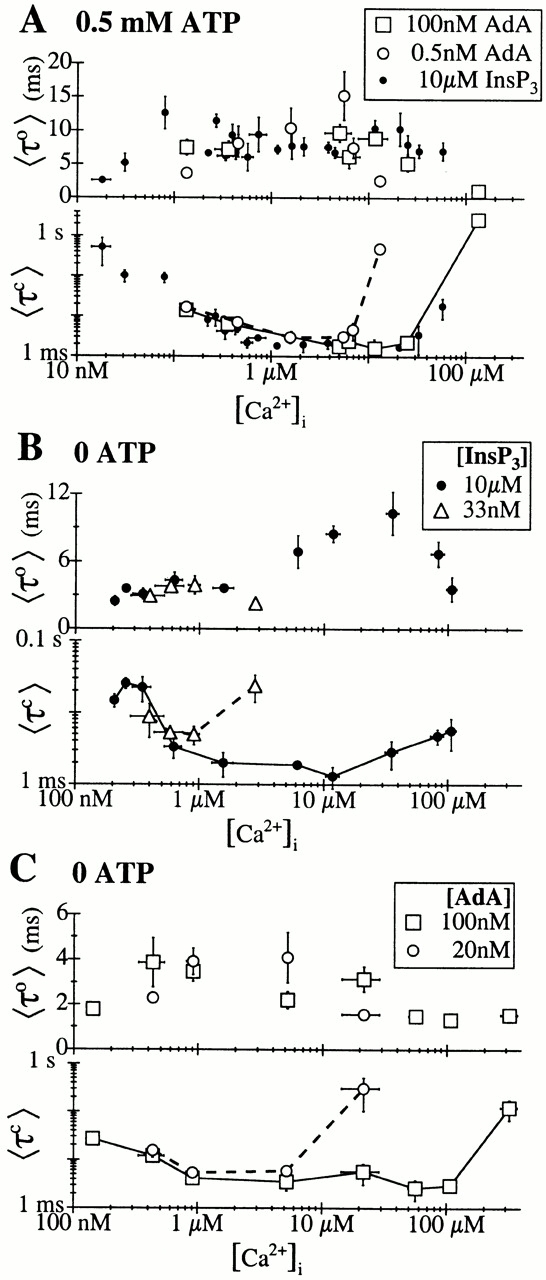
[Ca2+]i dependencies of the mean open (〈τo〉) and closed (〈τc〉) dwell times of the X-InsP3R-1 channel. In the 〈τc〉 graphs, data points from the same experimental conditions are connected with solid or dashed lines for clarity. (A) Channel activated by AdA or InsP3 (Mak and Foskett 1998), in 0.5 mM ATP. (B) Channel activated by saturating (10 μM) or subsaturating (33 nM) concentrations of InsP3 in the absence of ATP. (C) Channel activated by saturating (100 nM) or subsaturating (20 nM) concentrations of AdA in the absence of ATP.
The closed dwell time histograms of the X-InsP3R-1 channel revealed that it had at least four distinguishable closed kinetic states with time constants τc >100 ms, 20–60 ms, 2–10 ms, and <1 ms, respectively (Fig. 11 and Fig. 12). The decrease in 〈τc〉 associated with Ca2+ activation of InsP3-liganded channels in 0.5 mM ATP was caused by sequential destabilization and, therefore, reduction of the relative weights, of the three longer closed kinetic states, until the shortest closed kinetic state with τc < 1 ms became dominant in [Ca2+]i > 1 μM (Fig. 11, A–E). Reduction of τc of the longer closed kinetic states also contributed, to a lesser extent, to the decrease in 〈τc〉.
Figure 11.
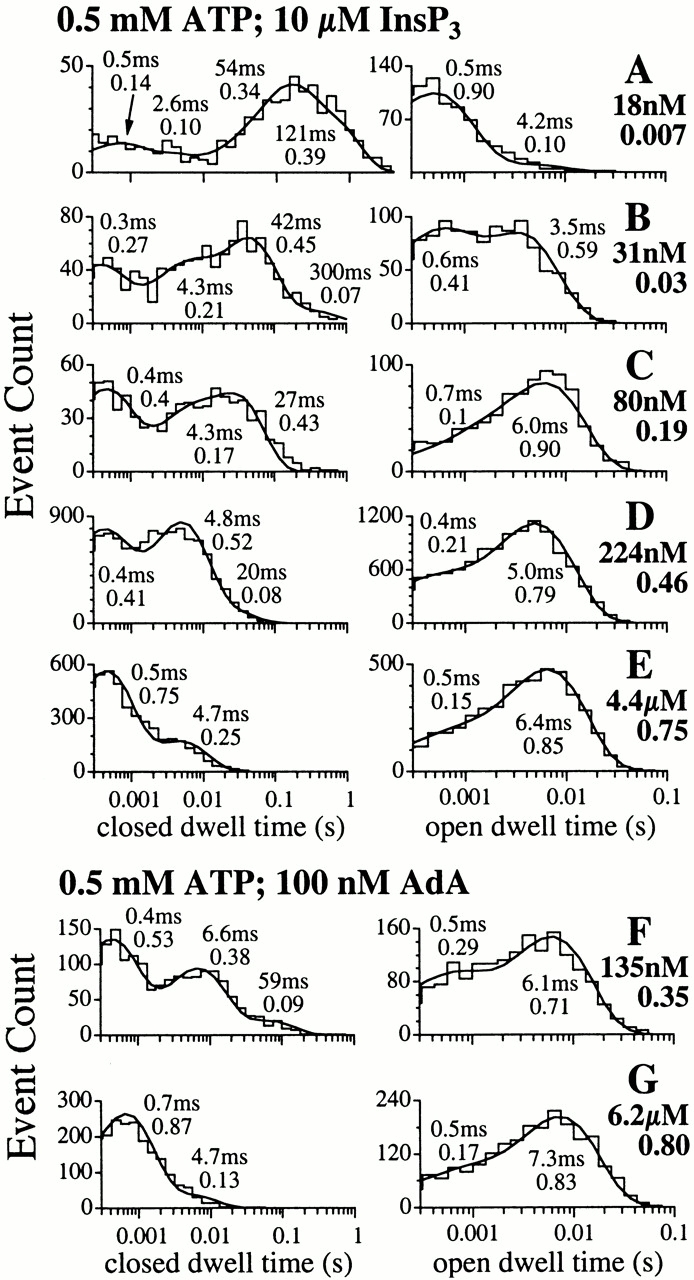
Open and closed dwell time histograms of the X-InsP3R-1 channel in 0.5 mM ATP and various [Ca2+]i, activated by saturating concentrations of InsP3 (10 μM) or AdA (100 nM). The smooth curves are the pdf. The time constant and relative weight of each exponential component of the pdf are tabulated next to the corresponding peak in the curves. [Ca2+]i used in each of the analyzed experiments and its P o are tabulated next to the corresponding graphs.
Figure 12.
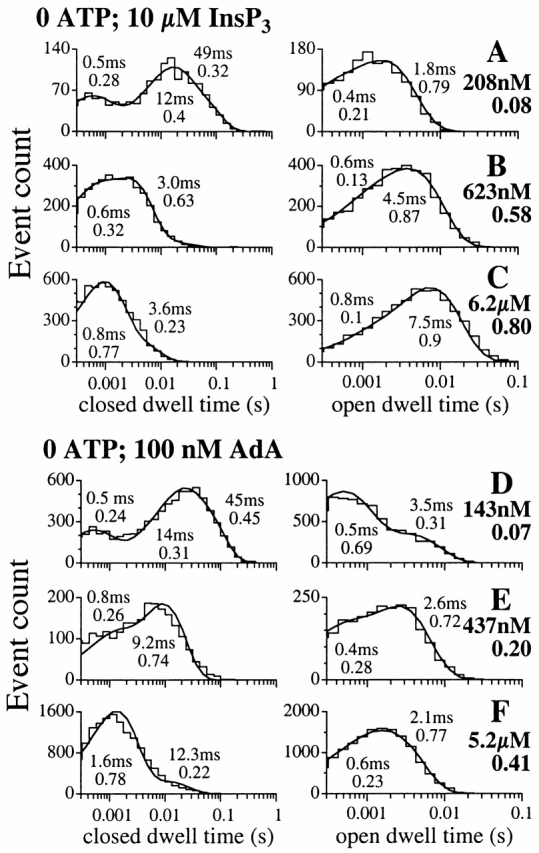
Open and closed dwell time histograms of the X-InsP3R-1 channel in the absence of ATP and various [Ca2+]i, activated by saturating concentrations of InsP3 (10 μM) or AdA (100 nM). The smooth curves are the pdf. The time constant and relative weight of each exponential component of the pdf are tabulated next to the corresponding peak in the curves. [Ca2+]i used in each of the analyzed experiments and its P o are tabulated next to the corresponding graphs.
As suggested by their essentially identical [Ca2+]i dependencies of the P o (Fig. 3) and 〈τc〉 (Fig. 10 A) of channels activated in 0.5 mM ATP by either AdA or InsP3, the closed channel dwell time distributions of the channels activated by either ligand in 0.5 mM ATP were very similar (Fig. 11). Although the sensitivity of the channels to Ca2+ activation was diminished in the absence of ATP, the closed dwell time distributions of InsP3-liganded channels in the absence of ATP resembled those in the presence of 0.5 mM ATP when compared at [Ca2+]i that gave comparable channel P o (compare Fig. 12, A–C, with Fig. 11, C–E).
Interestingly, although the gating kinetics of the InsP3R channel activated by AdA in the absence of ATP were very different from those of channels activated by AdA in 0.5 mM ATP or activated by InsP3 (Fig. 2), Ca2+ activation of the AdA-liganded InsP3R in the absence of ATP was still caused by destabilization of the longer closed kinetic states (Fig. 12, D–F), although the closed channel time constants were generally longer than in other conditions.
Open Channel Dwell Time Distributions of X-InsP3R-1 Channels Activated by Various Ligands
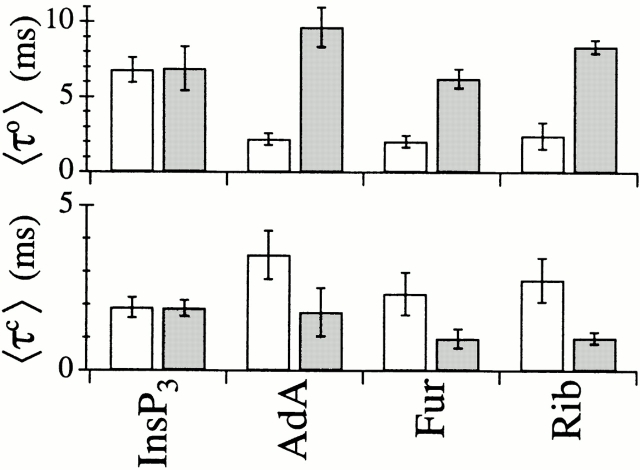
The mean open channel duration (〈τo〉) of the X-InsP3R-1 channel activated by saturating concentrations of InsP3 in 0.5 mM ATP remained within a narrow range, between 5 and 15 ms, over a wide range of [Ca2+]i (50 nM–50 μM; Fig. 10 A). 〈τo〉 dropped below 5 ms at very low or very high [Ca2+]i. In subsaturating concentrations of InsP3, 〈τo〉 dropped below 5 ms at lower [Ca2+]i (Mak et al. 1998). This [Ca2+]i dependence of 〈τo〉 was mirrored in AdA-liganded channels in 0.5 mM ATP (Fig. 10 A). A similar [Ca2+]i dependence of 〈τo〉 was also observed in InsP3-liganded channels in 0 ATP, except that 〈τo〉 was >5 ms for [Ca2+]i between 300 nM and 100 μM in saturating concentrations of InsP3 because of the change in [Ca2+]i sensitivity of the channel in the absence of ATP (Fig. 10 B).
In contrast, a very different [Ca2+]i dependence was observed for 〈τo〉 of the AdA-liganded channel in 0 ATP. 〈τo〉 never rose above 5 ms over the entire wide range of [Ca2+]i examined (Fig. 10 C). This reduced 〈τo〉 accounted for the distinct channel gating kinetics of the InsP3R activated by AdA in 0 ATP. Thus, a typical opening event of the channel activated by AdA in the absence of ATP was significantly shorter than a typical opening event of the channel under other activating conditions examined. This was the major factor contributing to the low value of P max for the AdA-liganded channel in 0 ATP.
Open dwell time histograms of the fully activated X-InsP3R-1 channel generally contained two exponential components, corresponding to at least two distinguishable open kinetic states (Fig. 11 and Fig. 12). Over most [Ca2+]i in which the channel 〈τo〉 remained high, the long open kinetic state was the dominant one. At very low [Ca2+]i, 〈τo〉 of the channel was shorter because of either the sharp reduction in the relative weight of the long open kinetic state in favor of the short one (for InsP3-liganded channel in 0.5 mM ATP; Fig. 11A and Fig. B; and AdA-liganded channel in 0 ATP; Fig. 12 D), or the reduction of the time constant of the long open kinetic state (for InsP3-liganded channel in 0 ATP; Fig. 12 A).
The time constant τo of the dominating long open kinetic state was 5–8 ms for all experimental conditions in which the channel had P max of 0.8: channels in 0.5 mM ATP activated by InsP3 (Fig. 11, C–E) or AdA (Fig. 11F and Fig. G), and InsP3-liganded channels in 0 ATP (Fig. 12B and Fig. C). In contrast, τo of the dominating long open kinetic state was only ∼2 ms for AdA-liganded channel in 0 ATP with P max of 0.4 (Fig. 12E and Fig. F).
Comparison of 〈τo〉 and 〈τc〉 of the InsP3R channel in saturating concentrations of various ligands (Fig. 13) clearly indicated that the channel optimally activated by Rib or Fur exhibited the same gating kinetics as AdA-liganded channels, characterized by having a significantly shorter 〈τo〉 and a longer 〈τc〉 in the absence of ATP than in the presence of ATP. In contrast, InsP3-liganded channels exhibited the same 〈τo〉 and 〈τc〉 under both conditions.
Figure 13.
〈τc〉 and 〈τo〉 of X-InsP3R-1 channels in optimal [Ca2+]i (4.4–6.2 μM) and saturating concentrations of various ligands in 0 (white bars) and 0.5 mM (shaded bars) ATP.
DISCUSSION
Since its discovery as a potent, metabolically stable agonist of the InsP3R, AdA has been used in studies of the InsP3R and its regulation and in studies that examined intracellular Ca2+ release in cells (see introduction). Our study represents the first investigation of the single-channel properties of the InsP3R channel in its native membrane environment activated by AdA and its analogues.
ATP-dependent Differences in InsP3R Gating Activated by InsP3 and AdA
The major finding in our study is that AdA activates the InsP3R channel with distinct properties depending on the presence or absence of ATP. In the presence of 0.5 mM cytoplasmic free ATP, the endogenous Xenopus type 1 InsP3R channel activated by AdA was indistinguishable from the InsP3-liganded channel. The conductance properties, channel gating properties, biphasic Ca2+ activation and inhibition, and tuning of the sensitivity to Ca2+ inhibition by the agonist concentration were identical for InsP3- and AdA-liganded InsP3R. The efficacy of the two ligands (i.e., P max of the channel that the two ligands can elicit) was also comparable. However, the potency of AdA as an agonist to reduce the sensitivity of the channel to Ca2+ inhibition (i.e., increasing K inh) was ∼60 times that of InsP3. This figure agrees well with the affinity of the channel for AdA determined by binding and Ca2+ release assays (Takahashi et al. 1994; Hirota et al. 1995; Murphy et al. 1997). Thus, in the presence of ATP, the sole distinguishing feature between channels activated by the two ligands is the higher functional affinity of the channel for AdA compared with InsP3. Therefore, the liganded channel in the presence of ATP must attain comparable structural conformations that result in kinetically indistinguishable gating and regulatory behaviors, independent of the nature of the specific ligand.
On the other hand, the nature of the ligand was critically important in determining the kinetic and regulatory properties of the channel when ATP was absent. ATP has been previously shown to stimulate the activities of the InsP3-liganded type 1 InsP3R channels (Ferris et al. 1990; Iino 1991; Bezprozvanny and Ehrlich 1993; Missiaen et al. 1997; Landolfi et al. 1998). In a detailed study that used the same experimental conditions as those employed in the present study, ATP was demonstrated to enhance the sensitivity (lowering K act) of the type 1 InsP3R channels to Ca2+ activation (Mak et al. 1999). New data obtained in this study indicates that ATP also increases the sensitivity of the channel to Ca2+ inhibition (lowering K inh). However, the P max and the gating kinetics (〈τo〉 and 〈τc〉) of optimally activated InsP3-liganded channels are not affected by ATP (Mak et al. 1999; and this study). Furthermore, the affinity of the channel for InsP3 is also not substantially affected by ATP (up to 0.5 mM; this study).
In marked contrast, when the channels were activated by AdA, the presence or absence of ATP (0 vs. 0.5 mM) profoundly affected the P max of the channel and the gating kinetics, as well as the potency of AdA to activate the channel. Although ATP enhanced the sensitivities of the AdA-liganded X-InsP3R-1 channel to both Ca2+ activation and inhibition to the same extent as for InsP3-liganded channels, channels activated by AdA in the absence of ATP had a decreased P max, altered gating kinetics (mainly decreased 〈τo〉), and diminished functional affinity for AdA. Thus, in the absence of ATP, several features distinguish channels activated by either InsP3 or AdA. Both the efficacy and apparent affinity of AdA become significantly reduced in the absence of ATP. Whereas AdA is a full agonist in the presence of ATP, it is only a partial agonist in its absence. InsP3, on the other hand, is a full agonist in either the presence or absence of free ATP. Therefore, the InsP3-liganded and AdA-liganded channels in the absence of ATP must attain distinct structural conformations that result in kinetically distinguishable gating and regulatory behaviors.
Molecular Structural Basis of Interactions between the InsP3R and Its Agonists
Based on comparisons of the molecular structures of analogues of InsP3 (Irvine et al. 1984) and AdA (Takahashi et al. 1994; Wilcox et al. 1995; Marchant et al. 1997; Shuto et al. 1998; Beecroft et al. 1999; Hotoda et al. 1999) that activate Ca2+ release through the InsP3R channel, and on our study of the single-channel activities of InsP3R activated by AdA and its analogues under various conditions, three structural elements can be identified that contribute to the interactions between the channel and its agonists.
First, AdA and most of its structural analogues that activate the InsP3R with high potency have a glucose moiety with a 3′′,4′′-bisphosphate/2′′-hydroxyl motif (Fig. 1) that is structurally similar to the 4,5-bisphosphate/6-hydroxyl motif of InsP3 and its analogues that activate the InsP3R. Therefore, interactions between this structural element and the InsP3 binding site of the InsP3R are necessary for activation of InsP3R channel activity.
Second, although many structural analogues of AdA also bind and activate the InsP3R, their binding affinities (1/K d) and functional potencies (1/EC50) for the channel are all significantly lower than those of AdA. AdA has an adenosine 2′-phosphate moiety (Fig. 1) not present in any of its analogues. Thus, it has been proposed that interactions between this second structural element and the InsP3R enhance the affinity of the channel for AdA (Hotoda et al. 1999). As AdA and ATP share a common adenine moiety in their molecular structures (Fig. 1), we initially considered that some interaction of AdA with an ATP binding site(s) in the InsP3R might contribute to high affinity binding. However, our single-channel results provide no evidence for ATP being an antagonist competing with AdA for the same binding site(s) in the InsP3R, as the presence of cytoplasmic free ATP enhanced rather than reduced the functional potency of AdA to activate channel gating (Fig. 3 and Fig. 7). Therefore, we conclude that ATP and AdA must bind to distinct sites in the InsP3R. In support of this conclusion, peptides containing putative ATP-binding sequences in the InsP3R bind ATP but not AdA (Maes et al. 1999), and the NH2-terminal ligand-binding domain of the InsP3R itself contains the site(s) responsible for high affinity binding of AdA to the receptor (Glouchankova et al. 2000).
The third structural element contributing to AdA interaction with the InsP3R is the 2′-phosphoryl group in the ribose ring of AdA, Rib, and Fur. This element is probably in an analogous position as the 1-phosphoryl group in InsP3 (Wilcox et al. 1995), although it has a different physical location relative to the 3′′,4′′-bisphosphate/2′′-hydroxyl motif in AdA and its analogues compared with the 1-phosphoryl group in InsP3 relative to the 4,5-bisphosphate/6-hydroxyl motif (Hotoda et al. 1999). Interaction between this element and the InsP3R is necessary for the activation of the InsP3R by its agonists (Irvine et al. 1984)
Molecular Model for Allosteric Effects of ATP on Ligand Gating of InsP3R
How can we account for the dramatic effects of ATP on the functional interaction of AdA with the channel? How is it that, in the presence of ATP, AdA elicited identical channel activation and gating as InsP3 but with a much higher potency, whereas in the absence of ATP, AdA had only approximately twofold higher potency than InsP3 and could only activate the InsP3R half as efficaciously as InsP3?
As discussed above, there is no evidence for a direct interaction of AdA and ATP with the same sites in the InsP3R. Therefore, the effects of ATP on the functional interaction of AdA with the receptor are likely mediated by allosteric interactions. We suggest that ATP binds to a site in the InsP3R different from the NH2-terminal ligand-binding site, likely within the regulatory domain that links the ligand-binding domain to the channel domain. Binding of ATP to this site produces an allosteric conformational change in the ligand-binding site that enhances the binding of AdA to the channel, as illustrated in Fig. 14. The model shown in Fig. 14 assumes that this enhanced binding of AdA to InsP3R is caused by interaction between the receptor and the adenine moiety in AdA (Hotoda et al. 1999), but it is possible that the enhanced functional affinity of AdA to the InsP3R in the presence of ATP is due to the adenine structure in AdA positioning the 2′-phosphoryl group in the ribose ring of AdA for a more optimal interaction with the receptor (Hotoda et al. 1999).
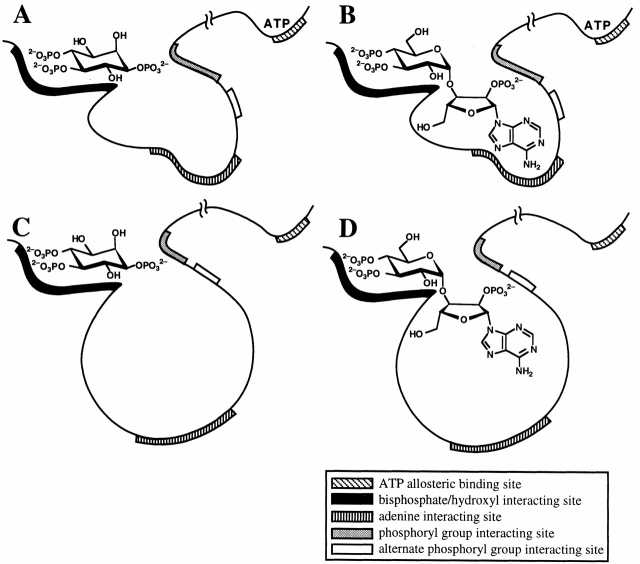
Figure 14.
Schematic diagrams representing interactions between the X-InsP3R-1 molecule and various ligands. (A) Interaction between X-InsP3R-1 and InsP3 in the presence of ATP. (B) Interaction between X-InsP3R-1 and AdA in the presence of ATP. (C) Interaction of X-InsP3R-1 and InsP3 in the absence of ATP. (D) Interaction of X-InsP3R-1 and AdA in the absence of ATP.
As shown in Fig. 14, the 1-phosphoryl group in InsP3 interacts equally well with the conformations of the ligand-binding site of the InsP3R in either the presence or absence of ATP. This interaction elicits the same channel gating kinetics independent of ATP (Fig. 2). In the presence of ATP, the 2′-phosphoryl group in AdA, Rib, or Fur can bind to the same phosphoryl group-binding site in the receptor that InsP3 interacts with, so that the channel gating kinetics evoked by AdA, Fur, and Rib are indistinguishable from those evoked by InsP3 (Fig. 2 A and 8). Under these conditions, AdA has equal efficaciousness as a full agonist as InsP3. In contrast, in the distinct conformation that the InsP3R assumes in the absence of ATP, the 2′-phosphoryl group in AdA, Fur, or Rib has a different interaction with the InsP3R ligand-binding site (possibly through an alternate phosphoryl group interacting site). When AdA is bound to the channel in this conformation, the channel gates differently (Fig. 2 B and 8) because the interaction is less able to stabilize the channel open state as when the channel is bound to InsP3 or AdA in the presence of ATP. Therefore, AdA gates the channel less efficaciously, behaving as a partial agonist. The interaction between this element and the InsP3R is not only necessary for the activation of the InsP3R by its agonists, but also determines the gating kinetics of the activated channel.
The regulatory region of the InsP3R, where ATP likely binds, has been regarded as a transduction region which links the NH2-terminal ligand-binding domain to the gating machinery associated with the COOH-terminal channel pore region. Our results suggest that the regulatory region influences the properties of the ligand-binding domain as well as the coupling between ligand binding and channel gating. Binding of either ligand, InsP3 or AdA, activates channel gating by destabilizing channel closed states (Mak et al. 1998). ATP activates the liganded channel also by destabilizing closed states, tuning the Ca2+ sensitivity of distinct activating Ca2+-binding sites (Mak et al. 1999). Regulation of open channel states has not been previously implicated in the mechanisms by which InsP3R channel gating is regulated by ligands, Ca2+, and ATP (Mak et al. 1998; Mak et al. 1999; and this study). The present study has identified distinct channel open times as the major kinetic feature that accounts for the significant reduction in the efficacy of AdA as an agonist. This result now suggests that ligand binding plays a role in stabilizing channel open states, in addition to destabilizing closed kinetic states.
Relationship of Channel Gating to Kinetics of AdA-induced Ca2+ Release through the InsP3R Observed in Xenopus Oocytes
Previous studies of AdA-induced intracellular Ca2+ release in Xenopus oocytes by confocal imaging (Marchant and Parker 1998) or measurements of plasma membrane Ca2+-activated Cl− currents (DeLisle et al. 1997; Hartzell et al. 1997; Machaca and Hartzell 1999) indicated that Ca2+ release through InsP3R activated by AdA was qualitatively different from that activated by InsP3. Our results demonstrate that the properties of the X-InsP3R-1 channel activated by AdA are indistinguishable from those activated by InsP3 in the presence of 0.5 mM cytoplasmic ATP. The profound effects of ATP on the AdA-liganded channels observed in this study were due to free ATP, as Mg2+ was not present. Thus, the distinct cytoplasmic calcium signals measured in oocytes activated by AdA may suggest that the level of free ATP in Xenopus oocyte cytoplasm was lower than 0.5 mM in those studies. Total ATP content in cells is 4–8 mM, most of which is complexed with Mg2+ (Flatman 1991). With 4 mM each of ATP and Mg2+, free ATP is predicted to be ∼0.35 mM; with 8 mM of each, free ATP is predicted to be ∼0.5 mM. Thus, free ATP concentrations in the oocyte cytoplasm may realistically be expected to be <0.5 mM, as our results predict.
Nevertheless, some features of the calcium signals evoked by AdA in oocytes are consistent with a high affinity of the InsP3R for AdA, which our results suggest is dependent on the presence of ATP. The slower rate of propagation of calcium waves activated by AdA (Bird et al. 1999; Machaca and Hartzell 1999) and the more spatially restricted calcium signals observed in the presence of AdA (Bird et al. 1999) were both interpreted to reflect a substantially reduced diffusion coefficient of AdA in the oocyte cytoplasm because of high affinity binding to the InsP3R (Machaca and Hartzell 1999). Our study suggests that the affinity of the InsP3R for AdA is significantly higher than that for InsP3 only in the presence of cytoplasmic free ATP (Fig. 3 and Fig. 7). Thus, the oocyte cytoplasm, although having a free ATP concentration <0.5 mM, must nevertheless contain a finite concentration of free ATP, as expected. Therefore, we conclude that the Xenopus oocytes have cytoplasmic free ATP concentrations between 0 and 0.5 mM.
Our results demonstrate that AdA may or may not elicit a similar response from the InsP3R as InsP3, depending on the concentration of cytoplasmic free ATP. Without knowledge or control of the cytoplasmic free ATP concentration in experiments using AdA to investigate intracellular calcium signaling, AdA cannot be regarded simply as a nonmetabolizable, more potent substitute for InsP3 as an agonist of the InsP3R.
With this in mind, the single-channel gating kinetics of the X-InsP3R-1 activated by AdA observed in our nuclear patch-clamp experiments can reasonably account for results obtained in the in vivo Ca2+ release studies. First, the rate of Ca2+ release in oocytes stimulated with a high concentration of AdA (∼2 μM) was only half of that stimulated by high concentrations of InsP3 (∼20 μM; Machaca and Hartzell 1999). This can be explained by our observation that P max of the InsP3R channel activated by AdA was substantially lower than that activated by InsP3 at the suboptimal ATP concentrations in the oocyte cytoplasm. Second, 5 nM AdA elicited a significantly slower rate of Ca2+ release than 2 μM AdA (Machaca and Hartzell 1999), although both concentrations would have been predicted to be saturating for binding to the channel (Takahashi et al. 1994; Hirota et al. 1995; Murphy et al. 1997; and this study). Our patch-clamp experiments revealed that at suboptimal free ATP concentrations in the oocyte cytoplasm, the affinity of AdA for the channel is likely reduced, such that 5 nM AdA may become subsaturating and, therefore, elicit a significantly lower rate of Ca2+ release than 2 μM AdA, as observed. Third, whereas InsP3 invariably stimulated the Ca2+-activated Cl− current I Cl1-S (Hartzell 1996; Hartzell et al. 1997; Kuruma and Hartzell 1999; Machaca and Hartzell 1999), AdA was insufficient to stimulate I Cl1-S but still generate store-operated Ca2+ influx (DeLisle et al. 1997; Hartzell et al. 1997; Machaca and Hartzell 1999). Our experimental results offer a likely explanation. The rate of Ca2+ release activated by InsP3 was invariably high enough to activate I Cl1-S because of the high P max of InsP3-liganded InsP3R channels. In contrast, in suboptimal cytoplasmic-free ATP concentrations, the reduced P max of the AdA-liganded channels gave rise to a slower rate of Ca2+ release that was insufficient to stimulate I Cl1-S. This slow rate of release activated by AdA was nevertheless sufficient over time to deplete the InsP3 sensitive Ca2+ stores, thereby generating store-operated Ca2+ influx.
Of considerable interest is the possibility to correlate distinct single-channel properties of the InsP3R activated by either InsP3 or AdA with the distinct kinetics of elementary Ca2+ release events (puffs) triggered by these ligands in Xenopus oocytes. Puffs mediated by the X-InsP3R-1 have been imaged in oocytes activated sequentially by InsP3 and AdA (Marchant and Parker 1998). These spatially restricted puffs reflect the activation of several InsP3R channels within a cluster of channels. The variable amplitudes of puffs can be understood as reflecting variable numbers of InsP3R channels within clusters (Mak and Foskett 1997; Sun et al. 1998; Thomas et al. 1998) and the stochastic nature of the channel gating (Mak and Foskett 1997). Puffs have been characterized by quantitative determinations of the peak change in fluorescence, as a measure of the peak rate of Ca2+ liberation; the duration and the rise time, both reflecting the length of time the channels were open to release Ca2+; and signal mass, representing the total amount of Ca2+ released (Marchant and Parker 1998; Sun et al. 1998). A major unresolved question in calcium signaling is the nature of the mechanisms that regulate the duration of Ca2+ release during a puff. Puffs elicited by activation with low concentrations of AdA had similar peak rates of Ca2+ liberation but were temporally shorter (faster rise time and shorter duration) and released less total Ca2+ compared with those activated by InsP3 (Marchant and Parker 1998). The principle conclusion from these results was that the duration of a Ca2+ puff bears no simple relationship to the affinity of the agonist, ruling out agonist dissociation as the mechanism which delimits the period of Ca2+ flux through the InsP3R channels during a puff (Marchant and Parker 1998). Therefore, it was speculated (Marchant and Parker 1998) that Ca2+-mediated inhibition (Parker and Ivorra 1990; Finch et al. 1991) and InsP3-induced channel inactivation (Hajnóczky and Thomas 1994; Mak and Foskett 1997) may be involved. Our results now suggest another possible mechanism. Our study of the single-channel properties of the X-InsP3R-1 has revealed that in suboptimal ATP concentrations, the main difference between InsP3R channels activated by AdA and InsP3 is the significantly shorter 〈τo〉 of the AdA-liganded channels (Fig. 10B and Fig. C). Thus, there is a correlation between 〈τo〉 of the single InsP3R channel and the rise time, duration, and total amount of Ca2+ released of a Ca2+ puff. Therefore, we suggest that a major determinant of the duration of Ca2+ release, and of the amount of Ca2+ released during a puff, is the ligand-dependent 〈τo〉, rather than Ca2+-mediated inhibition or ligand-induced channel inactivation. It is interesting to note that Ca2+ sparks mediated by ryanodine receptor Ca2+ release channels in frog skeletal muscle fibers have faster rise times and reduced total Ca2+ released when 〈τo〉 of the channels is prematurely shortened by membrane repolarization (Lacampagne et al. 2000). Thus, 〈τo〉 can be a major determinant of the duration of elementary Ca2+ release events mediated by both major families of intracellular Ca2+ release channels.
Based on our studies of the regulation of the single-channel activities of X-InsP3R-1, we consider the following scenario as one that can account for the correlation between 〈τo〉 and the duration of a puff. It is generally believed that each Ca2+ puff is initiated by the stochastic opening of one of the InsP3R channels clustered together in the Ca2+ release site (Yao et al. 1995). The Ca2+ released by one channel can diffuse to neighboring channels, increasing the local [Ca2+]i in the vicinity of those channels so that they too open, by Ca2+ activation (Ca2+ induced Ca2+ release). Other mechanisms may serve to couple the channels to affect a concerted opening of several of them (Mak and Foskett 1997; Marx et al. 1998). This rapid concerted activation of the channels in a cluster generates the Ca2+ puffs (Yao et al. 1995; Mak and Foskett 1997; Sun et al. 1998). The locally high [Ca2+]i near the channels as a result of this liberation can, in turn, feed back to inhibit them (Fig. 3, Fig. 4, and Fig. 7). Indeed, because puffs are generated under conditions of low agonist concentration (Yao et al. 1995; Berridge 1997; Marchant and Parker 1998), the channel has a high sensitivity to Ca2+ inhibition (Mak et al. 1998; and this study). Nevertheless, a critical observation is that 〈τo〉 has very little dependence on [Ca2+]i (Fig. 10). This lack of sensitivity of the open channel to Ca2+ inhibition implies that, once a channel has opened, it will stay open for a duration approximately equal to 〈τo〉, independent of the local [Ca2+]i in the vicinity of the channel. Only after the channel closes can cytoplasmic Ca2+ feed back to inhibit it from reopening, as Ca2+ inhibition of gating operates by stabilizing the channel closed state (Fig. 10). Thus, once a channel has opened during a puff, it will stay open for a duration approximately equal to 〈τo〉 and then close. At that time, it is possible that the high local [Ca2+]i, contributed by Ca2+ released from the channel itself as well as from its neighbors, will prevent it from reopening within the duration of the puff. Therefore, during a single puff, each channel in the Ca2+ release site likely opens at most once. As the conductance properties of the AdA- and InsP3-liganded channels are indistinguishable (Fig. 2), the mean amount of Ca2+ released in an opening of each InsP3R channel in a cluster is therefore predicted to be directly proportional to 〈τo〉. This model also predicts that the mean duration of the puff will be proportional to 〈τo〉.
Alternatively, the puff could terminate as a result of the stochastic nature of channel gating without invoking Ca2+ inhibition of reopening. When all the activated channels in the cluster become closed at the same time, simply as a result of the nonzero probability that the stochastic closed times of all the activated channels will coincide, the Ca2+ release needed for stimulation of further openings will be eliminated, thereby extinguishing the puff (Niggli 1999; Stern et al. 1999). This model is similar to the stochastic attrition model developed by Stern 1992 to help account for termination of Ca2+ release through clusters of ryanodine receptors. Analytical derivation of the time constant for the puff duration in the stochastic attrition model showed it to be directly proportional to the channel mean open duration (Stern et al. 1999). Thus, models using Ca2+ inhibition or stochastic attrition both suggest that the difference between the duration of puffs and the quantities of Ca2+ released by puffs activated by InsP3 and AdA (Marchant and Parker 1998) is a consequence of the difference in the mostly Ca2+-independent 〈τo〉 of the InsP3- and AdA-liganded InsP3R channels. Importantly, our results demonstrate that this difference is a function of the cytoplasmic free ATP concentration, suggesting that free ATP concentration helps to shape the properties of elementary Ca2+ release signals generated by AdA.
Acknowledgments
This work was supported by grants to J.K. Foskett from the National Institutes of Health (MH59937 and GM56328) and to D.-O.D. Mak from the American Heart Association (9906220U).
Footnotes
Abbreviations used in this paper: AdA, adenophostin A; [Ca2+]i, cytoplasmic free Ca2+ concentration; Fur, furanophostin; InsP3, inositol 1,4,5-trisphosphate; InsP3R, InsP3 receptor; pdf, probability density function; Rib, ribophostin.
References
- Adkins C.E., Morris S.A., De Smedt H., Sienaert I., Török K., Taylor C.W. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1,-2 and-3 inositol trisphosphate receptors. Biochem. J. 2000;345:357–363. [PMC free article] [PubMed] [Google Scholar]
- Baudet S.B., Hove-Madsen L., Bers D.M. How to make and use calcium-specific mini- and microelectrodes. In: Nuccitelli R., editor. A Practical Guide to the Study of Calcium in Living Cells. Academic Press; San Diego, CA: 1994. pp. 94–114. [PubMed] [Google Scholar]
- Beecroft M.D., Marchant J.S., Riley A.M., Van Straten N.C.R., Van der Marel G.A., Van Boom J.H., Potter B.V.L., Taylor C.W. Acyclophostina ribose-modified analog of adenophostin A with high affinity for inositol 1,4,5-trisphosphate receptors and pH-dependent efficacy. Mol. Pharmacol. 1999;55:109–117. doi: 10.1124/mol.55.1.109. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Elementary and global aspects of calcium signalling. J. Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- Bird G.S., Takahashi M., Tanzawa K., Putney J.W., Jr. Adenophostin A induces spatially restricted calcium signaling in Xenopus laevis oocytes. J. Biol. Chem. 1999;274:20643–20649. doi: 10.1074/jbc.274.29.20643. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Broad L.M., Armstrong D.L., Putney J.W., Jr. Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (I crac) J. Biol. Chem. 1999;274:32881–32888. doi: 10.1074/jbc.274.46.32881. [DOI] [PubMed] [Google Scholar]
- DeLisle S., Marksberry E.W., Bonnett C., Jenkins D.J., Potter B.V.L., Takahashi M., Tanzawa K. Adenophostin A can stimulate Ca2+ influx without depleting the inositol 1,4,5-trisphosphate-sensitive Ca2+ stores in the Xenopus oocyte. J. Biol. Chem. 1997;272:9956–9961. doi: 10.1074/jbc.272.15.9956. [DOI] [PubMed] [Google Scholar]
- Ferris C.D., Huganir R.L., Snyder S.H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc. Natl. Acad. Sci. USA. 1990;87:2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E.A., Turner T.J., Goldin S.M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Flatman P.W. Mechanisms of magnesium transport. Annu. Rev. Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Glouchankova L., Krishna U.M., Potter B.V.L., Falck J.R., Bezprozvanny I. Association of the inositol (1,4,5)-trisphosphate receptor ligand binding site with phosphatidylinositol (4,5)-bisphosphate and adenophostin A. Mol. Cell Biol. Res. Commun. 2000;3:153–158. doi: 10.1006/mcbr.2000.0208. [DOI] [PubMed] [Google Scholar]
- Gregory R.B., Wilcox R.A., Berven L.A., Van Straten N.C.R., Van der Marel G.A., Van Boom J.H., Barritt G.J. Evidence for the involvement of a small subregion of the endoplasmic reticulum in the inositol trisphosphate receptor-induced activation of Ca2+ inflow in rat hepatocytes. Biochem. J. 1999;341:401–408. [PMC free article] [PubMed] [Google Scholar]
- Hagar R.E., Ehrlich B.E. Regulation of the type III InsP3 receptor by InsP3 and ATP. Biophys. J. 2000;79:271–278. doi: 10.1016/S0006-3495(00)76289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G., Thomas A.P. The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature. 1994;370:474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H.C. Activation of different Cl currents in Xenopus oocytes by Ca liberated from stores and by capacitative Ca influx. J. Gen. Physiol. 1996;108:157–175. doi: 10.1085/jgp.108.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H.C., Machaca K., Hirayama Y. Effects of adenophostin-A and inositol-1,4,5-trisphosphate on Cl− currents in Xenopus laevis oocytes. Mol. Pharmacol. 1997;51:683–692. doi: 10.1124/mol.51.4.683. [DOI] [PubMed] [Google Scholar]
- He C.L., Damiani P., Ducibella T., Takahashi M., Tanzawa K., Parys J.B., Fissore R.A. Isoforms of the inositol 1,4,5-trisphosphate receptor are expressed in bovine oocytes and ovariesthe type-1 isoform is down-regulated by fertilization and by injection of adenophostin A. Biol. Reprod. 1999;61:935–943. doi: 10.1095/biolreprod61.4.935. [DOI] [PubMed] [Google Scholar]
- Hirota J., Michikawa T., Miyawaki A., Takahashi M., Tanzawa K., Okura I., Furuichi T., Mikoshiba K. Adenophostin-medicated quantal Ca2+ release in the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett. 1995;368:248–252. doi: 10.1016/0014-5793(95)00659-w. [DOI] [PubMed] [Google Scholar]
- Hotoda H., Murayama K., Miyamoto S., Iwata Y., Takahashi M., Kawase Y., Tanzawa K., Kaneko M. Molecular recognition of adenophostin, a very potent Ca2+ inducer, at the d-myo-inositol 1,4,5-trisphosphate receptor. Biochemistry. 1999;38:9234–9241. doi: 10.1021/bi990114r. [DOI] [PubMed] [Google Scholar]
- Huang Y., Takahashi M., Tanazawa K., Putney J.W., Jr. Effect of adenophostin A on Ca2+ entry and calcium release-activated calcium current (Icrac) in rat basophilic leukemia cells. J. Biol. Chem. 1998;273:31815–31821. doi: 10.1074/jbc.273.48.31815. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Effects of adenine nucleotides on inositol 1,4,5-trisphosphate-induced calcium release in vascular smooth muscle cells. J. Gen. Physiol. 1991;98:681–698. doi: 10.1085/jgp.98.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F., Brown K.D., Berridge M.J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem. J. 1984;222:269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T., He C.L., Wu H., Parys J.B., Fissore R.A. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev. Biol. 2000;223:238–250. doi: 10.1006/dbio.2000.9675. [DOI] [PubMed] [Google Scholar]
- Kashiwayanagi M., Tatani K., Shuto S., Matsuda A. Inositol 1,4,5-trisphosphate and adenophostin analogues induce responses in turtle olfactory sensory neurons. Eur. J. Neurosci. 2000;12:606–612. doi: 10.1046/j.1460-9568.2000.00948.x. [DOI] [PubMed] [Google Scholar]
- Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptorstructure, function, and localization in oocytes and eggs. Cell. 1993;73:555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Kuruma A., Hartzell H.C. Dynamics of calcium regulation of chloride currents in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1999;276:C161–C175. doi: 10.1152/ajpcell.1999.276.1.C161. [DOI] [PubMed] [Google Scholar]
- Lacampagne A., Klein M.G., Ward C.W., Schneider M.F. Two mechanisms for termination of individual Ca2+ sparks in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2000;97:7823–7828. doi: 10.1073/pnas.97.14.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A.M. Ca2+ homeostasis in the agonist-sensitive internal storefunctional interactions between mitochondria and the ER measured in situ in intact cells. J. Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K., Hartzell H.C. Adenophostin A and inositol 1,4,5-trisphosphate differentially activate Cl− currents in Xenopus oocytes because of disparate Ca2+ release kinetics. J. Biol. Chem. 1999;274:4824–4831. doi: 10.1074/jbc.274.8.4824. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J. Biol. Chem. 1991;266:1109–1116. [PubMed] [Google Scholar]
- Maes K., Missiaen L., Parys J.B., Sienaert I., Bultynck G., Zizi M., De Smet P., Casteels R., De Smedt H. Adenine-nucleotide binding sites on the inositol 1,4,5-trisphosphate receptor bind caffeine, but not adenophostin A or cyclic ADP-ribose. Cell Calcium. 1999;25:143–152. doi: 10.1054/ceca.1998.0011. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D, Foskett J.K. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- Marchant J.S., Beecroft M.D., Riley A.M., Jenkins D.J., Marwood R.D., Taylor C.W., Potter B.V.L. Disaccharide polyphosphates based upon adenophostin A activate hepatic d-myo-inositol 1,4,5-trisphosphate receptors. Biochemistry. 1997;36:12780–12790. doi: 10.1021/bi971397v. [DOI] [PubMed] [Google Scholar]
- Marchant J.S., Parker I. Kinetics of elementary Ca2+ puffs evoked in Xenopus oocytes by different Ins(1,4,5)P3 receptor agonists. Biochem. J. 1998;334:505–509. doi: 10.1042/bj3340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Ondrias K., Marks A.R. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Meas K., Missiaen L., De Smet P., Vanlingen G., Callewaert G., Parys J.B., De Smedt H. Differential modulation of inositol 1,4,5-trisphosphate receptor type 1 and type 3 by ATP. Cell Calcium. 2000;27:257–267. doi: 10.1054/ceca.2000.0121. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988;240:653–655. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Mignery G.A., Sudhof T.C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. Inositol 1,4,5-trisphosphate receptor. Trends Pharmacol. Sci. 1993;14:86–89. doi: 10.1016/0165-6147(93)90069-v. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Parys J.B., De Smedt H., Sienaert I., Sipma H., Vanlingen S., Maes K., Casteels R. Effect of adenine nucleotides on myo-inositol-1,4,5-trisphosphate-induced calcium release. Biochem. J. 1997;325:661–666. doi: 10.1042/bj3250661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Parys J.B., Sienaert I., Maes K., Kunzelmann K., Takahashi M., Tanzawa K., De Smedt H. Functional properties of the type-3 InsP3 receptor in 16HBE14o- bronchial mucosal cells. J. Biol. Chem. 1998;273:8983–8986. doi: 10.1074/jbc.273.15.8983. [DOI] [PubMed] [Google Scholar]
- Murphy C.T., Riley A.M., Lindley C.J., Jenkins D.J., Westwick J., Potter B.V.L. Structural analogues of d-myo-inositol-1,4,5-trisphosphate and adenophostin Arecognition by cerebellar and platelet inositol-1,4,5-trisphosphate receptors. Mol. Pharmacol. 1997;52:741–748. doi: 10.1124/mol.52.4.741. [DOI] [PubMed] [Google Scholar]
- Niggli E. Localized intracellular calcium signaling in musclecalcium sparks and calcium quarks. Annu. Rev. Physiol. 1999;61:311–335. doi: 10.1146/annurev.physiol.61.1.311. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberationa possible mechanism for oscillatory release of Ca2+ . Proc. Natl. Acad. Sci. USA. 1990;87:260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto S., Tatani K., Ueno Y., Matsuda A. Synthesis of adenophostin analogues lacking the adenine moiety as novel potent IP3 receptor ligandssome structural requirements for the significant activity of adenophostin A. J. Org. Chem. 1998;63:8815–8824. [Google Scholar]
- Sigworth F.J., Sine S.M. Data transformations for improved display and fitting of single channel dwell time histograms. Biophys. J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D. Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Song L.S., Cheng H.P., Sham J.S.K., Yang H.T., Boheler K.R., Ríos E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 1999;113:469–489. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.-P., Callamaras N., Marchant J.S., Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J. Physiol. 1998;509:67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Tanzawa K., Takahashi S. Adenophostins, newly discovered metabolites of Penicillium brevicompactum, act as potent agonists of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1994;269:369–372. [PubMed] [Google Scholar]
- Thomas D., Lipp P., Berridge M.J., Bootman M.D. Hormone-evoked elementary Ca2+ signals are not stereotypic, but reflect activation of different size channel clusters and variable recruitment of channels within a cluster. J. Biol. Chem. 1998;273:27130–27136. doi: 10.1074/jbc.273.42.27130. [DOI] [PubMed] [Google Scholar]
- Toescu E.C. Temporal and spatial heterogeneities of Ca2+ signalingmechanisms and physiological roles. Am. J. Physiol. 1995;269:G173–G185. doi: 10.1152/ajpgi.1995.269.2.G173. [DOI] [PubMed] [Google Scholar]
- Vanlingen S., Sipma H., De Smet P., Callewaert G., Missiaen L., De Smedt H., Parys J.B. Ca2+ and calmodulin differentially modulate myo-inositol 1,4,5-trisphosphate (IP3)-binding to the recombinant ligand-binding domains of the various IP3 receptor isoforms. Biochem. J. 2000;346:275–280. [PMC free article] [PubMed] [Google Scholar]
- Wilcox R.A., Erneux C., Primrose W.U., Gigg R., Nahorski S.R. 2-hydroxyethyl a-D-glucopyranoside 2,3',4'-trisphosphatea novel metabolically resistant adenophostin A and myo-inositol 1,4,5-trisphosphate analogue potently interacts with the myo-inositol 1,4,5-trisphosphate receptor. Biochem. Soc. Trans. 1995;23:420S. doi: 10.1042/bst023420s. [DOI] [PubMed] [Google Scholar]
- Yao Y., Choi J., Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]