Abstract
Human central core disease (CCD) is caused by mutations/deletions in the gene that encodes the skeletal muscle ryanodine receptor (RyR1). Previous studies have shown that CCD mutations in the NH2-terminal region of RyR1 lead to the formation of leaky SR Ca2+ release channels when expressed in myotubes derived from RyR1-knockout (dyspedic) mice, whereas a COOH-terminal mutant (I4897T) results in channels that are not leaky to Ca2+ but lack depolarization-induced Ca2+ release (termed excitation-contraction [EC] uncoupling). We show here that store depletion resulting from NH2-terminal (Y523S) and COOH-terminal (Y4795C) leaky CCD mutant release channels is eliminated after incorporation of the I4897T mutation into the channel (Y523S/I4897T and Y4795C/I4897T). In spite of normal SR Ca2+ content, myotubes expressing the double mutants lacked voltage-gated Ca2+ release and thus exhibited an EC uncoupling phenotype similar to that of I4897T-expressing myotubes. We also show that dyspedic myotubes expressing each of seven recently identified CCD mutations located in exon 102 of the RyR1 gene (G4890R, R4892W, I4897T, G4898E, G4898R, A4905V, R4913G) behave as EC-uncoupled release channels. Interestingly, voltage-gated Ca2+ release was nearly abolished (reduced ∼90%) while caffeine-induced Ca2+ release was only marginally reduced in R4892W-expressing myotubes, indicating that this mutation preferentially disrupts voltage-sensor activation of release. These data demonstrate that CCD mutations in exon 102 disrupt release channel permeation to Ca2+ during EC coupling and that this region represents a primary molecular locus for EC uncoupling in CCD.
Keywords: excitation-contraction coupling, skeletal muscle, calcium channel, muscle disease
INTRODUCTION
Excitation-contraction (EC)* coupling in skeletal muscle involves a mechanical interaction between dihydropyridine receptors (DHPR) in the sarcolemma and ryanodine receptors (RyR1) or Ca2+ release channels located in the terminal cisternae of the SR (Melzer et al., 1995). DHPR-RyR1 mechanical coupling is a bidirectional signaling interaction since DHPRs trigger the opening of SR Ca2+ release channels (orthograde coupling) and the presence of RyR1 enhances the Ca2+-conducting activity and modifies the L-channel gating properties of the DHPR (retrograde coupling) (Nakai et al., 1996; Avila and Dirksen, 2000; Dirksen, 2002). The central roles of DHPR and RyR1 proteins in normal skeletal muscle Ca2+ signaling is emphasized by the fact that mutations in both of these proteins account for several different human congenital muscle diseases (e.g., hypokalemic periodic paralysis, malignant hyperthermia [MH], and central core disease [CCD]) (Lehmann-Horn and Jurkat-Rott, 1999).
CCD is a human autosomal dominant, nonprogressive myopathy characterized by hypotonia and proximal muscle weakness in infancy (Shuaib et al., 1987). At least 32 different point mutations or deletions in the gene that encodes RyR1 are known to cause CCD in humans (for review see Dirksen and Avila, 2002). All of the CCD mutations identified to date are located within three relatively restricted regions of the RyR1 protein: 3 mutations in MH/CCD region 1 (residues 35–614), 4 mutations in MH/CCD region 2 (residues 2129–2458), and 15 mutations in MH/CCD region 3 (COOH-terminal region, including residues 4136–4973).
Functional studies of MH and CCD mutant RyR1s proteins expressed in either heterologous systems (e.g., HEK-293 cells) or skeletal myotubes derived from RyR1-knockout (dyspedic) mice indicate that CCD mutations in MH/CCD regions 1 and 2 lead to the formation of SR Ca2+ release channels that exhibit varying degrees of Ca2+ leak that manifests as an elevation in resting calcium and a depletion of SR Ca2+ stores (Treves et al., 1994; Tong et al., 1997, 1999; Lynch et al., 1999; Monnier et al., 2000; Avila and Dirksen, 2001). As a consequence of store depletion, voltage-gated Ca2+ release during EC coupling is markedly reduced (Avila and Dirksen, 2001), presumably resulting from a reduction in both the driving force for Ca2+ release from the SR and the regulation of release channel activity by luminal Ca2+ (Sitsapesan and Williams, 1997).
In contrast to that found for CCD mutations in MH/CCD regions 1 and 2, resting Ca2+ levels and SR Ca2+ content are normal in dyspedic myotubes expressing a COOH-terminal mutation in RyR1 (I4897T) (Avila et al., 2001a). Nevertheless, I4897T-expressing dyspedic myotubes do not release SR Ca2+ after stimulation by either caffeine or voltage and coexpression of wild-type and I4897T mutant RyR1 proteins only partially restores caffeine and voltage-gated Ca2+ release. These results suggest that the I4897T mutation leads to a functional uncoupling of sarcolemmal depolarization from the efficient release of SR Ca2+ (Avila et al., 2001a). We have referred to this phenomenon by which voltage-gated SR calcium release is reduced in a manner independent of changes in SR Ca2+ content in I4897T-expressing dyspedic myotubes as “EC uncoupling.” However, our suggestion that muscle weakness arising from the I4897T mutation operates via a fundamentally distinct mechanism (EC uncoupling) from that of the NH2-terminal mutants (leaky channels) is controversial since other studies have suggested that I4897T mutant release channels act as leaky release channels in transfected HEK293 cells (Lynch et al., 1999) and lymphocytes obtained from CCD patients possessing this mutation (Tilgen et al., 2001).
We therefore set out to more rigorously test the validity of our hypothesis that the I4897T mutation operates via a fundamentally distinct cellular mechanism than that caused by CCD mutations that lead to enhanced SR Ca2+ leak. Our results indicate that normal levels of resting Ca2+ and SR Ca2+ content are restored after incorporation of the I4897T mutation into leaky channels. In addition, we found that all seven of the CCD mutations identified in exon 102 of the RyR1 gene lead to varying degrees of EC uncoupling following reconstitution in dyspedic myotubes, thus confirming this region of RyR1 as a primary molecular locus for EC uncoupling in CCD.
MATERIALS AND METHODS
Preparation and cDNA Injections of Dyspedic Myotubes
Primary cultures of myotubes were prepared from skeletal muscle of newborn dyspedic mice as described previously (Nakai et al., 1996; Avila and Dirksen, 2000). Each of the different CCD point mutations in RyR1 used in this study (Y523S, Y4795C, G4890R, R4892W, I4897T, G4898E, G4898R, A4905V, and R4913G) were introduced into a rabbit RyR1 cDNA using standard two-step site-directed mutagenesis strategies and the entire PCR-modified cDNA portion of each clone was ultimately confirmed by sequence analysis. Expression of wild-type RyR1 and the different CCD mutations in RyR1 was achieved by nuclear microinjection of cDNAs encoding CD8 (0.2 μg/μl) and the appropriate expression plasmid (0.5 μg/μl) 4–6 d after of initial plating of myoblasts (Avila et al., 2001a).
Measurements of Resting Ca2+ and Caffeine-, 4-Chloro-m-Cresol–, and CPA-induced Ca2+ Transients in Intact Myotubes
Resting Ca2+ and drug-induced changes in intracellular Ca2+ were measured in intact myotubes loaded with the fluorescent Ca2+ indicator Indo-1 AM (Molecular Probes) as described previously (Avila et al., 2001a,b). Indo-1–loaded myotubes were bathed in a normal rodent Ringer's solution (containing 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, pH 7.4) and excited at 350 nm. Fluorescence emission at 405 and 485 nm was collected using a photomultiplier detection system and the results presented as the ratio (R) of F405/F485. The resting F405/F485 ratio was converted to cytosolic Ca2+ levels using an in situ calibration approach described previously (Avila et al., 2001a). Relative changes in global Ca2+ levels were subsequently monitored after application of maximal concentrations of either caffeine (10 mM), 4-chloro-m-cresol (4-cmc, 500 μM), or cyclopiazonic acid (CPA, 30 μM) using a rapid perfusion system that permits fast, local application of agonist as well as rapid washout with control solution (Avila et al., 2001a,b). In some experiments, Indo-1 AM–loaded myotubes bathed in Ringer's were stimulated (8.0 V for 20 ms at 0.1 Hz for 30 s) using an extracellular pipette before the application of RyR1 agonists. Peak intracellular Ca2+ changes in response to agonist application are expressed as ΔRatio (Ragonist − Rbaseline).
Simultaneous Measurements of Macroscopic Ca2+ Currents and Ca2+ Transients
The whole-cell patch clamp technique was used to simultaneously measure L-currents and Ca2+ transients in expressing myotubes as described previously (Avila and Dirksen, 2000, 2001; Avila et al., 2001a,b). Four different series of experiments were carried-out, and the results for each experimental condition are plotted with the corresponding control condition (RyR1). Thus, results obtained from RyR1-expressing myotubes were collected in four different groups: group I (n = 18), group II, (n = 14), group III (n = 8), and group IV (n = 9). Peak L-currents (linear components subtracted using a P/3 protocol) were normalized to cell capacitance (pA/pF), plotted as a function of the membrane potential, and fitted according to: I = Gmax*(Vm − Vrev)/(1 + exp[(VG1/2 − Vm)/k G]) where Vrev is the extrapolated reversal potential of the Ca2+ current, Vm is the membrane potential during the test pulse, Gmax is the maximum L-channel conductance, VG1/2 is the voltage for half activation of Gmax, and k G is a slope factor. Relative changes in cytosolic Ca2+ in patch clamp experiments were recorded in Fluo-3 dialyzed myotubes. Fluorescence traces were analogue filtered (τ = 0.5 ms, RC filter) before digitization at 10 kHz and expressed as ΔF/F ([Fpeak − FBase]/FBase). The magnitude of maximal voltage-gated calcium release ((ΔF/F)max) was plotted as a function of the membrane potential, and fitted according to: ΔF/F = (ΔF/Fmax) /{1 + exp [(VF1/2 − Vm)/k F]}, where ΔF/Fmax is the calculated maximal fluorescence change during the test pulse, VF1/2 is the midpoint potential, and k F is a slope factor. The internal solution contained (in mM): 145 Cs-Aspartate, 10 CsCl, 0.1 Cs2-EGTA, 1.2 MgCl2, 5 Mg-ATP, 0.2 K5-Fluo-3, 10 HEPES, pH 7.4. The external solution contained (in mM): 145 TEA-Cl, 10 CaCl2, 0.003 TTX, and 10 HEPES, pH 7.4.
All experiments were performed at room temperature (20–24°C) and data are presented as mean ± SEM. When indicated, significant differences (unpaired t tests) were determined using the software package Sigma Plot 2000 (SPSS). Statistic analysis was carried out by comparing every experimental condition versus its corresponding control group (RyR1) collected concurrently. Unless specified, differences were determined to be significant at P < 0.05.
RESULTS
The I4897T Mutation Blocks SR Ca2+ Leak through Y523S and Y4795C CCD Mutant Ca2+ Release Channels
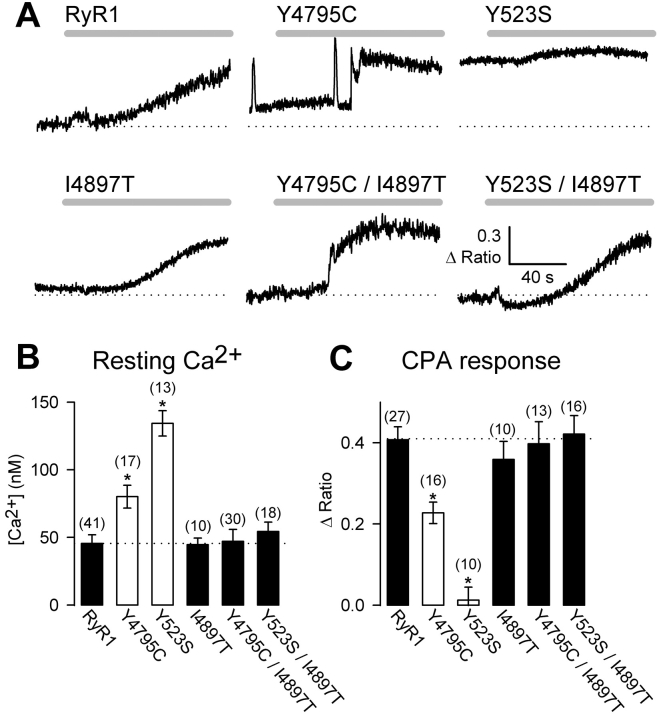
We hypothesized that if the I4897T mutation results in SR Ca2+ release channels that poorly permeate Ca2+, then incorporation of this mutation into a leaky channel should restore normal levels of resting Ca2+ and SR Ca2+ content. To test this idea, we evaluated the effects of incorporating the I4897T mutation into a severely leaky NH2-terminal CCD mutant release channel (Fig. 1 , Y523S). For these experiments, myoplasmic Ca2+ levels were monitored in Indo-1 AM–loaded RyR1- and Y523S-expressing dyspedic myotubes before and after application of a maximal concentration of CPA, an agent that increases cytosolic Ca2+ by reversibly inhibiting SR Ca2+-ATPase pumps. Compared with RyR1-expressing dyspedic myotubes, Y523S-expressing myotubes exhibit a substantially elevated resting Ca2+ level (Fig. 1 B) and a reduction in CPA-releasable SR Ca2+ stores (Fig. 1 C). Interestingly, increased SR Ca2+ leak can also result from a CCD mutations in the COOH-terminal region of RyR1 (MH and CCD region 3) since dyspedic myotubes expressing the Y4795C CCD mutation also exhibit elevated resting Ca2+ levels and a reduction in SR Ca2+ content (Fig. 1, Y4795C). Consistent with the hypothesis that the I4897T mutation disrupts Ca2+ permeation through SR Ca2+ release channels, expression of double mutant RyR1 proteins that incorporate both the I4897T and either Y523S (Y523S/I4897T) or Y4795C (Y4795C/I4897T) resulted in total restoration of normal levels of both resting Ca2+ and SR Ca2+ content (Fig. 1, B and C).
Figure 1.
The I4897T CCD mutation blocks SR Ca2+ leak through Y523S and Y4795C release channels. (A) Representative CPA-induced Ca2+ responses (30 μM CPA, gray bars) in Indo-1 AM–loaded dyspedic myotubes expressing wild-type RyR1, Y523S, Y4795C, I4897T, Y523S/I4897T, Y4795C/I4897T. A dotted line representing the average resting fluorescence of RyR1-expressing myotubes is shown for comparison. (B) Average resting Ca2+ levels of dyspedic myotubes expressing wild-type RyR1, Y523S, Y4795C, I4897T, Y523S/I4897T, and Y4795C/I4897T. (C) Average steady-state CPA-induced Ca2+ responses (Δ Ratio = RCPA − Rbaseline) for dyspedic myotubes expressing wild-type RyR1, Y523S, Y4795C, I4897T, Y523S/I4897T, Y4795C/I4897T. Asterisks indicate significant differences (P < 0.05) compared with RyR1.
Voltage-gated SR Ca2+ Release Is Abolished in Y523S/I4897T- and Y4795C/I4897T-expressing Dyspedic Myotubes
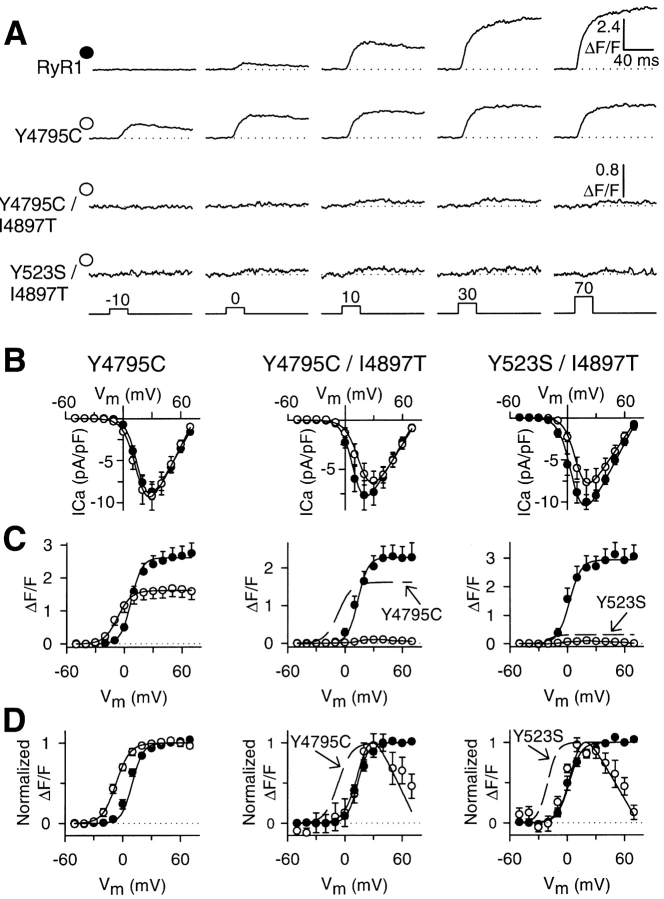
Fig. 1 demonstrates that the I4897T mutation blocked Ca2+ leak through both Y523S and Y4795C CCD mutant release channels, consistent with a mutation that disrupts Ca2+ permeation of the release channel. We next used the whole-cell patch clamp technique in conjunction with a Ca2+-sensitive dye to evaluate the impact of combining the I4897T mutation with NH2-terminal and COOH-terminal leaky SR Ca2+ channels on bidirectional coupling between DHPR and RyR1 proteins (Fig. 2) . L-type Ca2+ currents (L-currents) and relative changes in intracellular Ca2+ were simultaneously monitored during brief (30 ms) depolarizations to potentials between −50 and 70 mV. Similar to that observed for wild-type RyR1 and NH2-terminal leaky CCD mutant release channels (Avila and Dirksen, 2001), expression of the Y4795C CCD mutant in dyspedic myotubes fully restored the Ca2+ conducting activity of the DHPR (Fig. 2 B). However, maximal voltage-gated SR Ca2+ release was significantly reduced (maximal ΔF/F was 38% smaller in Y4795C-expressing myotubes) and shifted ∼15 mV to more hyperpolarizing potentials compared with that of RyR1 (Fig. 2, C and D). The large hyperpolarizing shift in the voltage dependence of SR Ca2+ release is most clearly seen at threshold potentials (e.g., −10 mV in Fig. 2 A). Thus, the effects of the Y4795C mutation were less severe than that of the Y523S mutation but quantitatively similar to that of the R164C CCD mutation (Avila and Dirksen, 2001). Importantly, dyspedic myotubes expressing double mutants of I4897T and either Y523S (Y523S/I4897T) or Y4795C (Y4795C/I4897T) exhibited normal L-type Ca2+ channel activity (Fig. 2 B), but lacked voltage-gated SR Ca2+ release similar to that of I4897T-expressing myotubes (Fig. 2, A and C). The extremely small Ca2+ transients observed in myotubes expressing the double mutants most likely arise from Ca2+ influx through the DHPR since they were barely detectable (maximal ΔF/F ∼0.1) and exhibited a bell-shaped voltage dependence identical to that of L-channel activation (Fig. 2, C and D), a result identical to that observed for I4897T-expressing myotubes (Avila et al., 2001a). Thus, the double mutants exhibit an EC uncoupling behavior identical to that of homozygous I4897T-expressing myotubes, demonstrating that the EC-uncoupling phenotype caused by the I4897T mutation dominates in myotubes expressing the double mutants.
Figure 2.
The I4897T mutation converts NH2-terminal (Y523S) and COOH-terminal (Y4795C) leaky SR Ca2+ release channels into EC uncoupled release channels. (A) Representative voltage-gated Ca2+ transients (ΔF/F) elicited by 30-ms test pulses to the indicated potentials in RyR1-, Y4795C-, Y4795C/I4897T-, and Y523S/I4897T-expressing dyspedic myotubes. Similar to that observed for Y523S-expressing myotubes (Avila and Dirksen, 2001), voltage-gated Ca2+ release activates at more negative potentials (i.e., −10 mV) in Y4795C-expressing dyspedic myotubes. (B) Average peak Ca2+ current activation curves for Y4795C- (left, open circles), Y4795C/I4897T- (middle, open circles), and Y523S/I4897T- (right, open circles) expressing dyspedic myotubes compared with that attributable to wild-type RyR1 (closed circles). (C) Average voltage dependence of peak intracellular Ca2+ transients (ΔF/F − V) for Y4795C- (left, open circles), Y4795C/I4897T- (middle, open circles), and Y523S/I4897T- (right, open circles) expressing dyspedic myotubes compared with that attributable to wild-type RyR1 (closed circles). The broken lines represent curves obtained from fitting data obtained for Y4785C- (middle) and Y523S- (right) expressing myotubes. (D) The ΔF/F − V data and curves in C are replotted following normalization to their respective maximal values. All data were acquired from three different sets of experiments and represent the average values from a total of 9 Y4795C-, 9 Y4795C/I4897T-, 8 Y523S/I4897T-, and 40 RyR1-expressing myotubes. The controls for each different set of experiments were plotted together with the corresponding experimental condition that was investigated simultaneously. The number of RyR1 controls collected for Y4795C-, Y4795C/I4897T-, and Y523S/I4897T-expressing myotubes were 18, 14, and 8, respectively.
CCD Mutations in Exon 102 of the RyR1 Gene Result in EC Uncoupling
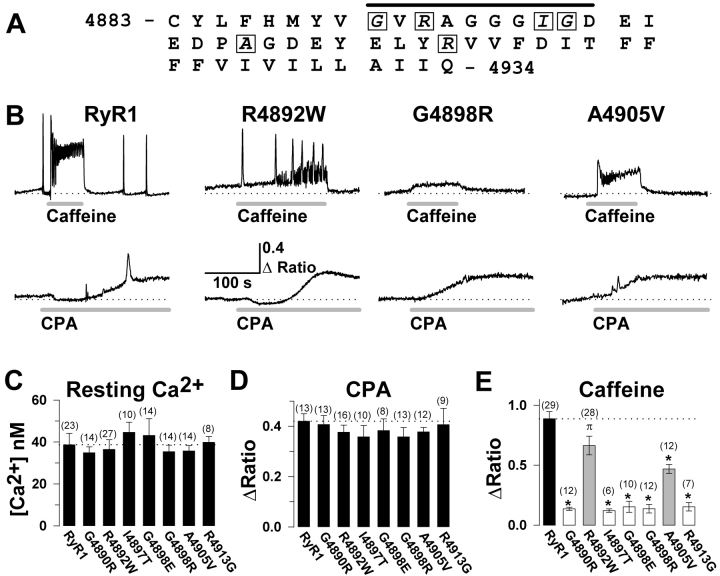
Of the different CCD mutations in RyR1 that have been reconstituted in dyspedic myotubes, six are known to promote varying degrees SR Ca2+ leak (R164C, I404M, Y523S, R2163H, R2435H, Y4795C), whereas only one (I4897T) has been shown to function as an EC uncoupled channel (Avila and Dirksen, 2001; Avila et al., 2001a). However, if EC uncoupling represents an important cellular mechanism for muscle weakness in CCD, then other CCD mutations in RyR1 might also be expected to result in EC uncoupling. A logical place to start would be an analysis of the six newly identified CCD mutations (G4891R, R4893W, G4899R, G4899E, A4906V, and R4914G) located in exon 102 of human RyR1 gene (Fig. 3 A), since this exon encodes the majority of the linker between RyR1 transmembrane regions 3 and 4 (in the model of Takeshima et al. [1989]) and the entire putative pore-lining/selectivity filter of the channel (Gao et al., 2000). Accordingly, we investigated effects on resting Ca2+ and changes in intracellular Ca2+ after exposure to caffeine (10 mM) and CPA (30 μM) in dyspedic myotubes expressing each of the seven different exon 102 CCD mutations in RyR1 (Fig. 3 B). Resting Ca2+ levels (Fig. 3 C) and CPA responses (Fig. 3 D) were not significantly different between dyspedic myotubes expressing wild-type RyR1 and any of the exon 102 CCD mutations in RyR1, indicating that these mutations do not cause significant SR Ca2+ depletion. In spite of the presence of normal SR Ca2+ content, caffeine-induced SR Ca2+ release was reduced for each of the exon 102 CCD mutations in RyR1 (Fig. 3 E). Interestingly, caffeine-induced Ca2+ release was only partially reduced for two of the exon 102 mutant release channels (see R4892W and A4905V in Fig. 3 E), indicating that SR Ca2+ release may only be partially reduced by these particular mutations. This effect was not due to a decrease in caffeine sensitivity since release was not greater for these constructs following the application of 30 mM caffeine (unpublished data).
Figure 3.
The exon 102 CCD mutations in RyR1 exhibit normal resting Ca2+ levels and CPA-sensitive Ca2+ release, but a significant reduction in caffeine-induced Ca2+ release. (A) Amino acid sequence encoded by exon 102 of the human RyR1 gene. Seven different CCD mutations (G4891R, R4893W, I4898T, G4899E, G4899R, A4906V, and R4914G) have been identified for six different exon 102 amino acids (boxed letters), four of which are located within the putative pore-lining region of RyR1 (black bar). (B) Representative maximal caffeine- (10 mM, top) and CPA- (30 μM, bottom) induced Ca2+ responses in Indo-1 AM–loaded dyspedic myotubes expressing wild-type RyR1, R4892W, G4898R, and A4905V (numbers correspond to rabbit RyR1 sequence). The dotted line represents the average resting ratio of RyR1-expressing myotubes. (C–E) Average resting Ca2+ level (C), steady-state CPA response (D), and peak caffeine response (E) for dyspedic myotubes expressing wild-type RyR1 and each of the seven different exon 102 CCD mutations in RyR1. *, P < 0.01 compared with RyR1; π, P < 0.05 compared with RyR1.
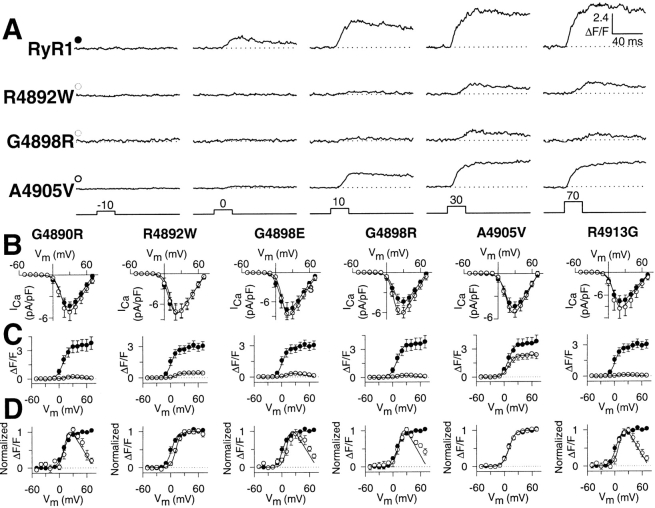
We next characterized the effects of expression in dyspedic myotubes of the exon 102 CCD mutations on RyR1-mediated restoration of both voltage-gated SR Ca2+ release (orthograde coupling, defined as Ca2+ transients that exhibit a sigmoidal voltage-dependence) and L-type Ca2+ channel activity (retrograde coupling). Fig. 4 A shows representative Ca transients recorded from myotubes expressing wild-type RyR1 and three different exon 102 CCD mutants. Average peak L-current density (Fig. 4 B), Ca2+-transient magnitude (Fig. 4 C), and the normalized voltage dependence of these Ca2+ transients (Fig. 4 D) for each exon 102 mutant is plotted (open circles) together with its matching control (RyR1, closed circles). Similar to that observed for I4897T (Avila et al., 2001a), homozygous expression of each of the other exon 102 CCD mutants fully restored L-type Ca2+ channel activity to a degree identical to that of wild-type RyR1 (Fig. 4 B). The complete restoration of retrograde coupling ¬indicates that the exon 102 CCD mutants were synthesized, properly targeted to sarcolemmal-SR junctions, and functionally interacted with DHPRs present within these junctions. In contrast to the complete restoration of retrograde coupling, voltage-gated SR Ca2+ release was absent in G4890R-, G4898E-, G4898R-, and R4913G-expressing dyspedic myotubes. Interestingly, maximal voltage-gated Ca2+ release was only partially reduced in dyspedic myotubes expressing R4892W (85%) and A4905V (36%), the same two constructs that also resulted in a partial reduction in caffeine-induced Ca2+ release (see Fig. 3). In spite of this reduction, voltage-gated Ca2+ release of R4892W- and A4905V-expressing myotubes exhibited a sigmoidal voltage dependence (Fig. 4, C and D, and representative traces in Fig. 4 A) that was not shifted significantly from that of wild-type RyR1 (VF1/2 was 8.6 ± 1.1, 11.3 ± 1.8, and 8.9 ± 1.9 for RyR1-, R4892W-, and A4905V-expressing myotubes, respectively, P > 0.05).
Figure 4.
Voltage-gated Ca2+ release is reduced by exon 102 CCD mutations in RyR1. (A) Representative voltage-gated Ca2+ transients (ΔF/F) elicited by 30-ms test pulses to the indicated potentials in RyR1-, R4892W-, G4898R-, and A4905V-expressing dyspedic myotubes. (B) Average peak Ca2+ current activation curves for G4890R-, R4892W, G4898E, G4898R, A4905V-, and R4913G-expressing myotubes (open circles) compared with that attributable to wild-type RyR1 (closed circles). (C) Average voltage dependence of peak intracellular Ca2+ transients for G4890R-, R4892W-, G4898E-, G4898R-, A4905V-, and R4913G-expressing myotubes (open circles) compared with that attributable to wild-type RyR1 (closed circles). (D) The ΔF/F − V data and curves in C normalized to their respective maximal values. Dyspedic myotubes expressing either the G4898E (n = 8), R4892W (n = 8), or the R4913G (n = 9) CCD mutants were compared with eight separate RyR1-expressing myotubes. Dyspedic myotubes expressing the G4890R (n = 8), A4905V (n = 10), or the G4898R (n = 8) CCD mutants were collected together with nine separate RyR1-expressing myotubes.
The R4892W Mutation Preferentially Disrupts Voltage Sensor Activation of the SR Ca2+ Release Channel
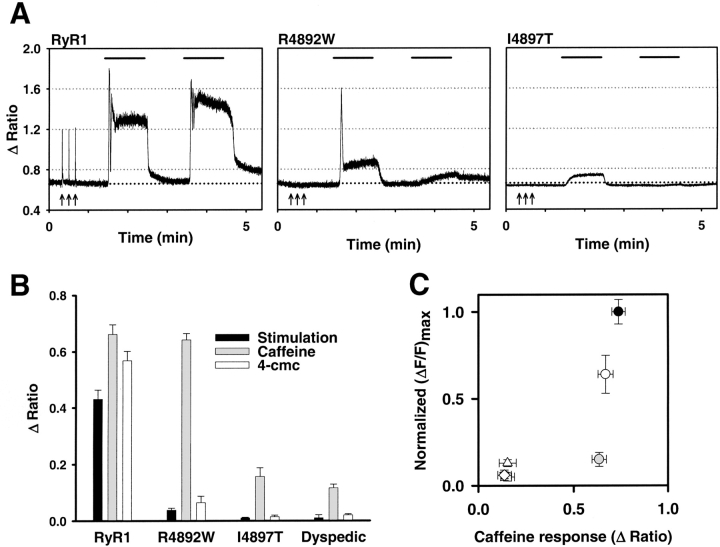
Results obtained in Figs. 3 and 4 indicate that both caffeine and voltage-gated SR Ca2+ release are reduced to a similar degree for most of the exon 102 CCD mutations in RyR1. However, the R4892W mutation appeared somewhat unique in this regard since R4892W-expressing myotubes exhibited a large reduction in maximal voltage-gated SR Ca2+ release (∼85%), whereas caffeine-induced Ca2+ release was only marginally reduced. To more rigorously test whether voltage-gated Ca2+ release is preferentially disrupted by the R4892W mutation in RyR1, we monitored electrically evoked and caffeine-induced Ca2+ release from individual Indo-1 AM–loaded myotubes expressing either RyR1, R4892W, or I4897T. For these experiments, voltage-gated Ca2+ release was first activated by a brief train of supramaximal electrical stimuli (8 V for 20 ms at 0.1 Hz for 30 s) using an extracellular electrode placed adjacent to the cell of interest (Fig. 5 A, arrows). Shortly thereafter, myotubes were sequentially exposed to 60-s applications of caffeine (10 mM), control Ringer's, 4-cmc (500 μM), and a final wash with control Ringer's (Fig. 5 A). In RyR1-expressing myotubes, electrical stimulation evoked rapid voltage-gated Ca2+ transients that were typically somewhat smaller than maximal Ca2+ release activated by 10 mM caffeine (Fig. 5 A, left). Similar to uninjected dyspedic myotubes, I4897T-expressing myotubes completely lacked Ca2+ transients in response to either electrical stimulation or exposure to the RyR1-selective agonist 4-cmc and responded only weakly or not at all to 10 mM caffeine (Fig. 5 A, right). The small caffeine response in uninjected and I4897T-expressing dyspedic myotubes (see also Fig. 3 E) presumably arises from a low endogenous expression of the type 3 ryanodine receptor in dyspedic myotubes (Takeshima et al., 1995). On the other hand, although R4892W-expressing myotubes exhibited very small or undetectable electrically evoked Ca2+ transients, 10 mM caffeine (but not 4-cmc) was still able to induce a significant release of SR Ca2+ (Fig. 5 A, middle). Average responses of RyR1-, I4897T-, and R4892W-expressing myotubes to electrical stimulation, caffeine, and 4-cmc are summarized in Fig. 5 B. There was a strong correlation (r = 0.98) between the magnitude of maximal caffeine-induced and voltage-gated SR Ca2+ release (as determined in Fig. 4) for all of the exon 102 CCD mutations in RyR1 except R4892W (Fig. 5 C). Even the partial reduction in voltage- and caffeine-induced Ca2+ release observed in A4905V-expressing myotubes was adequately described by this correlation. Although we have not directly determined if R4892W-expressing myotubes are indeed capable of firing action potentials in response to an extracellular stimulus, the data presented in Figs. 3–5 provide compelling evidence that the R4892W mutation disrupts voltage sensor activation of release to a greater extent than that triggered by caffeine, making it unique among the identified CCD mutations in exon 102 of RyR1.
Figure 5.
The R4892W mutation preferentially disrupts voltage-gated Ca2+ release. (A) Representative traces of electrical-, caffeine-, and 4-cmc–induced Ca2+ transients recorded from Indo-1 AM–loaded RyR1- (left), R4892W- (middle), and I4897T- (right) expressing myotubes. A brief train of supramaximal electrical stimuli (8 V for 20 ms at 0.1 Hz for 30 s) (arrows) was delivered before sequential applications of 10 mM caffeine (first bar), control Ringer's, 500 μM 4-cmc (second bar), and a final wash with control Ringer's. (B) Average electrically activated (black bars), caffeine-induced (gray bars), and 4-cmc–induced (white bars) Ca2+ transients for RyR1-, R4892W-, and I4897T-expressing myotubes. (C) The average magnitude of maximal voltage-gated Ca2+ release (obtained from experiments like those depicted in Fig. 4C) are plotted as a function of the average maximal caffeine response for RyR1 and each of the exon 102 CCD mutant RyR1 proteins. Symbols represent RyR1 (closed circle), G4890R (open diamond), R4892W (gray circle), G4898E (open triangle), G4898R (inverted open triangle), A4905V (open circle), and R4913G (open square). The symbols for the G4898R and R4913G mutants are plotted behind the symbol for G4890R.
DISCUSSION
Leaky Channels Can Result from CCD Mutations in the COOH-terminal Region of RyR1
Based on results obtained after reconstitution of CCD mutant RyR1 proteins in dyspedic myotubes, we have proposed two fundamentally distinct cellular mechanisms for muscle weakness in CCD (leaky and EC uncoupled SR Ca2+ release channels) (Avila and Dirksen, 2001; Avila et al., 2001a; Dirksen and Avila, 2002). Our previous work has shown that expression of NH2-terminal CCD mutants in dyspedic myotubes resulted in varying degrees of SR Ca2+ leak (reduced voltage-gated release resulting from SR Ca2+ depletion), while a COOH-terminal mutant (I4897T) results in EC uncoupling (reduced voltage-gated release in the absence of detectable store depletion). These observations raised the possibility that NH2-terminal CCD mutations (MH/CCD regions 1 and 2) result in leaky release channels while COOH-terminal CCD mutations result in EC uncoupling (MH/CCD region 3). However, such a clear structural division between mechanisms does not appear to be accurate since we now show that a COOH-terminal CCD mutation (Y4795C) also exhibits all the hallmarks of an NH2-terminal leaky SR Ca2+ release channel (elevated resting Ca2+, reduced SR Ca2+ content, hyperpolarized-shifted VF1/2, reduced maximal ΔF/F).
CCD Mutations in the Pore Region of RyR1 Markedly Reduce SR Ca2+ Release
Our proposal that the I4897T mutation operates via a mechanism distinct from that of leaky CCD mutant release channels is controversial since I4897T mutant release channels promote Ca2+ leak from intracellular Ca2+ stores after expression in HEK293 cells (Lynch et al., 1999). In addition, increased SR Ca2+ leak was also observed in human lymphocytes obtained from CCD patients heterozygous for the I4897T mutation (Tilgen et al., 2001). To more rigorously test our hypothesis that the I4897T mutation leads to SR Ca2+ release channels that poorly permeate Ca2+ in a skeletal muscle environment, we determined whether the I4897T mutation could block SR Ca2+ depletion caused by leaky CCD mutant release channels. Accordingly, we found that incorporation of the I4897T mutation into leaky release channels arising from either NH2-terminal (Y523S) or COOH-terminal (Y4795C) CCD mutations in RyR1 blocked the ability of these mutations to cause store depletion and an elevation in resting Ca2+. In addition, although the I4897T mutation prevented SR Ca2+ leak through leaky CCD mutant release channels, myotubes expressing the double mutants were EC uncoupled since they completely lacked voltage-gated SR Ca2+ release (i.e., Ca2+ transients that exhibit a sigmoidal voltage dependence). These results support the idea that the I4897T mutation greatly inhibits Ca2+ permeation through the SR Ca2+ release channel.
As a second test, we surmised that if EC uncoupling represents an important cellular mechanism for muscle weakness in CCD, then other CCD mutations in RyR1 would also be likely to operate via this mechanism. Exon 102 of the gene encoding the human RyR1 protein encodes 52 amino acids, including the entire putative RyR pore-lining/selectivity filter of the channel. Exon 102 apparently represents a highly susceptible region for CCD since seven different disease causing mutations within six different amino acid residues have been identified to date. We hypothesized that mutations to key pore-lining residues of the channel encoded by exon 102 that contribute significantly to Ca2+ permeation of the open channel would result in EC-uncoupled release channels. Our results indicate that all of the exon 102 CCD mutations in RyR1 result in a marked reduction in voltage-gated SR Ca2+ release that occurs in the absence of a measurable change in resting Ca2+ or SR Ca2+ content, confirming that this region of RyR1 represents a primary molecular locus for EC uncoupling in CCD.
Homozygous expression in dyspedic myotubes of five of the seven identified exon 102 CCD mutations in RyR1 (G4890R, I4897T, G4898E, G4898R, and R4913G) results in a complete loss of voltage-gated SR Ca2+ release that occurs in the absence of a significant change in either resting Ca2+, SR Ca2+ content, or retrograde coupling with sarcolemmal DHPRs (complete EC uncoupling). However, the other two exon 102 mutations were unique in that voltage-gated SR Ca2+ release was only partially reduced (∼85% for R4892W and ∼36% for A4905V; partial EC uncoupling). Although reduced in magnitude, the residual voltage-gated Ca2+ release attributable to these mutants exhibited a voltage dependence (VF1/2) that was not significantly different from that of wild-type RyR1. The normal voltage dependence of Ca2+ release in R4892W- and A4905V-expressing myotubes is similar to what we have shown previously for the partial restoration of release observed after coexpression of wild-type RyR1 and I4897T (Avila et al., 2001a). Thus, the normal voltage dependence of partially uncoupled release channels provides strong evidence in support for the conclusion that the exon 102 mutations result in a fundamentally distinct phenotype from that which arises from mutations that lead to leaky SR Ca2+ release channels.
Caffeine and voltage-gated Ca2+ release were reduced to a similar degree for all but one of the exon 102 mutants (Fig. 5 C). These results could be explained by exon 102 mutations that inhibit Ca2+ flux through the release channel in a manner that is independent of the precise activation mechanism. This could occur by mutations that alter the gating and/or Ca2+ permeation of the open release channel. The one exception to this rule was that voltage-gated Ca2+ release through R4892W mutant release channels was reduced to a far greater extent than that observed upon addition of caffeine (Fig. 5). These results demonstrate that R4892W mutant release channels are in fact able to conduct Ca2+ ions under certain activation paradigms (e.g., caffeine) but not others (voltage, 4-cmc). In addition, since the R4892W mutation inhibited voltage-gated and 4-cmc–induced Ca2+ release similarly, it is tempting to speculate that 4-cmc may activate RyR1 by a mechanism analogous to that of the voltage sensor. In any event, these results indicate that while voltage-gated Ca2+ release is reduced for all EC-uncoupled release channels, EC uncoupling is not necessarily synonymous with a Ca2+-impermeant channel. Thus, in addition to altering the pore of the release channel, it is conceivable that EC uncoupling (i.e., reduced voltage-gated release in the absence of store depletion) could also arise from CCD mutations in RyR1 (or the DHPR) that either (a) perturb RyR1 (or DHPR) junctional targeting, (b) result in DHPR/RyR1 expression level mismatch, (c) alter appropriate assembly of DHPR tetrads or RyR1 arrays, or (d) disrupt functional coupling between properly targeted/assembled DHPR and RyR1 proteins (Dirksen and Avila, 2002). Thus, while mutations within exon 102 of the RyR1 gene represent a hotspot for EC uncoupling, mutations within other functional domains of the channel could potentially lead to mechanistically distinct forms of EC uncoupling.
Therapeutic Implications for Two Distinct Mechanisms of Muscle Weakness in CCD
Currently, there are no effective therapies for the treatment of muscle weakness or core development in CCD. However, normalization of muscle Ca2+ homeostasis by interventions expected to block SR Ca2+ leak could potentially improve muscle performance in forms of CCD that result from excessive leak of SR Ca2+ through CCD mutant RyR1 channels. Our results in which resting Ca2+ and SR Ca2+ content are restored to normal levels in dyspedic myotubes expressing double mutants that combine the I4897T “EC uncoupled” mutation with NH2-terminal or COOH-terminal “leaky” channels (Fig. 1) provides sound preliminary rationale for such an idea. However, although levels of resting Ca2+ and SR Ca2+ content are normal, EC coupling is completely “uncoupled” (i.e., no voltage-gated release) in myotubes expressing the double mutants; a result not unexpected if the I4897T mutation results in release channels that only poorly permeate Ca2+. Thus, any effective therapeutic approach designed to counteract SR Ca2+ loss through leaky SR Ca2+ release channels would need to restore both resting Ca2+ and SR content and also promote normalization of voltage-gated SR Ca2+ release.
Dantrolene, an FDA-approved agent used in the treatment of MH crises (McCarthy et al., 2000), may represent an intriguing candidate for counteracting Ca2+ dysregulation resulting from leaky SR Ca2+ release channels. Dantrolene is thought to antagonize MH episodes by reducing skeletal muscle twitch force and shifting the sensitivity of contractile activation to more depolarized voltages (Hainaut and Desmedt, 1974), an effect diametrically opposed to that caused by CCD mutations in RyR1 that result in leaky SR Ca2+ release channels (where the voltage dependence of Ca2+ release is shifted to more hyperpolarized potentials) (Fig. 2; Avila and Dirksen, 2001). However, the precise mechanism by which dantrolene alters SR Ca2+ release is still unclear since a more recent report has shown that dantrolene reduces the amplitude of voltage-gated SR Ca2+ release without altering the voltage dependence of Ca2+ release in rodent skeletal muscle fibers (Szentesi et al., 2001). Nevertheless, it will be important for future experiments to determine whether dantrolene can normalize resting Ca2+, SR Ca2+ content, as well as the magnitude and voltage dependence of Ca2+ release attributable to leaky CCD mutant release channels. In such a case, dantrolene could potentially oppose muscle weakness that arises from leaky release channels (e.g., by inhibiting SR Ca2+ loss through leaky channels) while exacerbating muscle weakness resulting from mutations that lead to EC uncoupling (e.g., by further inhibiting Ca2+ release through EC uncoupled release channels). Such observations would emphasize the importance of combining information gleaned from genetic screening of kindreds with the development and application of specific therapeutic approaches for improving muscle strength in CCD.
Acknowledgments
We would like to thank Drs. Kurt G. Beam and Paul D. Allen for providing us access to the dyspedic mice used in this study and to Linda Groom for excellent technical assistance.
This work was supported by the National Institutes of Health (AR44657 to R.T. Dirksen) and a Neuromuscular Disease Research grant from the Muscular Dystrophy Association (to R.T. Dirksen).
Olaf S. Andersen served as editor.
Abbreviations used in this paper: CCD, central core disease; CPA, cyclopiazonic acid; DHPR, dihydropyridine receptor; EC, excitation-contraction; L-currents, L-type Ca2+ currents; MH, malignant hyperthermia.
References
- Avila, G., and R.T. Dirksen. 2000. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J. Gen. Physiol. 115:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, G., and R.T. Dirksen. 2001. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J. Gen. Physiol. 118:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, G., J.J. O'Brien, and R.T. Dirksen. 2001. a. Excitation-contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc. Natl. Acad. Sci. USA. 98:4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, G., K.M. O'Connell, L.A. Groom, and R.T. Dirksen. 2001. b. Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J. Biol. Chem. 276:17732–17738. [DOI] [PubMed] [Google Scholar]
- Dirksen, R.T. 2002. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front. Biosci. 7:d659–d670. [DOI] [PubMed] [Google Scholar]
- Dirksen, R.T., and G. Avila. 2002. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca2+ release channels? Trends Cardiovasc. Med. 12:189–197. [DOI] [PubMed] [Google Scholar]
- Gao, L., D. Balshaw, L. Xu, A. Tripathy, C. Xin, and G. Meissner. 2000. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca2+ release channel (ryanodine receptor) activity and conductance. Biophys. J. 79:828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut, K., and J.E. Desmedt. 1974. Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature. 252:728–730. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn, F., and K. Jurkat-Rott. 1999. Voltage-gated ion channels and hereditary disease. Physiol. Rev. 79:1317–1372. [DOI] [PubMed] [Google Scholar]
- Lynch, P.J., J. Tong, M. Lehane, A. Mallet, L. Giblin, J.J. Heffron, P. Vaughan, G. Zafra, D.H. MacLennan, and T.V. McCarthy. 1999. A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease. Proc. Natl. Acad. Sci. USA. 96:4164–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, T.V., K.A. Quane, and P.J. Lynch. 2000. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 5:410–417. [DOI] [PubMed] [Google Scholar]
- Melzer, W., A. Herrmann-Frank, and H.C. Luttgau. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. [DOI] [PubMed] [Google Scholar]
- Monnier, N., N.B. Romero, J. Lerale, Y. Nivoche, D. Qi, D.H. MacLennan, M. Fardeau, and J. Lunardi. 2000. An autosomal dominant congenital myopathy with cores and rods is associated with a neomutation in the RyR1 gene encoding the skeletal muscle ryanodine receptor. Hum. Mol. Genet. 9:2599–2608. [DOI] [PubMed] [Google Scholar]
- Nakai, J., R.T. Dirksen, H.T. Nguyen, I.N. Pessah, K.G. Beam, and P.D. Allen. 1996. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 380:72–75. [DOI] [PubMed] [Google Scholar]
- Shuaib, A., R.T. Paasuke, and K.W. Brownell. 1987. Central core disease. Clinical features in 13 patients. Medicine (Baltimore). 66:389–396. [PubMed] [Google Scholar]
- Sitsapesan, R., and A.J. Williams. 1997. Regulation of current flow through ryanodine receptors by luminal Ca2+. J. Membr. Biol. 159:179–185. [DOI] [PubMed] [Google Scholar]
- Szentesi, P., C. Collet, S. Sarkozi, C. Szegedi, I. Jona, V. Jacquemond, L. Kovacs, and L. Csernoch. 2001. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J. Gen. Physiol. 118:355–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima, H., S. Nishimura, T. Matsumoto, H. Ishida, K. Kangawa, N. Minamino, H. Matsuo, M. Ueda, M. Hanaoka, and T. Hirose. 1989. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 339:439–445. [DOI] [PubMed] [Google Scholar]
- Takeshima, H., T. Yamazawa, T. Ikemoto, H. Takekura, M. Nishi, T. Noda, and M. Iino. 1995. Ca2+-induced Ca2+ release in myocytes from dyspedic mice lacking the type-1 ryanodine receptor. EMBO J. 14:2999–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgen, N., F. Zorzato, B. Halliger-Keller, F. Muntoni, C. Sewry, L.M. Palmucci, C. Schneider, E. Hauser, F. Lehmann-Horn, C.R. Muller, and S. Treves. 2001. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum. Mol. Genet. 10:2879–2887. [DOI] [PubMed] [Google Scholar]
- Tong, J., T.V. McCarthy, and D.H. MacLennan. 1999. Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J. Biol. Chem. 274:693–702. [DOI] [PubMed] [Google Scholar]
- Tong, J., H. Oyamada, N. Demaurex, S. Grinstein, T.V. McCarthy, and D.H. MacLennan. 1997. Caffeine and halothane sensitivity of intracellular Ca2+ release is altered by 15 calcium release channel (ryanodine receptor) mutations associated with malignant hyperthermia and/or central core disease. J. Biol. Chem. 272:26332–26339. [DOI] [PubMed] [Google Scholar]
- Treves, S., F. Larini, P. Menegazzi, T.H. Steinberg, M. Koval, B. Vilsen, J.P. Andersen, and F. Zorzato. 1994. Alteration of intracellular Ca2+ transients in COS-7 cells transfected with the cDNA encoding skeletal-muscle ryanodine receptor carrying a mutation associated with malignant hyperthermia. Biochem. J. 301:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]