Abstract
Incorporation of BK Ca2+–activated K+ channels into planar bilayers composed of negatively charged phospholipids such as phosphatidylserine (PS) or phosphatidylinositol (PI) results in a large enhancement of unitary conductance (gch) in comparison to BK channels in bilayers formed from the neutral zwitterionic lipid, phospatidylethanolamine (PE). Enhancement of gch by PS or PI is inversely dependent on KCl concentration, decreasing from 70% at 10 mM KCl to 8% at 1,000 mM KCl. This effect was explained previously by a surface charge hypothesis (Moczydlowski, E., O. Alvarez, C. Vergara, and R. Latorre. 1985. J. Membr. Biol. 83:273–282), which attributed the conductance enhancement to an increase in local K+ concentration near the entryways of the channel. To test this hypothesis, we measured the kinetics of block by external and internal Ba2+, a divalent cation that is expected to respond strongly to changes in surface electrostatics. We observed little or no effect of PS on discrete blocking kinetics by external and internal Ba2+ at 100 mM KCl and only a small enhancement of discrete and fast block by external Ba2+ in PS-containing membranes at 20 mM KCl. Model calculations of effective surface potential sensed by the K+ conduction and Ba2+-blocking reactions using the Gouy-Chapman-Stern theory of lipid surface charge do not lend support to a simple electrostatic mechanism that predicts valence-dependent increase of local cation concentration. The results imply that the conduction pore of the BK channel is electrostatically insulated from the lipid surface, presumably by a lateral distance of separation (>20 Å) from the lipid head groups. The lack of effect of PS on apparent association and dissociation rates of Ba2+ suggest that lipid modulation of K+ conductance is preferentially coupled through conformational changes of the selectivity filter region that determine the high K+ flux rate of this channel relative to other cations. We discuss possible mechanisms for the effect of anionic lipids in the context of specific molecular interactions of phospholipids documented for the KcsA bacterial potassium channel and general membrane physical properties proposed to regulate membrane protein conformation via energetics of bilayer stress.
Keywords: electrostatics, Gouy-Chapman theory, lipid modulation, phospholipid, Slowpoke
INTRODUCTION
Ion transport proteins are responsible for movement of ions across a cell membrane that separates the external and internal aqueous milieu by ∼40 Å. The two membrane interfaces on the outside and inside of the cell exist as charged surfaces due to the presence of ionized chemical groups on protein, carbohydrate, and lipid. Discrete charges located at the membrane–water interface give rise to an electrostatic surface potential that varies in a complex fashion according to the distribution and density of fixed charges, electrolyte composition, and distance from the charged groups (McLaughlin, 1989; Honig and Nicholls, 1995). Because local concentrations of ionic substrates (e.g., Na+, K+, H+, Ca2+, Cl−) near the membrane are determined by this complex surface potential map, surface charge is considered to play an important role in modulating ion channel function (Green and Andersen, 1991; Latorre et al., 1992; Hille, 2001).
One of the first experiments to identify functional effects of lipid surface charge showed that the unitary conductance of the gramicidin cation channel is increased 4.3-fold, from 15 pS in phosphatidylcholine (PC)* to 65 pS in a phosphatidylserine (PS) membrane under conditions of 100 mM CsCl (Apell et al., 1979). According to electrostatic theory, the radius of the cylindrical gramicidin channel, ∼6–13 Å (Killian, 1992; Woolf and Roux, 1996), is close to the Debye length (λD 10 Å in 0.1 M salt solution) that determines the distance dependence of surface potential decay. Thus, this small peptide channel effectively “senses” the large increase in local Cs+ concentration that occurs near the surface of the PS membrane and responds with a proportionate increase in Cs+ conductance. Indeed, careful titration of phospholipid surface charge by changes in pH and mixtures of PC plus PS (Rostovtseva et al., 1998) showed that the effect of surface charge on gramicidin conductance is primarily explained by the Gouy-Chapman-Stern (GCS) theory of membrane surface potential (Eisenberg et al., 1979; McLaughlin et al., 1981).
In comparison to the test case of gramicidin, the effects of lipids and membrane surface charge on the activities of large ion channel proteins are considerably more complex. Using the planar bilayer system to measure the unitary conductance of the sarcoplasmic reticulum (SR) K+ channel in membranes formed from neutral zwitterionic lipids or negatively charged lipids, Bell and Miller (1984) observed a substantial enhancing effect of PS at KCl concentrations <400 mM. From a theoretical analysis based on GCS theory, they concluded that the cation entryway of the SR K+ channel behaved as if it sensed the local K+ concentration at a distance of 10–20 Å away from the electrified membrane interface. Such evidence that an ion channel may respond to less than the full value of the membrane surface potential has led to the idea that the mass disposition of a large channel protein may effectively “insulate” a channel's conducting pore from the electrostatic properties of the membrane surface.
According to this interpretation, various types of channel proteins would be differentially insulated from membrane surface charge. At one extreme, both the K+ conductance and Ca2+ sensitivity of the large conductance Ca2+-activated K+ channel (BK) are greatly enhanced in PS versus phosphatidylethanolamine (PE) bilayers (Moczydlowski et al., 1985). On the other hand, unitary conductance and the apparent blocking affinity of external divalent cations (Ca2+) and external decarbamoylsaxitoxin2+ are virtually unaffected by incorporation of voltage-gated Na+ channels (NaV) into negatively charged bilayers containing PS, as compared with a neutral lipid mixture of PE/PC (Green et al., 1987a; Guo et al., 1987; Worley et al., 1992). [Incidentally, the voltage activation process of the rat brain NaV channel is quite sensitive to bilayer PS as measured by shifts of the voltage-activation midpoint with increasing Ca2+ concentration and ionic strength (Cukierman et al., 1988; Cukierman, 1991).] In the case of L-type voltage–activated Ca2+ channels (CaV), only a small conductance increase is observed in PS bilayers when Na+ is the current carrier, but not when Ba2+ carries the current (Coronado and Affolter, 1986). These studies would suggest that conduction pores of large channel proteins are variably shielded from the electrostatic surface potential generated beyond the edge of the protein–lipid interface; however, the molecular basis of this phenomenon is poorly understood.
Since the protein complex of the tetrameric BK channel is larger in mass than structurally related NaV and CaV channel complexes, it is surprising that BK conductance is enhanced to a greater degree by negatively charged phospholipids. To explore the basis of this apparent paradox, we decided to reexamine the surface charge hypothesis previously used to explain the effect of PS on conductance of the BK channel (Moczydlowski et al., 1985). The association rate of a charged blocking ion to a site in the ion conduction pathway of a channel protein is generally expected to be proportional to the local concentration of the blocking ion in equilibrium with the binding site (Green et al., 1987b; Guo et al., 1987; MacKinnon et al., 1989; Escobar et al., 1993). Studies have shown that the apparent blocking affinity of charged peptide toxins, organic cations, and inorganic cation blockers is often dependent on electrostatic interactions between the blocking ion and the channel protein (Bell and Miller, 1984; Worley et al., 1986; MacKinnon and Miller, 1989; MacKinnon et al., 1989; Stocker and Miller, 1994; Escobar et al., 1993). Therefore, measurements of cation-blocking kinetics in membranes composed of charged versus neutral lipids can be used to monitor the relative fraction of membrane surface potential that falls in the vicinity of the entrance of an ion channel. In this study, we used Ba2+ and TEA+, two well-known blockers of K+ channels, as probes of phospholipid surface charge sensed by the rat muscle BK channel. We find that K+ conductance of the BK channel is enhanced more strongly by negatively charged phospholipids than the affinity or kinetics of pore-blocking cations. These observations suggest that lipid interactions with the BK channel are more complex than expected for a simple electrostatic mechanism based on surface charge. We propose that certain K+ channels may be subject to lipid-dependent tuning of protein conformational changes associated with rate-determining barriers to ion movement.
MATERIALS AND METHODS
Planar Lipid Bilayers and Measurement of Surface Charge Density
Planar lipid bilayers were formed on a 0.2-mm diameter hole in a polystyrene partition by spreading a solution of phospholipid (25 mg/ml) in decane with a small glass rod. The lipid composition of neutral bilayers (PE membrane) was 100% 1-palmitoyl-2-oleoyl PE and that of negatively charged bilayers (PS membrane) was 80% 1-palmitoyl-2-oleoyl phosphatidylserine plus 20% PE. In some experiments, negatively charged bilayers composed of 80% phosphatidylinositol (PI) from bovine liver plus 20% PE were also used (PI membrane). Lipid mixtures rather than pure PS or PI were used because addition of 20% PE improved bilayer stability during channel incorporation and long duration experiments. Phospholipids were purchased from Avanti Polar Lipids.
The negative charge density of phospholipid bilayers was measured with the use of the K+ ionophore nonactin as described previously (McLaughlin et al., 1970). This method gave the following values of surface charge density in units of e−/nm2: 0.04 ± 0.01 (mean ± SE, n = 5), 0.98 ± 0.04 (n = 4), and 1.07 ± 0.06 (n = 3) for PE, PS, and PI membranes, respectively. Based on a molecular surface area of 0.7 nm2 per phospholipid molecule (Loosley-Millman et al., 1982), the expected value of charge density for a membrane containing 80% negatively charge lipid is 1.143 e−/nm2. Our measured values correspond to 3% (PE), 69% (PS membrane), and 75% (PI membrane) in percentage of negatively charged lipid. These results are close to expectation; similar deviations from theoretical charge densities were found in previous studies (Bell and Miller, 1984; Coronado and Affolter, 1986; Rostovtseva et al., 1998).
Channel Incorporation and Electrical Recording
Membrane vesicles for bilayer incorporation of large conductance Ca2+-activated K+ channels (BK) were prepared from rat skeletal muscle using a sucrose density step gradient as described previously (Guo et al., 1987). The polystyrene partition on which the bilayer was formed was a cup and well-type chamber (Warner Instruments). The solution on both sides of the partition contained 10 mM HEPES adjusted to pH 7.2 with 7 mM KOH and the following range of KCl concentrations: 3, 10, 20, 30, 50, 100, 200, 500, 700, and 1,000 mM. Ca2+ was added to the same chamber (cis) as the membrane vesicles to a free concentration of 100 μM (0.6 mM CaCl2 plus 0.5 mM EGTA) for the experiments of Figs. 1 and 2 or 200 μM CaCl2 (without EGTA) for all other experiments. A cis-internal orientation of BK channel incorporation was confirmed by an increase in open state probability with increasing positive voltage applied on the cis side. For Ba2+-blocking experiments, BaCl2 was added to the external (trans) side at 0.5–60 mM or to the internal (cis) side at 1–100 μM. For TEA+-blocking experiments, TEA chloride was added to the external side at 0.03–1 mM.
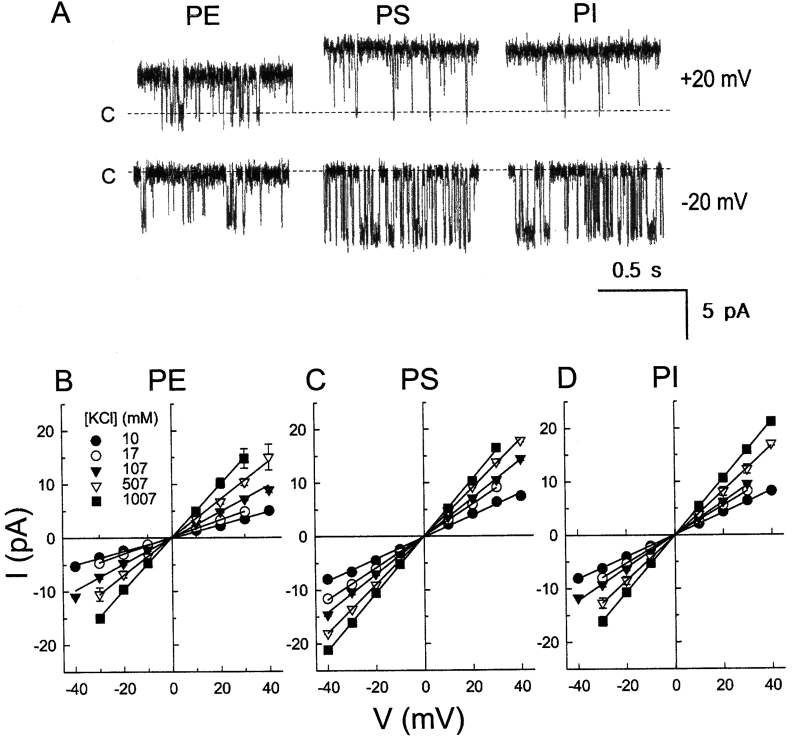
Figure 1.
Effect of negatively charged lipids on single-channel current-voltage behavior. (A) Current records of single BK channels from rat skeletal muscle in neutral PE and negatively charged PS and PI bilayers at 20 and −20 mV. The solution on both sides of the membrane contained 107 mM K+ (100 mM KCl, 0.5 mM EGTA, 0.6 mM CaCl2, 10 mM HEPES, adjusted to pH 7.2 with KOH). Current records were low-pass filtered at 0.5 kHz and digitized at 2 kHz. The dashed line marks the zero-current or closed level marked c. Single-channel current-voltage relations in the range of −40 to 40 mV are shown in symmetrical solutions containing 10, 17, 107, 507, and 1,007 mM K+ for PE (B), PS (C), and PI (D) bilayers. Solid lines correspond to respective slope conductances from low to high K+ of 130, 169, 252, 359, and 481 pS for PE bilayers, 217, 282, 360, 454, and 519 pS for PS bilayers, and 216, 268, 356, 458, and 533 pS for PI bilayers. Data points and error bars represent mean ± SE for 3–11 single channels. Most error bars were smaller than the symbol.
Figure 2.
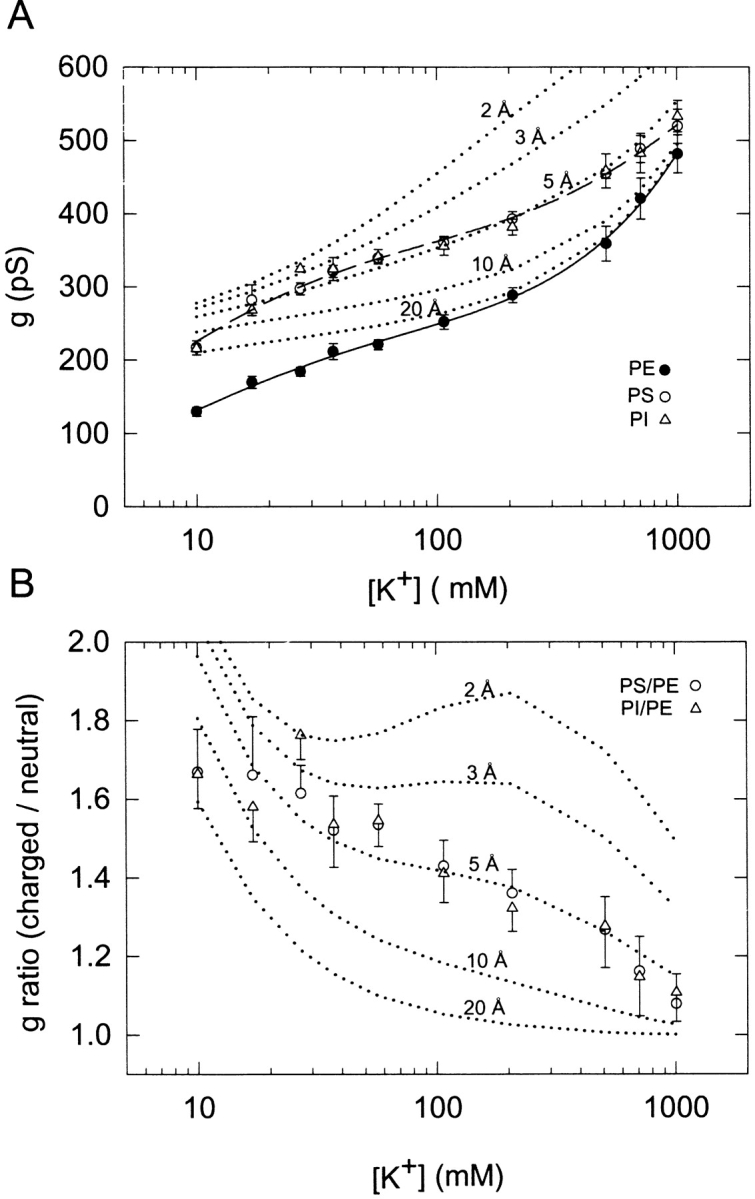
Unitary conductance of the rat BK channel as function of symmetrical K+ concentration in PE, PS, and PI bilayers. (A) Conductance versus [K+]. Single-channel conductance was measured in PE, PS, or PI bilayers as described in Fig. 1. Data points correspond to the mean ± SE for 3–11 single channels. The solid line and dashed lines are respective nonlinear fits of the PE and PS data to Eq. 7 in the text. The model and best-fit parameters are given in Table I. The dotted lines are computed simulations based on Eq. 7 and the best-fit parameters for the PE data listed in Table I using [K+]local at various distances (2, 3, 5, 10, and 20 Å) from a PS membrane as calculated according to GCS theory given in materials and methods. (B) Ratio of conductance data plotted in A for comparison of PS/PE or PI/PE bilayers vs. [K+]. The dotted lines are the ratio of the individual dotted line simulations in A to the fitted curve for the PE data in A.
The cis and trans compartments were connected to the amplifier via Ag/AgCl electrodes and KCl-agar bridges. BK channel activity was measured at room temperature (∼22°C) using a BC525A bilayer amplifier (Warner Instruments). Single-channel current data was stored on VCR tape via a digital data recorder (VR-10; Instrutech) for off-line analysis. The stored data was replayed either at 200 or 10 Hz using an 8-pole Bessel filter and digitized at 2 kHz using a PC-based data acquisition system from Axon Instruments, Inc.
Measurement of Single-channel Conductance and Blocking Parameters
Single-channel current amplitude was measured in the voltage range of −80 to 80 mV using pClamp 6 analysis software from Axon Instruments, Inc. that facilitated accurate measurement of mean closed state (defined as zero current) and mean open state current levels. Single-channel conductance was calculated from linear slopes of individual current-voltage datasets obtained at different K+ concentrations. Fast block by external Ba2+ and TEA+ was measured as the apparent reduction of unitary current. Time-averaged open state probability in the absence and presence of the discrete blocker, Ba2+, was measured for current records at 20 mV as the total time in the open state divided by the duration of the record. Dwell time histograms containing a minimum of 250 Ba2+-blocked or unblocked events were constructed from long records filtered at 10 Hz. A filter setting of 10 Hz imposed an effective dead time of 18 ms for the shortest detectable closed or blocked state event. This procedure eliminated the contribution of gating closures (Vergara and Latorre, 1983) since the longest component of closed state gating events typically had a time constant of ∼2 ms under these conditions. This method effectively isolated consistently sampled populations of Ba2+-blocked events that were fit to a sum of two exponentials and Ba2+-unblocked events that were fit to a single exponential function. The use of a dead time of 18 ms for the shortest acceptable blocked event results in a small artificial lengthening (<10%) of the measured unblocked duration due to exclusion of a small number of short-lived Ba2+-blocked states. We did not correct for this error, since this sampling effect only results in an ∼10% underestimate of the true association rate for Ba2+.
Modeling of Surface Charge Effects
To first explore how underlying rate constants of ion translocation might be quantitatively affected in a PS vs. PE membrane, we fit conductance vs. [K+] data to a simple discrete-state kinetic model of a symmetrical two-site channel with double occupancy (Andersen, 1989). Nonlinear least squares fitting was performed using SigmaPlot 2000 software (SPSS, Inc.).
To assess the relative effect of PS in enhancing K+ conductance and block by Ba2+ (and TEA+) by a surface charge mechanism, we calculated the expected local ion concentration at a distance, x, from the surface of a phospholipid membrane from the Boltzmann distributions for a monovalent cation (e.g., K+, TEA+) and a divalent cation (e.g., Ba2+):
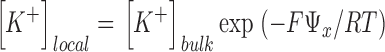 |
(1) |
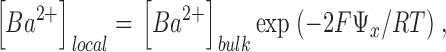 |
(2) |
where [K+]local and [Ba2+]local are the respective local concentrations of K+ and Ba2+, [K+]bulk and [Ba2+]bulk are the respective bulk concentrations of K+ and Ba2+ far from the membrane, Ψx, is the electrostatic potential at a distance x from the membrane surface, F is the Faraday, R is the gas constant, and T is the absolute temperature. The electrostatic potential at the membrane surface, Ψx = 0 or Ψ0, was calculated from the Grahame (1947) equation of Gouy-Chapman theory as outlined in McLaughlin et al. (1981) and Latorre et al. (1992):
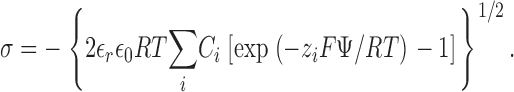 |
(3) |
Eq. 3 relates Ψ0 to the membrane surface density of negative charge, σ, and the indicated sum over the bulk concentrations, Ci, of i ions of valence zi in the aqueous phase (e.g., K+, Cl+, Ba2+), where ɛr is the dielectric constant of water and ɛ0 is the permittivity of free space. To simplify the calculations, 10 mM HEPES-KOH used as the buffer in all solutions was approximated as 7 mM KCl. The density of negative surface charge at the membrane surface, σ, is further equal to the theoretical surface density of negatively charged PS in a phospholipid membrane containing 80% PS, {PS} in e−/nm2, multiplied by a factor to correct for the known binding association constants of K+ to PS (K1 = 0.15 M−1), Ba2+ to PS (K2 = 20 M−1), and Ca2+ to PS (K2 = 12 M−1) as described (Eisenberg et al., 1979; McLaughlin et al., 1981; Latorre et al., 1992):
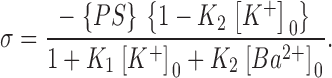 |
(4) |
In Eq. 4, we used {PS} = 1.143 e−/nm2 as the theoretical unneutralized surface density of 80% PS. [K+]0 and [Ba2+]0 are given, respectively, by Eqs. 1 and 2 at x = 0 Å from the surface. The potential at the membrane surface, Ψ0, was numerically computed by solving Eqs. 3 and 4 with the root function of Mathcad 8 software (MathSoft).
The decay of the surface potential as a function of linear distance from the membrane was computed according to McLaughlin (1989):
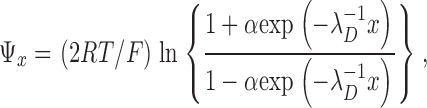 |
(5) |
where α =[exp(FΨ0/2RT) − 1]/[exp(FΨ0/2RT + 1]. The reciprocal Debye length, λD −1, is given by:
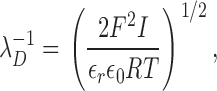 |
(6) |
where I is the ionic strength of the solution. Eq. 5 is not strictly applicable to solutions of mixed electrolytes containing both monovalent and divalent cations. For this reason, we limited distance calculations based on Eq. 5 to solution conditions where BaCl2 is <1 mM and is only a minor contribution to the total ionic strength. Under these conditions, monovalent ion interactions predominate and Eq. 5 is a good approximation of the distance function.
RESULTS
Enhancement of Unitary Conductance by Negatively Charged Phospholipids
Fig. 1 A illustrates the behavior of single BK channels from rat skeletal muscle incorporated into planar bilayers formed either from pure PE, 80/20 PS/PE, or 80/20 PI/PE. As described previously (Moczydlowski et al., 1985; Park and Ryu, 1998; Turnheim et al., 1999), the BK channel exhibits a large increase in unitary conductance and open state probability in membranes containing negatively charged phospholipids. Under ionic conditions of Fig. 1 A (symmetrical: 100 mM KCl, 10 mM HEPES-KOH, pH 7.2, cis: 100 μM free Ca2+), K+ conductance is increased by ∼40% in a bilayer composed of 80% PS (g = 360 ± 9 pS, mean ± SE) or 80% PI (g = 356 ± 12 pS) as compared with 100% PE (g = 252 ± 10 pS). Enhancement of K+ conductance, measured from the linear slope of single-channel current-voltage data at low voltage (less than ±40 mV), is observed over a wide range of [K+] from 10 to 1,007 mM (Figs. 1 B and 2). The data of Fig. 2 also show that conductance values in PS and PI bilayers are virtually indistinguishable, suggesting that the chemical nature of the phospholipid head group is less important than its net charge in promoting this lipid-based enhancement. In Fig. 2 B, a plot of the measured ratio of unitary conductance in PS or PI bilayers to that of PE as a function of [K+] shows that the enhancement is inversely dependent on K+ concentration or ionic strength, ranging from ∼1.7-fold at 10 mM K+ to ∼1.1-fold at 1,007 mM K+. In principle, such an inverse dependence is consistent with a mechanism based on surface charge, since increasing ionic strength and K+ concentration acts to diminish the surface potential through screening of negative charges and binding of K+ to the carboxylate head groups of PS−.
In an earlier study, the dependence of unitary conductance on [K+] for the BK channel was empirically modeled as a sum-of-two Langmuir isotherms (Moczydlowski et al., 1985). In retrospect, it is apparent that this latter function is an inappropriate description of multiion conduction through K+ channels. Recent crystallographic studies have identified four possible K+ binding sites within in the single-filing selectivity filter of the S. lividans K+ channel, KcsA (Zhou et al., 2001). Ion conduction through this channel has been modeled by a linear, four-site system allowing single or double occupancy by K+, with states of two bound K+ ions partitioned into 1,3 and 2,4 configurations separated by intervening H2O molecules (Morais-Cabral et al., 2001). At its simplest level, K+ conduction in this system can also be represented by the basic kinetic scheme for a two-site channel with both single and double occupancy. The latter model has been previously applied to the functionally symmetric gramicidin channel (Finkelstein and Andersen, 1981; Andersen, 1989; Becker et al., 1992) and to Cs+ block of the BK channel (Cecchi et al., 1987). Here, we used this computationally simple scheme (Table I) to investigate how elementary rates of K+ movement might be altered to produce the conductance enhancement of the BK channel observed with negatively charged lipids. [Note that this approach does not take into account the electrostatic effect of charged protein residues (e.g., Asp, Glu, Lys, and Arg) located near the mouth of the channel on K+ conduction. Our purpose here is not to develop a physically precise model of K+ conduction, but simply to obtain a rough idea of how K+ rate constants might be expected to change in a lipid environment of PS vs. PE.]
TABLE I.
Rate Constants for Two-site K+ Channel with Double Occupancy
Values of rate constants defined by the symmetrical kinetic scheme for K+ permeation shown above were obtained by independently fitting conductance vs. [K+] data for PE and PS bilayers shown in Fig. 2 A to Eq. 7 as described in the text. K1, K2, K3, and K4 are calculated from the ratio of rate constants, e.g., K1 = k−1/k1.
Table I shows the kinetic scheme for a two-site channel with four states: one unoccupied state (00), two singly occupied states (K0, 0K), and one doubly occupied state (KK). For a functionally symmetric channel exhibiting linear current-voltage behavior in the low voltage range, this scheme of 10 rate constants can be further simplified to a system of 5 independent rate constants by setting k1 = k2, k−1 = k−2, k3 = k4, k−3 = k−4, and k5 = k6. The theoretical solution for the conductance of this system at the limit of zero voltage as a function of potassium concentration, [K+], is given by the following equation (equivalent to Eq. 101 cited in Andersen, 1989):
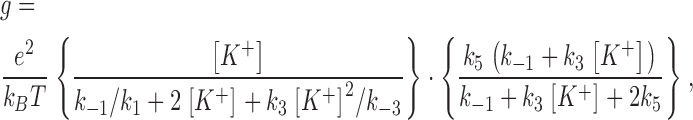 |
(7) |
where e is the elementary charge and kB is the Boltzmann constant. The best-fit lines plotted according to Eq. 7 in Fig. 2 A show that this theory is capable of closely simulating the conductance-concentration data of the BK channel in PE and PS.
The values of rate constants derived from independently fitting the conductance data for PE and PS to the symmetric two-site model are given in Table I. These results show that the conductance increase observed in a PS vs. PE bilayer can be accounted for by rather modest increases in elementary rate constants. For example, the bimolecular association rates for K+ binding to the unoccupied (k1, k2) and the singly occupied channel (k3, k4) are increased, respectively, by 2.1-fold and 1.4-fold for PS relative to PE. Since apparent rates of K+ association from solution to the outer K+ binding sites may reflect changes in the local K+ concentration at the mouth of the channel, the fitting results suggest that as little as a twofold increase in the local K+ concentration seen by the channel in a PS membrane could be sufficient to generate the observed conductance enhancement. Table I further shows almost no change in the best-fit values for the K+ translocation step (k5, k6), suggesting that PS may not necessarily affect site-to-site ion movement within the channel. It is more difficult to draw conclusions concerning the K+ dissociation rate constants (k−1, k−2, k−3, k−4), since the fitting procedure yields a large uncertainty (152%) on the value of one pair of these constants in PE (k−3, k−4). However, a 1.5-fold increase in the k−1, k−2 pair and the fourfold decrease in the k−3, k−4 pair show that a PS membrane could potentially affect elementary rates of dissociation of K+ from the channel. In addition, results of Table I indicate that a possible overall effect of PS would be to produce a differential increase in apparent binding affinity of K+ to the unoccupied channel (K1, K2 = 12.2 mM for PS vs. 17.4 mM for PE) and to the singly occupied channel (K3, K4 = 1.24 M for PS vs. 6.85 M for PE). In principle, a change in lipid environment could alter the equilibrium binding affinity at 0 mV of a channel for ions.
We also used the model of the two-site symmetrical channel to explore the expected effect of membrane surface potential predicted by GCS theory. This was performed by using the best-fit parameters for the neutral PE bilayer (Table I) to compute the expected conductance based on the local concentration of K+ at various distances from the surface of a membrane containing 80% PS. This approach assumes that PS does not change any of the underlying rate constants of K+ conduction that hold in a PE membrane. It further assumes that PS only acts to increase the local concentration of K+ near the mouth of the channel. The local concentration of K+ at various distances from the PS membrane were calculated from GCS theory as described in materials and methods, except that the bulk Ca2+ concentration was set equal to 100 μM and a Ca2+ binding association constant of 12 M−1 (McLaughlin et al., 1981) was used to account for binding of Ca2+ to PS. The calculated curves at 2, 3, 5, 10, and 20 Å corresponding to the dotted lines in Fig. 2, A and B, show that a simulation based on a distance of 5 Å closely matches the observed conductance behavior in PS. [Note that the inability of the Gouy-Chapman model to simulate surface charge effects on channel conductance at low K+ concentration in Fig. 2 A is due to the fact that the planar geometry of this model predicts a finite surface cation concentration in this limit (Apell et al., 1979; Green and Andersen, 1991), unlike the case for a protein.] Thus, this approach leads to the suggestion that the BK channel behaves as if it senses the K+ concentration located at 5 Å from the surface of the PS membrane. To put this result in perspective, GCS theory predicts that the electrostatic potential at 5 Å from the surface of an 80% PS bilayer is −55 mV at 0.1 mM CaCl2 and a bulk KCl concentration of 27 mM and −38 mV at a bulk KCl concentration of 107 mM KCl. According to the Boltzmann distribution of Eq. 1, surface potentials of this magnitude would increase the local concentration of K+ at the mouth of the channel by factors of 4.5 and 8.7, respectively, over that in the bulk solution.
In summary, the modeling results of Fig. 2 offer two different mechanisms and approaches to account for the enhancement of K+ conductance by PS. On one hand, PS might affect various intrinsic rate constants that underlie multiion conduction, perhaps by promoting a protein conformational change that perturbs elementary steps in K+ movement. On the other hand, PS might simply act by generating a more negative electrostatic potential at the mouth of the channel, which increases the local K+ concentration available for binding to the outermost K+ sites. However, both approaches predict a significant increase in the apparent association rate of K+ to the channel; i.e., a 2.1-fold increase in the k1 (k2) rate constant (Table I) or a 4.4- to 8.7-fold increase in local K+ concentration due to a partially shielded electrostatic potential.
Effect of Phosphatidylserine on Slow Block of the BK Channel by Extracellular Ba2+
The divalent cation Ba2+ is a blocker of many different K+ channels and has been extensively studied as a probe of multiion conduction in the BK channel (Neyton and Miller, 1988). Crystallographic analysis has also shown that Ba2+ binds within the selectivity filter of the KcsA K+ channel (Jiang and MacKinnon, 2000). Here, we used Ba2+ block to address questions raised by the conductance enhancement of the BK channel in PS. If a PS bilayer acts by increasing local K+ concentration at the mouth of the channel via a through-space electrostatic mechanism, one would expect the local Ba2+ concentration to be similarly increased, resulting in enhanced block. As pointed out by Bell and Miller (1984), the exponential dependence on charge valence for a Boltzmann distribution requires that the local concentration of a multivalent cation is increased more strongly by surface potential than that of a monovalent cation. Eqs. 1 and 2 lead to the following exact relationship:
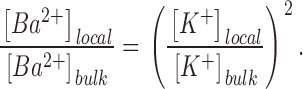 |
(8) |
Thus, according to Eq. 8, if membrane surface potential increases the local concentration of K+ at the mouth of a channel in a PS membrane by a factor of 4 compared with a PE membrane, the local concentration of Ba2+ at the mouth is predicted to increase by a factor of 16. Such an increase in [Ba2+]local should be reflected in the apparent bimolecular association rate of Ba2+. In principle, this relationship provides a sensitive test of a surface potential mechanism.
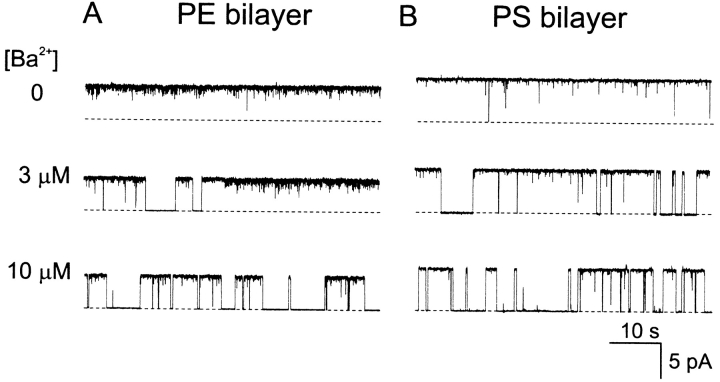
Fig. 3 shows examples of single-channel current recorded at 20 mV from experiments in which block by extracellular Ba2+ at 100 mM symmetrical KCl was compared in bilayers composed of pure PE (Fig. 10 A) or 80/20 PS/PE (Fig. 10 B). As described previously (Vergara and Latorre, 1983), Ba2+ added to the external side of the BK channel produces a slow block characterized by the appearance of discrete blocking events. Extracellular Ba2+ in the millimolar range also produces a second type of fast blocking effect that is evident from the progressive decrease in apparent unitary current at 2 and 20 mM Ba2+ (Fig. 3). At a first glance, simple inspection of the records in Fig. 3 suggests that block by external Ba2+ block is not markedly different in PE vs. PS bilayers.
Figure 3.
Effect of external Ba2+ on single BK channels in PE vs. PS bilayers. Examples of current records at 20 mV in PE (A) or PS (B) bilayers in the presence of 0, 2, or 20 mM BaCl2 on the external side. Conditions: symmetrical 100 mM KCl and 10 mM Hepes-KOH, pH 7.2, internal 200 μM CaCl2, and various concentrations of BaCl2 on the external side as indicated. Records are filtered at 20 Hz and the dashed line indicates the closed current level.
Figure 10.
Effect of internal Ba2+ on single BK channels in PE vs. PS bilayers. Examples of current records at 20 mV in PE (A) or PS (B) bilayers in the presence of 0, 3, or 10 μM BaCl2 on the internal side. Conditions: symmetrical 100 mM KCl and 10 mM HEPES-KOH, pH 7.4, internal 200 μM CaCl2, and various concentrations of BaCl2 on the external side as indicated. Records are filtered at 20 Hz and the dashed line indicates the closed current level.
We first analyzed the slow blocking effect of external Ba2+ by comparing the time-averaged blocking probability in PE vs. PS at ionic conditions of 100 and 20 mM KCl. The probability that the channel is not blocked, Punblocked or Pun, was defined as the total time in the closed-blocked state (equivalent to the zero current level) divided by the total time of the record. For each experiment, Pun was normalized by dividing it by a similar control probability measurement (P0) taken in the absence of added Ba2+. This normalization procedure corrects for a small probability of long closures due to normal gating behavior and Ba2+ contamination in the internal solution (Neyton, 1996) in the absence of added Ba2+. Fig. 4 presents titration curves of the normalized probability of being unblocked for the range of 0.5–60 mM BaCl2 at 100 mM KCl and 0.1–6 mM BaCl2 at 20 mM KCl. The data of Fig. 4 were fit to the following empirical Hill function described by a blocking constant, KB, that reflects the apparent blocking affinity of Ba2+, and a Hill coefficient, n, that describes the steepness of the curve:
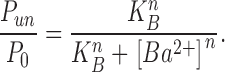 |
(9) |
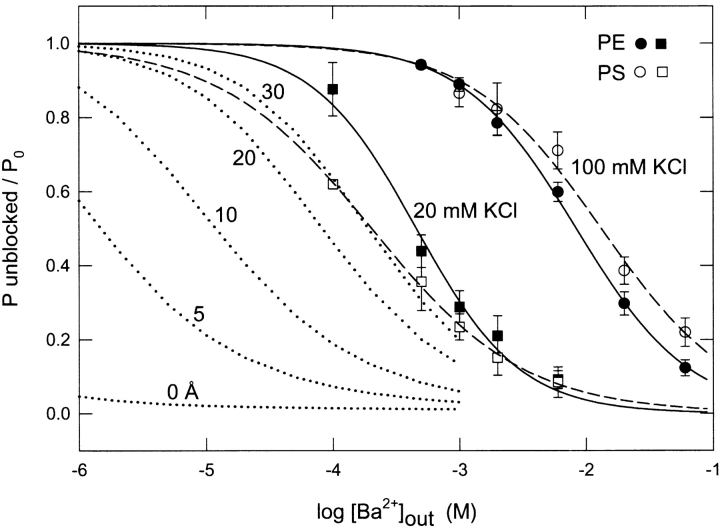
Figure 4.
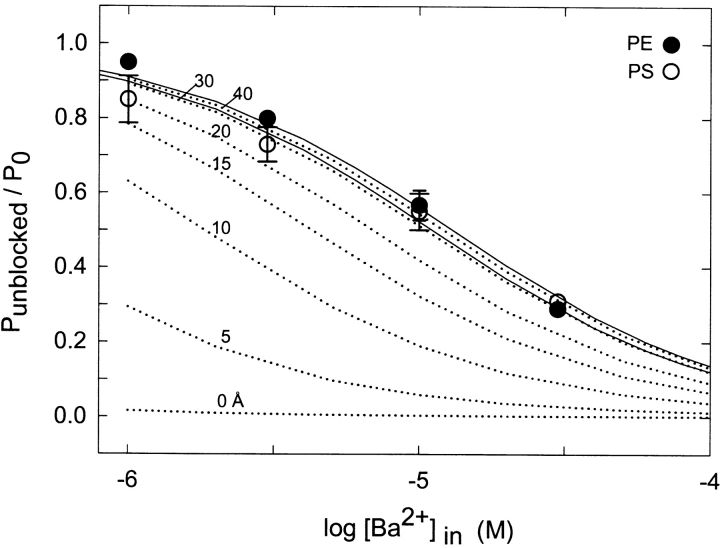
Probability that the channel is not blocked as a function of external Ba2+ concentration in PE and PS bilayers. Inhibition of single-channel activity due to discrete block by external Ba2+ was computed from the time-averaged reduction in open-state probability from long recordings at 20 mV. The ordinate value is a normalized ratio corresponding to the probability that the channel was not blocked by Ba2+ at a given Ba2+ concentration divided by the corresponding open state probability in the absence of Ba2+. Conditions: symmetrical 10 mM HEPES-KOH, pH 7.2, 100 mM KCl (squares) or 20 mM KCl (circles), internal 200 mM CaCl2, for PE (filled symbols) or PS bilayers (open symbols). The solid and dashed lines are fits to the empirical Hill equation (Eq. 9) with parameters given in the text. The dotted lines correspond to simulations computed according to Eq. 9 using KB = 0.46 mM based on the fit to PE data at 20 mM KCl, n = 1, and [Ba2+]local calculated from GCS theory (materials and methods) at various distances (0, 5, 10, 20, and 30 Å) from a PS bilayer at 20 mM KCl, and plotted as a function of bulk Ba2+ concentration.
At 20 mM KCl, the respective best-fit parameters were: PE, KB = 0.46 ± 0.05 mM, n = 1.06 ± 0.12; PS, KB = 0.20 ± 0.01, n = 0.72 ± 0.02. At 100 mM KCl, we obtain: PE, KB = 8.4 ± 0.3 mM, n = 0.97 ± 0.03; PS, KB = 13.3 ± 1.3 mM, n = 0.85 ± 0.07. Thus, the PS bilayer produces a 2.3-fold decrease in KB at the lower ionic strength of 20 mM KCl and a 1.6-fold increase in KB at 100 mM KCl. The Hill coefficients (n) of the Ba2+ titrations in PE are close to 1.0, consistent with a single class of sites. The n values for Ba2+ titrations in PS are somewhat lower than 1.0. Such deviations (n < 1.0) of titrations of divalent cations from simple Langmuir behavior may occur when surface potential decreases during the course of a titration due to increasing ionic strength or divalent cation binding (Ravindran et al., 1991; Latorre et al., 1992).
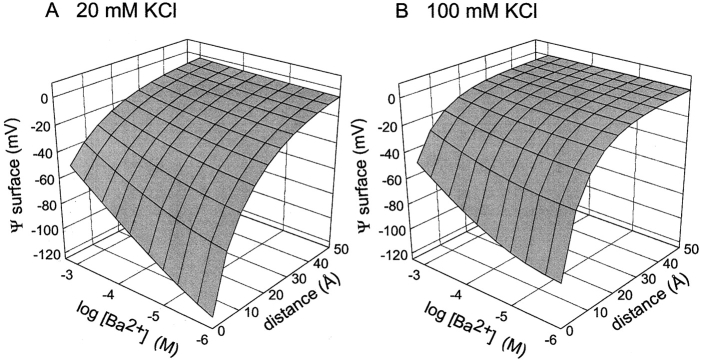
To interpret these results, we must consider changes in electrostatic potential at the membrane surface under the ionic conditions of the experiment. Although Ba2+ block can be used to monitor surface potential, Ba2+ also acts in two ways to reduce the surface potential. As a mobile counterion, high concentrations of Ba2+ reduce the surface potential by electrostatic screening. Ba2+ can also bind directly to PS to reduce the effective negative charge density. These effects are well-described by Eqs. 3 and 4 of GCS theory (McLaughlin et al., 1981). The three-dimensional surface plots of Fig. 5 illustrate the theoretical dependence of surface potential, ψ, on distance at 20 mM KCl (Fig. 5 A) and 100 mM KCl (Fig. 5 B) for bulk Ba2+ concentration ranging from 10−6 to 10−3 M Ba2+. At 20 mM KCl, the calculated potential at the surface (distance = 0) is reduced from −116 mV at 10−6 M Ba2+ to −46 mV at 10−3 M Ba2+. At 100 mM KCl, the respective surface potentials at these two Ba2+ concentrations are −91 and −44 mV. Using the approximate distance decay function of Eq. 5, Fig. 5 also shows that the electrostatic potential decays to less than −5 mV at a distance of 50 Å away from the surface in 20 mM KCl and decays to ∼0 mV at 50 Å in 100 mM KCl.
Figure 5.
Three-dimensional plot of electrostatic surface potential at various distances from a membrane composed of 80% PS and 20% PE. Surface potential was calculated at 5-Å intervals in the range of 0 to 50 Å from the membrane surface at 20 mM KCl (A) or 100 mM KCl (B) in the presence of different bulk BaCl2 concentrations ranging from 10−6 to 10−3 M using GCS theory described in materials and methods.
Although increasing bulk Ba2+ concentration reduces the surface potential, electrostatic potentials of this magnitude still produce very large increases in local Ba2+ concentration near the membrane. For example, according to Eq. 2, a surface potential of −116 mV would increase [Ba2+]local by 9,200-fold over bulk concentration; a surface potential of −44 mV would increase [Ba2+]local by 32-fold. Since Ba2+ blocking affinity is only slightly enhanced at 20 mM KCl, the results of Fig. 4 imply that the PS surface potential is greatly diminished at the mouth of the BK channel. This finding appears to be at odds with the distance estimate of 5 Å based on the K+ conductance and surface potential modeling results of Fig. 2.
Looking more closely at Fig. 4, the small rightward shift of the Ba2+ titration curve in PS vs. PE at 100 mM KCl suggests that the relevant surface potential for slow block by Ba2+ is actually more positive in PS than PE. This cannot be easily explained by lipid surface charge since our calculations indicate that the expected positive reversal of membrane surface charge that eventually occurs due to Ba2+ binding to PS− only commences at a bulk Ba2+ concentration above 50 mM. However, such a rightward shift could also occur if Ba2+ binding increases to nonblocking extracellular sites on the protein surface surrounding the pore entrance in the presence of PS. This could create an additional source of electrostatic repulsion and reduce the probability of Ba2+ binding to blocking site(s) in the selectivity filter.
At 20 mM KCl, the data of Fig. 4 suggests that a significant effect of lipid surface charge in the PS bilayer is present at the lower concentration range of bulk Ba2+. To model the effect of lipid surface charge under these conditions, we calculated expected titration curves based on Eq. 9 assuming an intrinsic blocking affinity equal to that measured in PE (KB = 0.46 mM) and using [Ba2+]local at various distances from the PS surface as calculated from GCS equations given in the materials and methods. Theoretical curves for distances of 0, 5, 10, 20, and 30 Å from the membrane are shown by the dotted lines in Fig. 4. This set of simulations shows that the Ba2+ titration at 20 mM KCl in a PS membrane most closely matches the enhancement expected to occur at a distance of ∼30 Å from the surface. Thus, this analysis suggests that the slow Ba2+ blocking process at 20 mM KCl senses less surface potential than the K+ conduction process analyzed in Fig. 2.
Another important consideration in the interpretation of these experiments is the effect of binding competition between and Ba2+ and K+. Since negative surface potential simultaneously increases the local concentration of K+ and Ba2+ according to Boltzmann distributions (Eqs. 1 and 2), Ba2+ block in a PS bilayer could also be attenuated by increased binding competition of Ba2+ and K+ for relevant sites in the BK channel. However, since local Ba2+ concentration increases as the square of the local K+ concentration according to Eq. 8, the surface charge effect on Ba2+ concentration is expected to predominate over the effect of K+ competition. In view of the uncertainty associated with the effect of K+ competition, distance estimates applied to Ba2+ titrations using the simple GCS-based theory described above should be considered as maximal estimates of the surface charge effect on Ba2+ block.
Kinetics of Block by External Ba2+ in Neutral and Negatively Charged Bilayers
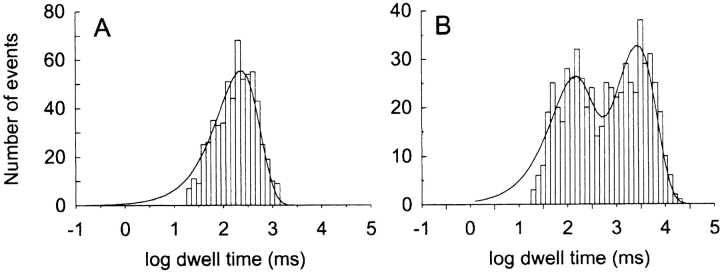
The effect of PS on the kinetics of slow Ba2+ block was further investigated by analyzing dwell time distributions of discrete Ba2+-blocking events. Fig. 6, A and B , show unblocked and blocked state histograms, respectively, of dwell times collected from a single-channel recorded in the presence of 20 mM external Ba2+ and filtered at 10 Hz. The population of unblocked events defined as dwell times between adjacent Ba2+-blocked states is well described by a single exponential function with a time constant of 623 ms (Fig. 6 A). However, the population of blocked events observed in the presence of Ba2+ is best fit by a sum-of-two-exponential function with 68% of the events described by a short time constant of 206 ms and 32% of the events described by a long time constant of 6.84 s (Fig. 6 B). The presence of two time constants in histograms of Ba2+-blocked events of the BK channel has been described previously (Sohma et al., 1996; Sugihara, 1998) and appears to reflect binding of Ba2+ to two different sites within the multiion conduction pathway of K+ channels.
Figure 6.
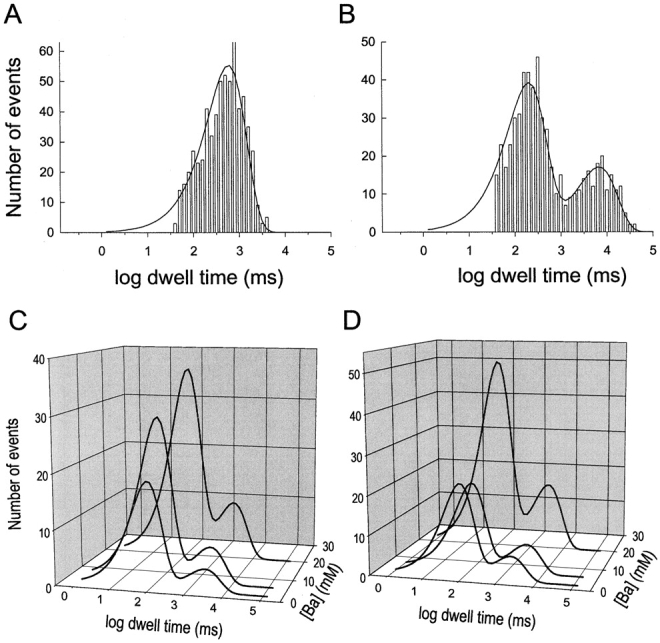
Dwell time distributions of discrete blocking events associated with slow block by external Ba2+ compared for PE and PS bilayers. (A and B) Dwell time histograms compiled for a single BK channel in a PE membrane recorded at 20 mV in the presence of symmetrical 100 mM KCl, 10 mM HEPES-KOH, pH 7.2, internal 200 μM CaCl2, and external 20 mM BaCl2. Populations of unblocked (A) and blocked (B) events were kinetically isolated from normal gating events by filtering the record at 10 Hz (Vergara and Latorre, 1983) and using PClamp analysis software to automatically compile blocked and unblocked events using a 50% threshold criterion for event detection. The solid line in A is a fit to a single exponential with a lifetime of 623 ms for a population of 585 events. The solid line in B is a fit to a sum-of-two exponentials with lifetimes (amplitudes) of 206 ms (68%) and 6.84 s (32%) for 585 events. (C and D) Examples of blocked time histograms are compared for PE (C) and PS (D) bilayers at 2, 6, and 20 mM external Ba2+. The solid lines are fits to a sum of two exponentials with respective lifetimes (amplitudes) at 2, 6, and 20 mM Ba2+ for PE (C): 850 ms (82%), 2.97 s (18%); 94 ms (81%), 2.65 s (19%); 115 ms (77%), 2.7 s (23%), and for PS (D): 85 (79%), 2.1 s (21%); 105 ms (75%), 3.6 s (25%); 104 ms (74%), 3.5 s (26%).
Fitted histograms of Ba2+-blocked events collected at 2, 6, and 20 mM external Ba2+ for BK channels incorporated into a PE bilayer or a PS bilayer are compared in Fig. 6, C and D, respectively. In all cases distributions of Ba2+-blocked times are well described by a sum of two exponentials. The relative contributions and magnitudes of the short and long time constants appear to be independent of lipid composition. Complete results for blocked-state distributions collected in the presence of external Ba2+ are shown in Fig. 7 . Fig. 7, A–C, summarizes the mean values and relative proportions of short and long time constants of Ba2+-blocking events obtained at either 20 or 100 mM KCl. The short and long blocked time constants are essentially independent of Ba2+ concentration. This is expected for a process that reflects dissociation of a single Ba2+ ion that transits between two different sites in the conduction pathway. The data also show that there is virtually no difference in the time constants or relative proportions of short and long Ba2+-blocked states for BK channels incorporated into PE vs. PS bilayers. This finding indicates that membrane lipids or electrostatics do not significantly influence the Ba2+ dissociation process.
Figure 7.
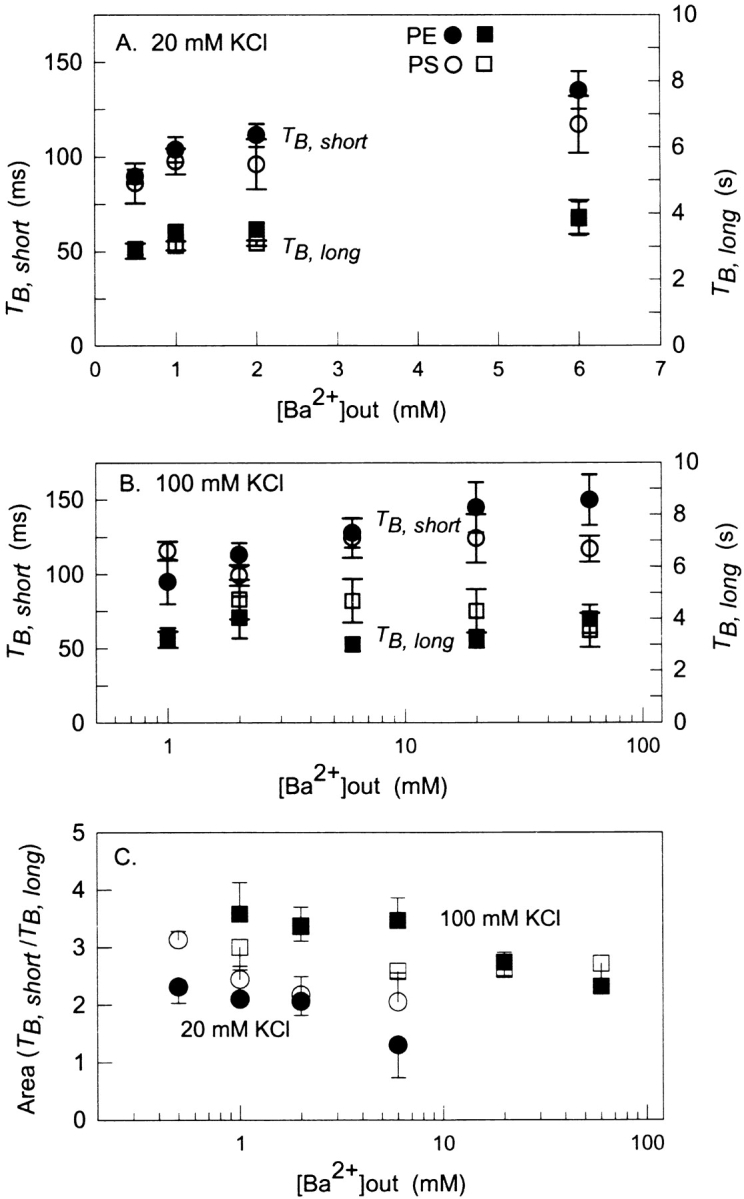
Lifetimes of blocked state events as a function of external Ba2+ concentration compared for PE and PS bilayers. (A and B) Mean values of short (circles) and long (squares) lifetimes of Ba2+-blocked events in PE (filled symbols) and PS (open symbols) bilayers measured at various concentrations of external Ba2+ in the presence of 20 mM (A) or 100 mM (B) symmetrical KCl. (C) Corresponding plot of the ratio of the amplitudes of the short lifetime component to the long lifetime component at 20 mM KCl (circles) and 100 mM KCl (squares). Data points and error bars represent the mean ± SE of 3–9 experiments.
Fig. 8 is a plot of the reciprocal lifetime of the unblocked state as a function of external [Ba2+] in PE and PS bilayers and for ionic conditions of 20 mM KCl (Fig. 8 A) or 100 mM KCl (Fig. 8 B). For a simple bimolecular blocking process, the reciprocal of the unblocked time constant is expected to be a linear function of blocker concentration (Vergara and Latorre, 1983). The linear fits of the data in Fig. 8 correspond to the following apparent association rate constants (kon) for external Ba2+: 20 mM KCl, kon = 1,180 ± 50 s−1M−1 for PE, kon = 1,470 ± 180 s−1M−1 for PS; 100 mM KCl, kon = 116 ± 3 s−1M−1 for PE and 79.6 ± 6.9 s−1M−1 for PS. These results essentially mirror the affinity behavior of the Ba2+ titration curves of Fig. 4. An increase in apparent kon for Ba2+ in PS vs. PE at 20 mM KCl reflects slightly higher Ba2+ blocking affinity in PS as observed in the titration of Fig. 4. Assuming that the apparent Ba2+ association rate is proportional to the local concentration of Ba2+ controlled by a negative surface potential according to Eq. 2, GCS theory can be used to calculate the expected enhancement of kon due to the predicted surface potential at various distances from a membrane composed of 80% PS. The dotted lines in Fig. 8 show simulations of the expected reciprocal unblocked time in a PS bilayer at distances of 5–30 Å from the membrane based on electrostatic enhancement of the Ba2+ association rate in a neutral PE bilayer. These simulations show that the observed Ba2+ association rate in PS behaves as if it responds to the local concentration of Ba2+ at a distance of more than 30 Å away from the PS surface. Thus, this kinetic analysis demonstrates that the process of association of external Ba2+ to slow blocking site(s) located within the BK channel is rather unresponsive to lipid surface charge. Again, this finding may be contrasted with the strong effect of PS on K+ conductance shown in Fig. 2.
Figure 8.
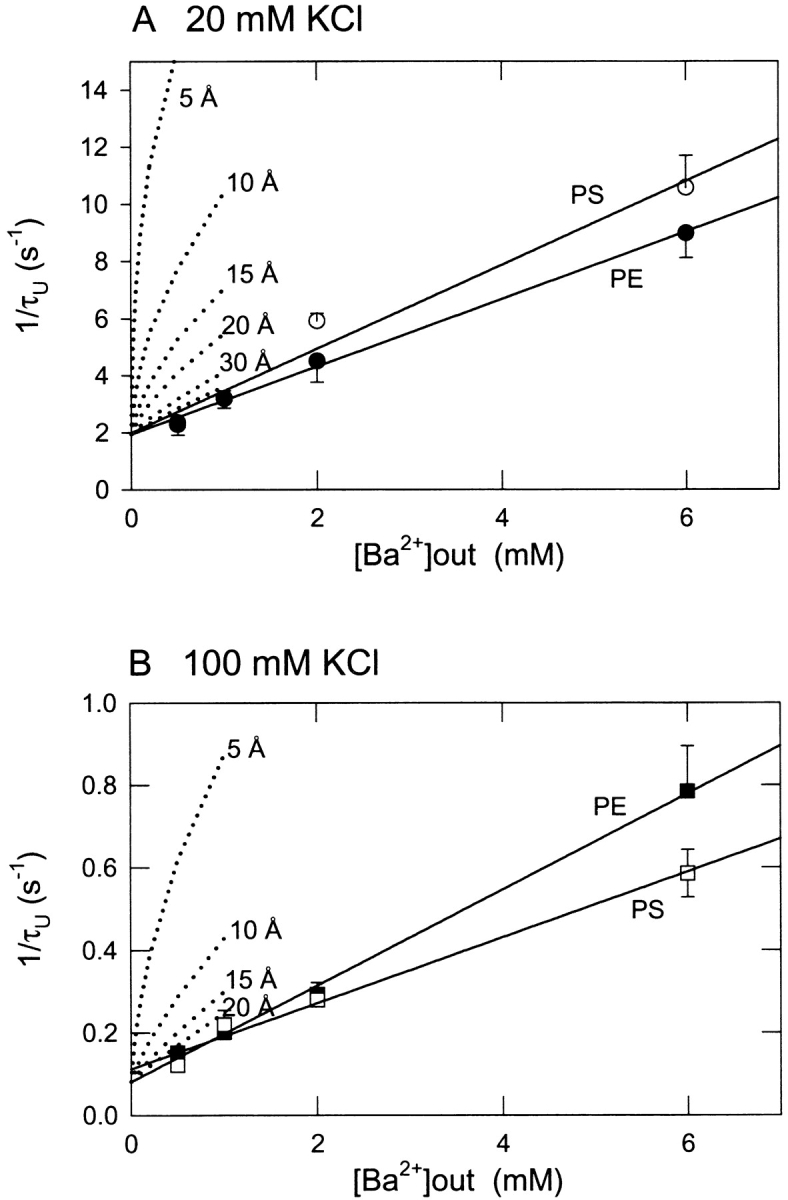
Ba2+ association rate measured from the dependence of reciprocal lifetime of unblocked events as a function of external Ba2+ concentration in PE and PS bilayers. (A and B) Mean values of the reciprocal lifetime of unblocked events at different concentrations of external Ba2+ and 20 mM (A) or 100 mM (B) symmetrical KCl. Note that the data points for PE and PS in A at 0.5 and 1 mM Ba2+ are superimposable. Solid lines are linear fits used to obtain the association rate constant for slow block by external Ba2+ as described in the text. The dotted lines are theoretical curves plotted according to τU −1 = kon[Ba2+]local + b, showing the behavior expected for a process governed by the measured association rate in PE (kon) and the expected local concentration of Ba2+ at various distances in Å from a bilayer containing 80% PS as calculated from GCS theory (materials and methods).
Effect of Phosphatidylserine on Fast Block by Extracellular Ba2+ and TEA+
As mentioned previously in regard to Fig. 3, relatively high concentrations of external Ba2+ also induce a fast blocking effect characterized by an apparent reduction in unitary conductance. Such “fast block” by divalent metal cations has been described for many different cation-selective channels; e.g., fast block by Mg2+ on the inside of the BK channel (Ferguson, 1991) and fast block by Ca2+ and Zn2+ on the outside of NaV channels (Green et al., 1987a; Ravindran et al., 1991; Worley et al., 1992). At the single-channel level, fast block results from rapid binding and binding of a blocking molecule at rates much faster than the shortest resolvable dwell time and at a site that interferes with conduction of permeant ions (Hille, 2001). Fast block of the BK channel by external Ba2+ is likely to involve a peripheral cation binding site (or sites) located external to the selectivity filter. Two such peripheral cation binding sites for K+ located on axis with the central pore but external to the selectivity filter of the KcsA K+ channel were identified recently in a high resolution crystallographic study (Zhou et al., 2001). Since Ba2+ binding to such an external fast-blocking site might be sensitive to lipid surface potential, we also analyzed titration curves for fast block by Ba2+ in PE and PS membranes.
Fig. 9 A summarizes titrations of the Ba2+-dependent reduction in apparent unitary current in PE and PS bilayers measured at 20 mV in symmetrical solutions of 100 and 20 mM KCl. The decrease in unitary current is measured as the ratio, I/I0, of single-channel current at a given concentration of Ba2+ to that of the same channel in the absence of external Ba2+. The results show that the apparent affinity of Ba2+ for the fast blocking(s) sites is increased in PS relative to PE at 20 and 100 mM KCl. The titration curves of Fig. 9 A were separately fit to the empirical Hill function of Eq. 9 using n = 0.7 to adequately describe the rather shallow [Ba2+] dependence. At 100 mM KCl, the best-fit values of the apparent blocking constants are: PE, KB = 36.5 ± 5.9 mM; PS, KB = 22.3 ± 1.7 mM. At 20 mM KCl, we obtain: PE, KB = 7.2 ± 0.5 mM; PS, KB = 2.1 ± 0.3 mM. Overall, these results support the idea that there is a small but significant effect of lipid surface charge on fast block by external Ba2+ since the PS-dependent enhancement of Ba2+ affinity as measured by KB is greater at the lower ionic strength of 20 mM vs. 100 mM KCl. To evaluate the observed enhancement of fast Ba2+ block within the context of surface charge theory, we calculated a series of expected Ba2+-titration curves based on [Ba2+]local at various distances from a PS membrane under conditions of 20 mM KCl using the procedure described above for the slow-block Ba2+ titration of Fig. 4. As shown by the calculated dotted line curves in Fig. 9 A, the observed enhancement of fast Ba2+ block in 80% PS and 20 mM KCl would be similar to that expected if the fast blocking site senses [Ba2+]local at a distance of 20–30 Å from the membrane.
Figure 9.
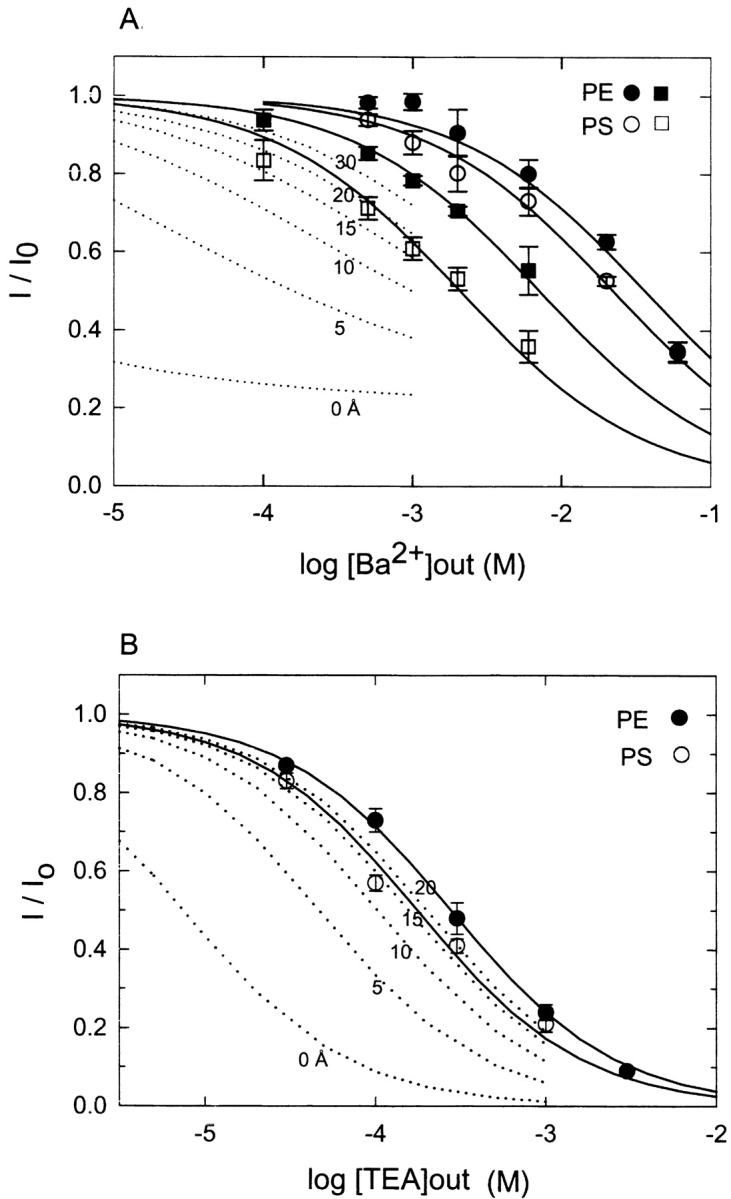
Apparent reduction in unitary BK channel current due to fast block by external Ba2+ and TEA+ compared in PE and PS bilayers. Ordinate values (I/I0) correspond to single-channel current at the indicated Ba2+ (or TEA+) concentration (I) divided by the unitary current of the same channel (I0) in the absence of Ba2+ (or TEA+). Data point are the mean ± SE for 4–9 experiments. Fast block by external Ba2+ (A) or external TEA+ (B) measured as the apparent reduction in unitary current at 20 mV. Solid symbols, PE; open symbols, PS; circles, symmetrical 100 mM KCl; squares, symmetrical 20 mM KCl. Solid lines in A and B are fits to the empirical Hill equation (Eq. 9) as described in the text. Dotted lines in A are theoretical curves using the KB parameter obtained for the fit of the PE data at 20 mM KCl and the local concentration of Ba2+ at various distances (in Å) from a bilayer containing 80% PS as calculated from GCS theory outlined in materials and methods. Dotted lines in B show similar theoretical curves based on the KB parameter for the fit to the TEA+ titration in PE bilayers and the calculated local concentration of TEA+ at various distances from a PS bilayer.
The organic cation, TEA+, is also known to produce a fast block of the BK channel that is easily measured by an apparent decrease in unitary current (Villarroel et al., 1988). We similarly analyzed the effect of a negatively charged bilayer on fast block by external TEA+ under conditions of 20 mV and 100 mM symmetrical KCl. As shown in Fig. 9 B, the TEA+ titration curves in PE and PS are well described by Eq. 9 using n = 0.9 and KB = 0.28 ± 0.01 mM for PE and KB = 0.18 ± 0.02 mM for PS. Using GCS theory, the small enhancement of blocking affinity in PS vs. PE is similar to that expected if the blocking site senses [TEA+]local at distance of ∼15–20 Å from the membrane, as indicated by the dotted line curves of Fig. 9 B.
Effect of Phosphatidylserine on Slow Block of the BK Channel By Intracellular Ba2+
Thus far, we have considered the effect of a negatively charged membrane surface on the reactions of blockers applied to the extracellular side of the rat BK channel. Depending on the location and disposition of the outer and inner pore entrances with respect to the phospholipid surface on each side of the membrane, membrane surface charge could potentially have different effects on the kinetics of blockers added to the external vs. internal sides of the channel. Therefore, we also studied the effect of PS on slow block by Ba2+ added to the internal side. The BK channel is well known to exhibit a higher affinity for slow block by internal Ba2+ in comparison to external Ba2+ (Vergara and Latorre, 1983). This allows internal block to be studied at a much lower range of Ba2+ concentration where self-screening by Ba2+ is insignificant.
Fig. 10 shows examples of single-channel current recorded at 20 mV from experiments in which block by intracellular Ba2+ at 100 mM symmetrical KCl was compared in bilayers composed of pure PE (Fig. 10 A) or 80% PS (Fig. 10 B). Visual comparison of records taken in the presence of 3 and 10 μM Ba2+ suggests that the kinetics of slow Ba2+ block are similar in PE and PS membranes. Fig. 10 also shows little evidence of fast block at micromolar concentrations of internal Ba2+ in comparison to the substantial reduction of unitary current at the higher concentrations of external Ba2+ used in the experiments of Fig. 3.
Fig. 11 shows corresponding Ba2+-titration curves of the time-averaged probability that the channel is not blocked by internal Ba2+. The data are presented in a similar format as measurements of Punblocked normalized to P0 in the absence of Ba2+ as described previously for Fig. 4. The PE and PS data were separately fit to Eq. 9 using n = 0.9 and KB = 13.1 ± 1.0 μM for the PE bilayer and KB = 11.1 ± 1.1 μM for the PS bilayer. As described above for titration curves of Figs. 4 and 9, we simulated the expected enhancement by PS of the Ba2+ titration by using GCS theory and Eq. 9, the KB value obtained for the Ba2+-titration in PE, and [Ba2+]local at various distances from a bilayer containing 80% PS. As shown by the dotted line curves of Fig. 11, the lack of significant enhancement of internal Ba2+ block by 80% PS can be simulated by a situation in which the blocking reaction responds to [Ba2+]local at a distance of 30 Å or greater from the membrane surface.
Figure 11.
Probability that the channel is not blocked as a function of internal Ba2+ concentration in PE and PS bilayers. Inhibition of single-channel activity due to discrete block by external Ba2+ at 20 mV was computed from the time-averaged reduction in open-state probability from long recordings. The ordinate value is a normalized ratio corresponding to the probability that the channel was not blocked by Ba2+ concentration divided by the corresponding open state probability in the absence of Ba2+. Conditions: symmetrical 10 mM HEPES-KOH, pH 7.2, 100 mM KCl, internal 200 μM CaCl2, for PE (filled circle) or PS bilayers (open circle). Solid lines are fits to the empirical Hill equation (Eq. 9) with parameters given in the text. Dotted lines correspond to simulations computed according to Eq. 9 using KB = 13 μM based on the fit to PE data and [Ba2+]local calculated from GCS theory (materials and methods) at various distances (0–40 Å) from a bilayer containing 80% PS.
The effect of PS on the kinetics of internal Ba2+ block was analyzed in the same fashion as described above for external Ba2+ block. Fig. 12 shows examples of dwell time histograms of unblocked (Fig. 12 A) and blocked events (Fig. 12 B) collected at 20 mV in the presence of 10 μM internal Ba2+ from a single-channel record filtered at 10 Hz to isolate populations of discrete Ba2+-blocking events. The histogram of unblocked events is well fit by a single exponential with a time constant of 222 ms (Fig. 12 A) and the blocked state histogram is well fit by a sum of two exponential distribution with respective short and long time constants of 127 ms (41% of the events) and 2.73 s (59% of the events). A summary of measured lifetimes for internal Ba2+ block is shown in Fig. 13 . The short and long lifetimes describing the Ba2+-blocked state population were essentially independent of Ba2+ concentration in the range of 1–30 μM and were not significantly different for PE vs. PS. As expected for a bimolecular process, the reciprocal lifetimes of the unblocked state exhibited a linear dependence on Ba2+ concentration (Fig. 13 B). Linear fits of the data in Fig. 13 B correspond to Ba2+ association rate constants of 5.91 × 104 s−1M−l for PE and 5.94 × 104 s−1M−1 for PS. A series of dotted line curves plotted in Fig. 13 B show the enhancement of Ba2+ association rate that would be predicted if the internal entryway to channel sensed the local Ba2+ concentration at various distances from a membrane composed of 80% PS. The lack of a significant effect of PS on the Ba2+ association rate shows that the internal Ba2+ blocking reaction is completely isolated from the enhancing effect of PS on [Ba2+]local.
Figure 12.
Dwell time distributions of discrete blocking events associated with slow block by internal Ba2+. Dwell time histograms were compiled as described in Fig. 6 for a single BK channel in a PE membrane at 20 mV in the presence of symmetrical 100 mM KCl, 10 mM HEPES-KOH, pH 7.2, internal 200 μM CaCl2, and internal 100 μM BaCl2. (A) Unblocked events. (B) Blocked events. The solid line in A is a fit to a single exponential with a lifetime of 222 ms for a population of 638 events. The solid line in B is a fit to a sum-of-two exponentials with lifetimes (amplitudes) of 127 ms (41%) and 2.73 s (59%) for 639 events.
Figure 13.
Lifetimes of blocked (A) and reciprocal unblocked (B) events as a function of internal Ba2+ concentration in PE and PS bilayers. (A) Mean values of short (circles) and long (squares) lifetimes of Ba2+-blocked events in PE (filled symbols) and PS bilayers (open symbols) measured at various concentrations of external Ba2+ in the presence of symmetrical 100 mM KCl. (B) Mean values of the reciprocal lifetime of unblocked events at different concentrations of internal Ba2+ and symmetrical 100 mM KCl. Solid lines are linear fits used to obtain the association rate constant for slow block by internal Ba2+ as given in the text. The dotted lines are theoretical curves plotted according to τU −1 = kon[Ba2+]local + b, showing the behavior expected for a process governed by the measured association rate (kon) in PE and the expected local concentration of Ba2+ at various distances in Å from a bilayer containing 80% PS as calculated from GCS theory (materials and methods).
DISCUSSION
This study was undertaken to investigate the mechanism of a remarkable enhancing effect of negatively charged phospholipids on the conductance of the BK channel. This effect is interesting for several reasons. All K+-selective ion channels are known to be structural homologues by virtue of their highly conserved pore domain and selectivity filter. However, the BK channel has the highest known unitary conductance of any K+-channel. The 1.4-fold enhancement from 252 pS at 100 mM KCl in a PE bilayer to 360 pS in a PS bilayer corresponds to flux rates of 1.6 × 108 and 2.2 × 108 ions/s, respectively, at 100 mV of driving force. These rates surpass those of many other K+-channels by 2- to 10-fold. For example, the KcsA K+ channel whose crystal structure is known in atomic detail (Doyle et al., 1998) has a conductance of 97 pS in 100 mM K+, only one-third that of BK (Le Masurier et al., 2001). The specific molecular features of the BK channel that are responsible for this phenomenal K+-selective flux rate are currently unknown; however, it has been proposed that unique acidic residues located at the intracellular vestibule may play a role (Brelidze et al., 2003; Nimigean et al., 2003). Since negatively charged phospholipids act on the BK channel protein to further augment an already substantial K+ flux, biophysical studies of this lipid–channel interaction may help to uncover principles for understanding the basis of rapid ion conduction and conductance modulation. Furthermore, while the lipid dependence of relatively few channel proteins has been investigated at the single-channel level, it appears that the BK channel is exceptionally responsive to phospholipid composition in comparison to NaV and CaV channels (Coronado and Affolter, 1986; Green et al., 1987a). Whether this is a general property of K+ channel proteins or a unique feature of the BK channel remains to be determined.
Second Thoughts about the Surface Charge Hypothesis
According to the surface charge hypothesis for the effect of PS on BK channel conductance (Moczydlowski et al., 1985), enhancement of K+ flux was proposed to originate from the well-known double-layer phenomenon in which a smeared surface containing negatively charged lipid molecules produces an accumulation of cations and depletion of anions in the aqueous phase adjacent to the membrane (McLaughlin, 1977; Latorre et al., 1992). This idea was inspired by classic work on gramicidin (Apell et al., 1979), a small channel-forming peptide that, like other ionophore molecules (McLaughlin et al., 1970), clearly acts as a molecular sensor of the increase in local alkali cation concentration near a PS-containing membrane. More recently, however, critical examination of the effect of PS on gramicidin conductance has revealed that surface charge theory is not sufficient to describe the full magnitude of the conductance enhancement. Unspecified “structural factors” (presumably related to PS-dependent changes in channel structure relative to the average structure in neutral lipids such as PC) are responsible for a unitary conductance increase on the order of ∼33% (Rostovtseva et al., 1998). Also, gramicidin conductance is affected by other chemical properties of the lipid headgroup, and the length and degree of saturation of acyl chains of phospholipid molecules that form the bilayer (Fonseca et al., 1992; Killian, 1992; Girshman et al., 1997).
Questions regarding the surface charge hypothesis also emerged with the recognition that the minimal size of a BK channel protein without its accessory β subunits is ∼500 kD, corresponding to a tetrameric complex of four 125-kD α subunits (Butler et al., 1993). The ion conduction pore of a protein complex this large is likely to be laterally separated from the lipid surface by a considerable distance over which the surface potential decays. For example, the measured external radius of the cone-shaped KcsA K+-channel is 27 Å (Doyle et al., 1998). If we assume that the lateral decay of surface potential is an approximately exponential function of distance relative to the Debye length, λD (Apell et al., 1979), then the residual magnitude of the membrane surface potential at the pore entrance of KcsA in 100 mM KCl (λD = 9.6 Å) would be only 6% of its value in the surrounding lipid. The membrane domain of the BK α subunit of the BK channel is predicted to have seven membrane-spanning segments (S0 + S1–S6) (Meera et al., 1997) versus two such segments (M1, M2 homologous to S5, S6) for the 17.6-kD protein monomer of the KcsA tetramer. Since the whole integral membrane protein domain of BK is clearly much larger than that of KcsA, one would expect the lipid surface potential to be virtually extinguished at the mouth of the BK pore by distance decay. Nevertheless, one might consider the possibility that one or more negatively charged phospholipid molecules could assemble in crevices among transmembrane segments of the BK channel protein at sites close to the pore and produce a localized electrostatic effect that would behave much like lipid-dependent surface potential. Such issues led us to reexamine the lipid surface charge hypothesis. In this work we aimed to determine whether PS acts on the BK channel to increase the local concentration of cations near the pore entrance.
Ba2+ Block as a Probe for Lipid-based Surface Potential at the Mouth of a K+ Channel: Conclusions and Limitations of the Analysis
Much evidence indicates that the blocking reactions of ion channel proteins are controlled by Coulombic interactions with the protein surface. For example, the association rates of the cationic guanidinium toxin blockers of NaV channels, tetrodotoxin1+ and saxitoxin2+, exhibit a dependence on Na+ concentration that is well described by a through-space electrostatic interaction with negatively charged surface residues located in the external mouth of the channel (Green et al., 1987b; Ravindran and Moczydlowski, 1989). In the case of the Shaker K+ channel, the affinity and blocking kinetics of analogs of charybdotoxin are determined by long-range electrostatic interactions between various charged groups on the toxin and the external channel surface (Escobar et al., 1993; Stocker and Miller, 1994). Likewise, the binding of divalent metal cations such as Ba2+ and zwitterionic peptide toxins (charybdotoxin and iberiotoxin) to liposomes containing acidic phospholipids in aqueous salt solution is well described by classical electrostatic theory of a planar surface (McLaughlin et al., 1981; Ben-Tal et al., 1997). From this standpoint, Ba2+, a well-known pore blocker of K+ channels, may be considered an ideal probe of the electrostatic influence of lipids on the interaction of permeant ions with the BK channel. Ba2+ blocks the BK channel by binding within the selectivity filter (Neyton and Miller, 1988; Jiang and MacKinnon, 2000) upon entering the pore from either the external and internal sides of the membrane (Vergara and Latorre, 1983). Rates of Ba2+ block are readily measured from single-channel kinetics. A validated electrostatic theory for PS-containing bilayers (McLaughlin et al., 1981) also allows one to predict how membrane surface potential varies as a function of KCl and BaCl2 concentration along the distance axis normal to the membrane (e.g., Fig. 5).
Using this approach, we generally find that Ba2+-blocking interactions with the BK channel are markedly insensitive to the electrostatic influence of PS. In interpreting the magnitude of the observed effects, we attempted to estimate the maximum effect of PS on Ba2+ block that would be expected from electrostatics alone. As in previous studies (Bell and Miller, 1984; Moczydlowski et al., 1985; Coronado and Affolter, 1986; Rostovtseva et al., 1998), we used surface potential theory and an approximate model for the dependence of channel conductance on bulk cation concentration in a neutral lipid membrane to estimate the effective magnitude of the surface potential acting on the ion conduction process.
The kinetic model shown in Table I is the simplest possible discrete state model of ion conduction for a multiion channel. This model was chosen not only for its computational simplicity and minimal number of free parameters, but also because it evokes double occupancy by K+ and the energetics of ion conduction observed in the crystal structure of KcsA as captured at low and high K+ concentrations (Berneche and Roux, 2001; Morais-Cabral et al., 2001; Zhou et al., 2001). The assumption of symmetry in the steps for inward and outward K+ flux is also reasonable considering the ohmic current behavior in the low voltage range (Fig. 1 B) and the rather symmetric structure of the K+ channel selectivity filter formed by four stacked rings of peptide carbonyl groups from each of the four subunits (Morais-Cabral et al., 2001; Zhou et al., 2001). This model (Eq. 7) is able to closely describe the conductance [K+] data in PE and PS (Fig. 2 A), although the best-fit value of one of the pairs of rate constants (k−3, k−4) is not well defined (Table I). Assuming that the effect of PS is solely due to increased local concentration of K+, we used standard Gouy-Chapman-Stern theory for a bilayer composed of 80% PS (McLaughlin et al., 1981) to estimate the effective surface potential that might be involved. According to this paradigm, we find that the local concentration of K+ present at a distance of 5 Å normal to the surface would be necessary to achieve the enhanced conductance [K+] behavior in PS (Fig. 2 A). This value is even smaller than the 9 Å estimated previously in the original study of Moczydlowski et al. (1985) that used an unrealistic conduction model (a sum of two independent pores).
An electrostatic distance of ∼5 Å obtained from the conventional approach described above seems impossibly small compared with the measured lateral external radius of the KcsA channel protein (27 Å) and the projected radius of the substantially larger BK channel protein. In quantitative terms, the estimate of ∼5 Å must not be construed as a measurement of the actual distance of the conduction pathway from the lipid because the geometry of the Gouy-Chapman membrane model is obviously very different from that of a pore embedded in a membrane protein. Rather, this is simply a useful and internally consistent way to quantify the residual membrane surface potential that would be required to raise the local K+ concentration high enough to achieve the observed conductance enhancement by PS. Although this sort of estimate is subject to many uncertainties, it suggests that the effect of negatively charged lipids on K+ conductance is too large to result purely from electrostatic enhancement of local K+ concentration near the pore. Various assays of Ba2+ block in PE and PS membranes that we performed generally support this conclusion, since the affinity and apparent association rates for Ba2+ are weakly enhanced, if at all, by PS vs. PE.
Application of GCS theory to the titration data for slow and fast modes of block by external Ba2+ as shown in Figs. 4, 8, and 9 A indicates that the comparable level of lipid surface potential sensed by these reactions is equivalent to that found at a distance greater than 20–30 Å from the membrane surface. These relative distance values are four- to sixfold larger than the estimate of 5 Å derived from the conductance analysis (Fig. 2). The corresponding results for titration of slow block by internal Ba2+ (Figs. 11 and 13 B) do not show any significant effect of PS, implying that the internal entryway to the channel pore is even more isolated from membrane surface charge than the external entryway. In addition, the small PS-dependent enhancement observed for the blocking affinity of external TEA+ at 100 mM KCl (Fig. 9 B) is consistent with the results for external Ba2+ and shows that the findings are not peculiar to one cation probe of surface potential. To put these results into perspective, GCS theory can be used to calculate (i.e., Eq. 2) that if the enhancing effect of PS on K+ conductance were purely due to electrostatic enhancement of local K+ concentration, then the local concentration of Ba2+ at the mouth of the channel would be increased by 59-fold over bulk Ba2+ in our 20 mM KCl solution containing 0.1 mM Ba2+ (Ψx = −52 mV at 5 Å from the membrane) and by 17-fold in our 100 mM KCl solution containing 0.1 mM BaCl2 (Ψx = −36 mV at 5 Å from the membrane). Thus, we would expect to observe much a larger enhancement of Ba2+ block by PS, if the mechanism of the enhancement of K+ conductance were simply due to surface electrostatics.
Before rejecting the surface charge hypothesis, an additional factor must be considered in the quantitative interpretation of the blocker titration experiments. As mentioned in results, this concerns the fact that Ba2+ binding to its blocking site(s) is competitive with respect to K+ binding. Such Ba2+-K+ competition will attenuate the expected effect of surface potential on Ba2+ block since enhancement of local K+ concentration in PS also reduces the apparent affinity of Ba2+ via binding competition. Such competition is evident by the increase in apparent KB for Ba2+ block in PE from 0.46 mM at 20 mM KCl to 8.4 mM at 100 mM KCl as seen in Fig. 4 and the similar [K+] dependence of fast Ba2+ block seen in the titrations of Fig. 9 A. Aside from mutually exclusive binding competition, part of the increase in KB for Ba2+ may also be due to the ∼5-fold increase in ionic strength from 20 to 100 mM KCl solutions. This increase in ionic strength would also act to screen surface potential originating from negatively charged protein groups near the channel mouth that exert a direct electrostatic influence on blocker affinity. In the absence of detailed structural information, the effect of K+ competition on Ba2+ block cannot readily be separated from the effect of surface potential screening by K+. Likewise, the effect of K+ competition on slow block by external Ba2+ also cannot easily be predicted the since KB for Ba2+ is a nonlinear function of [K+]; i.e., KB increases by 18-fold as [K+] is increased fivefold from 20 to 100 mM. Such a higher-order dependence on [K+] may reflect multiion occupancy of the channel by K+.
One way to view the results independently of K+ competition is to consider the ratio of the KB blocking parameters in PE and PS bilayers for fast block by external Ba2+ (Fig. 9 A) relative to fast block by external TEA+ (Fig. 9 B). Assuming that external K+ competes in a similar way for binding of external Ba2+ and TEA+ for their blocking sites, then the ratio of the KB ratio for PE/PS for Ba2+ to the KB ratio for PE/PS for TEA+ should reflect only the lipid electrostatic dependence, since the common terms describing K+ competition for the two blockers would cancel out of the ratio of KB ratios (Green et al., 1987b; Ravindran and Moczydlowski, 1989). At 100 mM KCl, this ratio of KB ratios equals 1.05, indicating that there is virtually no PS-dependent enhancement of the Ba2+ blocking affinity relative to the TEA+ blocking affinity (Fig. 9), as would be expected for a valence-dependent electrostatic mechanism. Thus, it appears that K+ competition cannot explain the weak effect of PS on the external Ba2+ blocking reaction.
Despite such uncertainties in the analysis, the overall results demonstrate that the discrete blocking reaction by internal Ba2+ is completely insensitive to the lipid surface potential and, at best, the slow and fast blocking reactions of external Ba2+ sense only a small fraction of the lipid surface potential. Thus, we are led to conclude that the mechanism of enhancement of BK conductance by negatively charged lipids is unlikely to result from electrostatic accumulation of K+ near the pore. As suggested previously for other types of large ion channel proteins (NaV, CaV), the ion conduction pathway of the BK channel is effectively insulated from the lipid-based surface potential (Coronado and Affolter, 1986; Worley et al., 1992), probably due to the fact that a lateral distance of separation in excess of 30 Å from the lipid–protein interface to the central pore allows the membrane surface potential to decay to values less than −10 mV before reaching the external and internal entryways to the channel.
What Other Mechanism Could Explain the Effect of PS on K+ Conductance?
In relatively low ionic strength solutions of 100 and 20 mM KCl, we find that the association and dissociation rate constants for discrete block by external and internal Ba2+ are virtually insensitive to the phospholipid head groups of PE vs. PS. Thus, it appears that the process of K+ conduction responds to lipid substitution quite strongly but Ba2+ block does not. Since both processes of Ba2+ block and K+ conduction undoubtedly involve cation binding and translocation (or dissociation) reactions among the same multiple sites in the selectivity filter region of the BK channel, how can we account for divergence in the lipid sensitivity of these two reactions? One might propose that the effect of PS on K+ conductance does not involve an increase in surface K+ concentration but rather a long-range electrostatic modulation (charge- or dipole-based) of ion translocation rates within the selectivity filter. Such a mechanism would still require all cations in the pore (K+ and Ba2+) to behave similarly with respect to charge interactions, and there is no significant effect of PS on Ba2+ dissociation steps. Therefore, the unlikelihood of a long-range electrostatically based mechanism leads us to consider that certain phospholipids may modulate BK conductance by an effect on the conformation of the channel protein that might more aptly be described as a lipid-dependent tuning of protein conformation. What sort of conformational change could be involved?
Aside from the steps involved in diffusion of ions up to the pore and exit from the pore, the rate determining steps that underlie K+ conductance take place within the narrow selectivity filter region identified in the structure of KcsA (Doyle et al., 1998). This selectivity filter also determines relative differences in elementary rates of translocation and block of permeant and impermeant cations such as K+, Rb+, Cs+, Na+, and Ba2+. Therefore, we hypothesize that the selective effect of anionic lipids on enhancement of K+ conductance versus Ba2+ block arises from an ion-selective conformational change of the selectivity filter. According to this model, addition of PS to the bilayer promotes a conformational change of the BK selectivity filter that differentially increases certain rate constants governing K+ flux without greatly affecting the elementary steps of Ba2+ block. Is this a reasonable proposition?
The process of K+ translocation occurs on a time scale that is many orders of magnitude faster than the measured rate constants of Ba2+ block. The high rate of K+ flux through K+ channels is now understood to arise from the energetic balance of ion binding and ion repulsion within the single-file region of the filter that undergoes rapid concerted transitions between several ion occupancy states involving two or three K+ ions (Berneche and Roux, 2001; Morais-Cabral et al., 2001). Thus, structural changes of the filter region that affect the dynamic balance of K+ binding and K+ interaction energies could modulate K+ conductance differently than Ba2+ block. The Ba2+ blocking process involves occupancy states that are relatively more stable, involving durations in the millisecond to seconds range, in comparison to the nanosecond range for much shorter states of K+ occupancy. In addition, the relevant chemical and hydration energies associated with interactions of peptide carbonyl oxygens, water, and inorganic cations are undoubtedly different for Ba2+ and K+. According to this reasoning, we are led to suspect that the effect of anionic phospholipids on the K+ conduction process may be related to fast structural-dynamic changes of the selectivity filter shown to be required for rapid, diffusional, K+ flux (Berneche and Roux, 2001). Molecular dynamics simulations using the KcsA structure indicate that dynamic fluctuations of the sixteen carbonyl oxygens atoms that form the selectivity filter of KcsA are much larger than the difference in the radius of K+ and Na+ (Berneche and Roux, 2001). Such fluctuations of the single-filing region appear to be an important factor in determining the high flux rate for K+. Furthermore, a ring-like hydrogen-bond network of aromatic residues surrounding the selectivity filter also appears to be coupled to dynamic motions of the pore and, thus, indirectly affects the K+ conduction rate (Doyle et al., 1998; Berneche and Roux, 2001). Therefore, we hypothesize that dynamic relaxations of the filter and residues coupled to the filter may affect the rate determining steps of K+ flux more strongly than the microscopic steps involved in the long-duration waiting and residence times of a slow-blocking ion such as Ba2+. How could anionic lipids promote conformational changes in the protein domains coupled to functional processes of the selectivity filter?
Phospholipid Modulation of the BK Channel: Specific Ligand Interaction or a More General Effect of Membrane Curvature Stress
At present, the complex interactions of lipids with membrane proteins are only partially understood. Current discussion of lipid–protein interactions appears to reflect two different schools of thought (Dowhan, 1997; Gil et al., 1998; Popot and Engleman, 2000; Fyfe et al., 2001). From one perspective, the lipid molecule may be regarded as any other small molecule or ligand capable of binding to a specific site on the protein surface. In this sense, discrete binding of single or multiple lipid molecules at the protein–membrane, protein–protein, or protein–solution interfaces may modulate channel activity in classic enzymological fashion by inducing ligand-dependent conformational changes of the protein; i.e., as an allosteric effector of protein function. Another viewpoint emphasizes the self-organizing material properties of lipids and proposes that the lipid bilayer affects membrane proteins in a more general way, by exerting elastic stress forces on the protein that affect its shape and function. These two mechanisms are not mutually exclusive and it is certainly possible that lipids affect BK channel conductance in one or both ways. To introduce the lipid-tuning hypothesis with a view toward designing further experiments to test it, we briefly discuss some known aspects of lipid interactions with K+ channels and relevant model systems. Corresponding to the two different perspectives of lipid–protein interactions, we describe how a specific lipid interaction or a general bilayer effect may account for our results.
Case I: A Specific Binding Site for Anionic Phospholipids
While many examples of specific lipid–protein interactions have been identified by activity assays, structural analysis of lipid binding to often irregularly shaped hydrophobic surfaces of membrane proteins has only recently been achieved with the use of X-ray crystallography. For example, phospholipid molecules such as cardiolipin, PE, phosphatidylcholine (PC), and phosphatidylglycerol (PG) have been identified in crystal structures of the bacterial reaction center and cytochrome oxidase (Fyfe et al., 2001). Atomic structures of these lipid–protein complexes show specific chemical interactions (H-bonding and ionic bonding) between the lipid head group and polar amino acids of the protein surface as well as hydrophobic interactions of lipid acyl chains lying in nonpolar grooves of the transmembrane regions of the protein.
Four phospholipid molecules (one per protein monomer) identified as PG have also been recently located within the crystal structure of the homotetrameric KcsA channel (Valiyaveetil et al., 2002). The negatively charged lipid head group of PG is thought to form close range electrostatic interactions with Arg64 and Arg89 residues of KcsA located near the outer leaflet of the bilayer. In addition, one of the lipid acyl chains is situated between a groove formed by the pore helix and the inner transmembrane helix (M2). This study also found that while anionic lipids are not an absolute requirement for refolding and tetramerization of denatured KcsA, PG is required for K+ transport function since KcsA tetramers reconstituted into liposomes lacking this lipid are inactive with respect to 86Rb+ flux. Based on the observation of a specific interaction of a lipid molecule with the transmembrane M2 helix involved in channel gating, Valiyaveetil et al. (2002) also proposed that PG may act as an important cofactor in the closed-open gating reaction of the channel. In line with these results, membrane association and tetramerization of newly synthesized KscA as well as tetramer stability with respect to temperature were found to exhibit a distinctive dependence on the nature of the phospholipid headgroup with a preferential effect of anionic lipids such as PG (Van Dalen et al., 2002).
Based on the observation of a specific PG “cofactor” intimately associated with the KcsA pore domain (Valiyaveetil et al., 2002), one may hypothesize that a similar binding interaction between one (or more) anionic lipids and a site(s) on the tetrameric BK channel protein is responsible for the conductance-enhancing effect of PS or PI studied here. From known principles of ligand–protein interactions, it is conceivable that the BK pore domain could adopt different conformations with PS vs. PE bound to a specific lipid modulatory site. According to this scenario, the PS-bound state would adopt a “high-flux” conformation relative to a “low-flux” conformation favored with PE bound to the modulatory lipid site. This lipid–protein interaction may also be inherently dependent on the solution ionic strength, which would account for the attenuation of the conductance-enhancing effect of PS and PI at high KCl concentration (Fig. 2).
Case II: A General Effect of Membrane Curvature Stress
Lipids are known to participate in many complex molecular interactions that are considered to be important for understanding membrane–protein interactions. Some of the phenomena associated with physical properties of lipids include: temperature-dependent liquid-crystalline to gel phase transitions, lateral separation of phases and lipid domain formation, changes in bilayer thickness resulting in hydrophobic mismatch between membrane proteins and the bilayer, and nonlamellar phase preferences of certain lipids such as inverted hexagonal or cubic phases (Dowhan, 1997; Epand, 1998; Gil et al., 1998; Killian, 1998; Rietveld and Simons, 1998). Changes in lipid packing associated with curvature stress and bilayer deformation energy are thought to account for large changes in the probability of channel formation by the model peptides, gramicidin and alamethicin, that occur when the elastic stress of the bilayer is altered by various experimental manipulations (Keller et al., 1993; Lundbaek et al., 1996, 1997; Bezrukov et al., 1998; Andersen et al., 1999; Bezrukov, 2000).
For example, the observed lifetime of the dimeric gramicidin channel decreases with treatments that promote negative (concave) monolayer curvature such as addition of PE, cholesterol, or limonene, and increased proton or divalent cation concentration for PS-containing bilayers (Lundbaek et al., 1996, 1997; Andersen et al., 1999). Conversely, the gramicidin lifetime increases with treatments that promote positive (convex) monolayer curvature, such as substitution of PC for PE, or addition of Triton X-100 or β-octylglucoside (Lundbaek et al., 1996, 1997; Andersen et al., 1999). In the case of alamethicin, a 20-residue peptide that forms channels by an aggregation mechanism, the probability of observing higher conductance states is dramatically increased as a function of the mole fraction of PE in bilayers formed from a binary mixture of PC and PE (Keller et al., 1993). This effect is thought to result from an increase in negative membrane curvature stress since PE is a comparatively cone-shaped lipid that favors formation of inverted hexagonal (HII) phases in relation to PC, a more cylindrically shaped lipid that favors bilayer formation. Interestingly, the probability of occurrence of higher conductance states of alamethicin is also observed as a function of decreasing solution pH for bilayers formed from PS (Bezrukov et al., 1998). This latter effect of pH correlates with protonation of the anionic PS headgroup and the accompanying reduction in negative surface charge. The decrease in headgroup repulsion concomitant with protonation of PS also increases negative curvature stress due to a relative change in lipid shape (i.e., protonated PS has a more conical average shape relative to the cylindrical PS anion subject to headgroup repulsion) that favors HII phase formation (Bezrukov et al., 1998).
The preceding two examples of the effect of elastic stress or membrane deformation energy on the probability of formation of model peptide channels provide certain ideas that may be useful for interpreting the effect of lipids on the BK channel. If different conformational states of the BK channel protein involve changes in shape or length of the hydrophobic membrane-spanning region, then functions of the channel (such as gating or conductance) associated with these shape changes would automatically be coupled to lipid-packing stress. Substitution of lipids that change the membrane curvature stress will thus promote changes in BK conductance or gating.
Specifically, in the present case, bilayers composed of 80:20 mixtures of PS:PE or PI:PE would be expected to have lower negative curvature stress than a bilayer composed of 100% PE, an HII phase–preferring lipid. The lower negative curvature stress of PS- and PI-containing bilayers may favor a “high flux” conformation of the selectivity filter, especially in KCl solutions at low ionic strength. As ionic strength is increased at higher KCl concentration, the reduction in head group repulsion of anionic lipids would tend to reduce the shape differences between PS, PI, and PE and diminish the lipid dependence of unitary conductance as observed in the experimental results for the BK channel in Fig. 2.
Although coupling of conformational changes associated with channel formation to changes in bilayer curvature stress seem to explain numerous phenomena of model peptide channels such as gramicidin and alamethicin, one may question the relevance of this concept to large integral membrane proteins. The relevance may depend on whether there are substantial changes in the shape or length of the hydrophobic-spanning region of channel proteins in different conformational states of ion conduction or gating. Recently, new insight to this question was glimpsed with the crystal structure of a prokaryotic Ca2+-activated K+ channel, MthK (Jiang et al., 2002a,b). This structure of this latter K+ channel, captured in the open channel conformation relative to the closed conformation of KcsA, demonstrates that there are indeed significant shape changes of the bilayer-spanning region of K+ channel proteins in the transition from closed (nonconducting) to open (conducting) conformations. In particular, it can be inferred that movement of the inner M2 helix at a gating-hinge site results in a dramatic change in shape of the K+ channel pore domain from a conically shaped form in the closed state (deriving from the “teepee” architecture described by Doyle et al. [1998]) to a more cylindrical shape in the open state (e.g., Fig. 6 of Yifrach and MacKinnon, 2002). Given such large apparent conformational changes in shape of the pore domain located within the bilayer, it follows that bilayer mechanical properties such as curvature stress may play a significant role in modulating of the conductance and gating of K+ channels. Further evidence for this possibility comes from fluorescence studies of KcsA reconstituted into PC bilayers of different acyl chain length (Williamson et al., 2002). This work suggests that the tilt angles of the transmembrane helix of KcsA decrease as a function of the thickness of the hydrophobic bilayer core. A mechanism based on matching the hydrophobic length of the protein to the lipid core could also be an important aspect of functional modulation of K+ channels.
Although the present study is primarily focused on the effect of anionic lipids on K+ conductance and Ba2+ block, our reexamination of the surface charge hypothesis probably also applies to the phenomenon of activation of BK channel gating by PS vs. PE, originally interpreted as an electrostatic enhancement of local Ca2+ concentration near the binding sites involved in Ca2+ activation (Moczydlowski et al., 1985). If we consider this later gating phenomenon in terms of bilayer mechanical properties rather than as a surface charge effect on Ca2+ binding, enhancement of the opening probability of the BK channel by PS may also be reinterpreted as an energetic stabilization of the cylindrically shaped open state versus the conically shaped closed state of the channel protein in a bilayer phase lipid such as PS versus an HII phase lipid such as PE.
Physiological Significance
Various pieces of evidence indicate that membrane lipids are powerful modulators of the BK channel gating process. Studies of the activation of smooth muscle BK channels by certain fatty acids, such as arachidonic and myristic acid, have concluded that such charged lipids promote channel opening by direct interaction with the channel protein rather than by an alteration of the membrane electric field or local electrostatic attraction of cations (Clarke et al., 2002, 2003). Studies of brain BK channels in planar bilayers found differences in mean channel open time depending on the size of the phospholipid head groups and sterol composition in bilayers formed from PE, PC, and cholesterol (Chang et al., 1995a,b). Addition of 11% cholesterol to a PE/PS bilayer was also found to reduce BK unitary conductance by 7% (Chang et al., 1995b). The activity of BK channels is also affected by membrane perturbants such as ethanol (Chu et al., 1998; Walters et al., 2000). In recent years a number of membrane channels and transport proteins have been found to be activated or inhibited by trivalent anionic lipid, phosphatidylinositol-4,5-bisphosphate (PIP2) (Hilgemann et al., 2001). The biophysical mechanism of PIP2 activation of inward rectifier K+ channels by PIP2 has not been completely elucidated; however, there is evidence that inositol lipids selectively bind to NH2- and COOH-terminal regions of the channel protein (Huang et al., 1998; Shyng and Nichols, 1998; Rohács et al., 2003). Membrane–channel interactions are also implicated in mechanisms underlying general anesthesia. For example, the volatile anesthetic isoflurane increases the unitary conductance of the voltage-activated Shaker channel by ∼20% (Li and Correa, 2001). Such examples suggest that interactions of lipids and lipid-soluble hydrophobic molecules with ion channels may be involved in many important physiological and pharmacological phenomena. Although our work leads us to doubt the functional importance of a direct effect of lipid surface charge on local K+ or Ca2+ concentration, mechanisms based on specific binding of lipid molecules or bilayer mechanical properties offer alternative paradigms for investigating the basis of such phenomena.
In retrospect, an indiscriminate electrostatic mechanism for control of ion channel activities by an effect of charged lipids on local ion concentrations could be considered impractical or even dangerous to cellular homeostasis. If the physical dimensions of most ion channel proteins were small enough so that the conduction process was under the direct influence of lipid surface potential, then large changes in ionic flux and excitability could result from a relatively small changes in membrane phospholipid composition and distribution. Since membrane proteins reside in a heterogeneous environment where rapid diffusion of lipids and hydrophobic solutes in the membrane results in a constant exposure to these substances at the protein–membrane boundary, such proteins must have means of protection from untoward electrostatic and molecular interactions. Electrostatic insulation based on the lateral separation of a central pore from charged lipid head groups probably provides built-in protection from many types of nonspecific surface charge effects. On the other hand, modulation by distinct chemical species of lipids or changes in bilayer physical properties offers the potential for individual isoforms of channel proteins to evolve greater specificity and selectivity with respect to functional changes that govern activity.
Summary and Predictions of New Models
In this study, we reexamined the mechanism of enhancement of unitary conductance of BK channels in PS vs. PE bilayers using block by Ba2+ and TEA+ as a probe of local cation concentration in the vicinity of the outer and inner entryways of the channel. Based on large disparities between the estimated magnitude of surface potential acting on ion conduction and blocking processes, it is unlikely that anionic lipids enhance conductance by increasing the local concentration of K+ near the channel vestibules. We propose that the effect of PS is produced by lipid-dependent tuning of the conformation of the channel protein. We outline two alternative hypotheses to explain the effect of negatively charged lipids. In the first mechanism, PS may bind to specific lipid modulatory sites on the BK channel and enhance conductance by acting as an effector of a “high flux” conformation of the selectivity filter through an allosteric ligand–protein interaction. In the second case, substitution of PS for PE could similarly stabilize a high conductance conformation of the BK channel protein through a change in bilayer mechanical properties such curvature stress. These two hypotheses make different predictions that are amenable to testing. In the first case, demonstration of specific lipid binding site(s) may be achieved by structural and mutational studies. In the second case, correlations between conductance modulation and membrane elastic stress may be explored by systematically examining the effect of chemical perturbants known to alter curvature stress or bilayer deformation energy.
Acknowledgments
The authors would like to thank Ramon Latorre and Rod MacKinnon for some charged discussions that led to this work.
This work was supported by grant awards to E. Moczydlowski from the U.S. National Institutes of Health (P01 NS42202) and the American Heart Association (0150058N) and to P.D. Ryu from Korea Science and Engineering Foundation (KOSEF 941-0700-002-2).
Olaf S. Andersen served as editor.
Jin Bong Park's present address is Department of Physiology, College of Medicine, Chungnam National University, Taejeon 301-131, Republic of Korea.
Footnotes
Abbreviations used in this paper: GCS, Gouy-Chapman-Stern theory; PC, phosphatidylcholine; PE, phospatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine.
References
- Andersen, O.S. 1989. Kinetics of ion movement mediated by carriers and channels. Methods Enzymol. 171:62–112. [DOI] [PubMed] [Google Scholar]
- Andersen, O.S., C. Nielsen, A.M. Maer, J.A. Lundbaek, M. Goulian, and R.E. Koeppe. 1999. Ion channels as tools to monitor lipid-bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 294:208–224. [DOI] [PubMed] [Google Scholar]
- Apell, H.-J., E. Bamberg, and P. Läuger. 1979. Effects of surface charge on the conductance of the gramicidin channel. Biochim. Biophys. Acta. 552:369–378. [DOI] [PubMed] [Google Scholar]
- Becker, M.D., R.E. Koeppe II, and O.S. Andersen. 1992. Amino acid substitutions and ion channel function: model dependent conclusions. Biophys. J. 62:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, J.E., and C. Miller. 1984. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys. J. 45:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tal, N., B. Honig, C. Miller, and S. McLaughlin. 1997. Electrostatic binding of proteins to membranes. Theoretical predictions and experimental results with charybdotoxin and phospholipid vesicles. Biophys. J. 73:1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2001. Energetics of ion conduction through the K+ channel. Nature. 414:73–77. [DOI] [PubMed] [Google Scholar]
- Bezrukov, S.M. 2000. Functional consequences of lipid packing stress. Curr. Opin. Coll. Interface Sci. 5:237–243. [Google Scholar]
- Bezrukov, S.M., R.P. Rand, I. Vodyanoy, and V.A. Parsegian. 1998. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 111:173–183. [DOI] [PubMed] [Google Scholar]
- Brelidze, T.I., X. Niu, and K.L. Magleby. 2003. Limits and contributing mechanisms for big single-channel currents in BK channels. Biophys. J. 84:92a. [Google Scholar]
- Butler, A., S. Tsunoda, D.P. McCobb, A. Wei, and L. Salkoff. 1993. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 261:221–224. [DOI] [PubMed] [Google Scholar]
- Cecchi, X., D. Wolff, O. Alvarez, and R. Latorre. 1987. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle. Biophys. J. 52:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.M., R. Reitstetter, and R. Gruener. 1995. a. Lipid-ion channel interactions: increasing phospholipid headgroup size but not ordering acyl chains alters reconstituted channel behavior. J. Membr. Biol. 145:13–19. [DOI] [PubMed] [Google Scholar]
- Chang, H.M., R. Reistetter, R.P. Mason, and R. Gruener. 1995. b. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as unifying concept. J. Membr. Biol. 143:51–63. [DOI] [PubMed] [Google Scholar]
- Chu, B., A.M. Dopico, J.R. Lemos, and S.N. Treistman. 1998. Ethanol potentiation of calcium-activated potassium channels reconstituted into planar lipid bilayers. Mol. Pharmacol. 54:397–406. [DOI] [PubMed] [Google Scholar]
- Clarke, A.L., S. Petrou, J.V. Walsh, and J.J. Singer. 2002. Modulation of BKCa channel activity by fatty acids and other charged lipids: structural requirements and mechanism of action. Am. J. Physiol. Cell Physiol. 283:C1441–C1453. [DOI] [PubMed] [Google Scholar]
- Clarke, A.L., S. Petrou, J.V. Walsh, and J.J. Singer. 2003. Site of action of fatty acids and other charged lipids on BKCa channels from arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 284:C607–C619. [DOI] [PubMed] [Google Scholar]
- Coronado, R., and H. Affolter. 1986. Insulation of the conduction pathway of muscle transverse tubule calcium channels from the surface charge of bilayer phospholipid. J. Gen. Physiol. 87:933–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman, S. 1991. Asymmetric electrostatic effects on the gating of rat brain sodium channels in planar lipid membranes. Biophys. J. 60:845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman, S., W.C. Zinkand, and R.J. French. 1988. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. J. Gen. Physiol. 92:431–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan, W. 1997. Molecular basis of membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199–232. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais-Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Eisenberg, M., T. Gresalfi, T. Riccio, and S. McLaughlin. 1979. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 18:5213–5223. [DOI] [PubMed] [Google Scholar]
- Epand, R.M. 1998. Lipid polymorphism and protein-ligand interactions. Biochim. Biophys. Acta. 1376:353–368. [DOI] [PubMed] [Google Scholar]
- Escobar, L., M.J. Root, and R. MacKinnon. 1993. Influence of protein surface charge on the bimolecular kinetics of a potassium channel peptide inhibitor. Biochemistry. 32:6982–6987. [DOI] [PubMed] [Google Scholar]
- Finkelstein, A., and O.S. Andersen. 1981. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J. Membr. Biol. 59:155–171. [DOI] [PubMed] [Google Scholar]
- Ferguson, W.B. 1991. Competitive Mg2+ block of a large-conductance, Ca2+-activated K+ channel in rat skeletal muscle: Ca2+, Sr2+, and Ni2+ also block. J. Gen. Physiol. 98:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca, V., P. Daumas, L. Ranjalahy-Rasoloarijoa, F. Heitz, R. Lazaro, Y. Trudelle, and O.S. Andersen. 1992. Gramicidin channels that have no tryptophan residues. Biochemistry. 31:5340–5350. [DOI] [PubMed] [Google Scholar]
- Fyfe, P.K., K.E. McAuley, A.W. Roszak, N.W. Isaacs, R.J. Cogdell, and M.R. Jones. 2001. Probing the interface between membrane proteins and membrane lipids by X-ray crystallography. Trends Biochem. Sci. 26:106–112. [DOI] [PubMed] [Google Scholar]
- Gil, T., J.H. Ipsen, O.G. Mouritsen, M.C. Sabra, M.M. Sperotto, and M.J. Zuckermann. 1998. Theoretical analysis of protein organization in lipid membranes. Biochim. Biophys. Acta. 1376:245–266. [DOI] [PubMed] [Google Scholar]
- Girshman, J., D.V. Greathouse, R.E. Koeppe, and O.S. Andersen. 1997. Gramicidin channels in phospholipid bilayers with unsaturated acyl chains. Biophys. J. 73:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame, D. 1947. The electric double layer and the theory of electrocapillarity. Chem. Rev. 41:441–501. [DOI] [PubMed] [Google Scholar]
- Green, W.N., and O.S. Andersen. 1991. Surface charges and ion channel function. Annu. Rev. Physiol. 53:341–359. [DOI] [PubMed] [Google Scholar]
- Green, W.N., L.B. Weiss, and O.S. Andersen. 1987. a. Batrachotoxin-modified sodium channels in planar bilayers: ion permeation and block. J. Gen. Physiol. 89:841–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, W.N., L.B. Weiss, and O.S. Andersen. 1987. b. Batrachotoxin-modified sodium channels in planar bilayers: characterization of saxitoxin- and tetrodotoxin-induced channel closures. J. Gen. Physiol. 89:841–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X., A. Uehara, A. Ravindran, S.H. Bryant, S. Hall, and E. Moczydlowski. 1987. Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry. 26:7546–7556. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D.W., S. Feng, and C. Nasuhoglu. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001:RE19. [DOI] [PubMed]
- Hille, B. 2001. Ion Channels of Excitable Membranes. Sinauer, Sunderland, MA. 814 pp.
- Honig, B., and A. Nicholls. 1995. Classical electrostatics in biology and chemistry. Science. 268:1144–1149. [DOI] [PubMed] [Google Scholar]
- Huang, C.-L., S. Feng, and D.W. Hilgemann. 1998. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 391:803–806. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., and R. MacKinnon. 2000. The barium site in a potassium channel by X-ray crystallography. J. Gen. Physiol. 115:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. a. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. b. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Keller, S.L., S.M. Bezrukov, S.M. Gruner, M.W. Tate, I. Vodyanoy, and V.A. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian, J.A. 1992. Gramicidin and gramicidin-lipid interactions. Biochim. Biophys. Acta. 1113:391–425. [DOI] [PubMed] [Google Scholar]
- Killian, J.A. 1998. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1376:401–416. [DOI] [PubMed] [Google Scholar]
- Latorre, R., P. Labarca, and D. Naranjo. 1992. Surface charge effects on ion conduction in ion channels. Methods Enzymol. 207:471–501. [DOI] [PubMed] [Google Scholar]
- LeMasurier, M., L. Heginbotham, and C. Miller. 2001. KcsA: it's a potassium channel. J. Gen. Physiol. 118:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., and A.M. Correa. 2001. Single-channel basis for conductance increase induced by isoflurane in Shaker H4 IR K+ channels. Am. J. Physiol. Cell Physiol. 280:C1130–C1139. [DOI] [PubMed] [Google Scholar]
- Loosley-Millman, M.E., R.P. Rand, and V.A. Parsegian. 1982. Effect of monovalent ion binding and screening and measured electrostatic forces between charged phospholipid bilayers. Biophys. J. 40:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek, J.A., P. Birn, J. Girshman, A.J. Hansen, and O.S. Andersen. 1996. Membrane stiffness and channel function. Biochemistry. 35:3825–3830. [DOI] [PubMed] [Google Scholar]
- Lundbaek, J.A., A.M. Maer, and O.S. Andersen. 1997. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry. 36:5695–5701. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., R. Latorre, and C. Miller. 1989. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 28:8092–8099. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., and C. Miller. 1989. Functional modification of a Ca2+-activated K+ channel by trimethyloxonium. Biochemistry. 28:8087–8092. [DOI] [PubMed] [Google Scholar]
- McLaughlin, S. 1977. Electrostatic potentials at membrane-solution interfaces. Curr. Top. Membr. Trans. 9:71–144. [Google Scholar]
- McLaughlin, S. 1989. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 18:113–136. [DOI] [PubMed] [Google Scholar]
- McLaughlin, S., N. Mulrine, T. Gresalfi, B. Vaio, and A. McLaughlin. 1981. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J. Gen. Physiol. 77:445–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, S.G.A., G. Szabo, G. Eisenman, and S.M. Ciani. 1970. Surface charge and conductance of phospholipid membranes. Proc. Natl. Acad. Sci. USA. 67:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera, P., M. Wallner, M. Song, and L. Toro. 1997. Large conductance voltage- and calcium dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), and extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc. Natl. Acad. Sci. USA. 94:14066–14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski, E., O. Alvarez, C. Vergara, and R. Latorre. 1985. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J. Membr. Biol. 83:273–282. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Neyton, J. 1996. A Ba2+ chelator suppresses long shut events in fully activated high-conductance Ca2+-dependent K+ channels. Biophys. J. 71:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton, J., and C. Miller. 1988. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J. Gen. Physiol. 92:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean, C., J. Chappie, and C. Miller. 2003. Why are BK channels so big? Biophys. J. 84:8a. [Google Scholar]
- Park, J.B., and P.D. Ryu. 1998. Increased activity of large conductance Ca2+-activated K+ channels in negatively-charged lipid membranes. Korean J. Physiol. Pharmacol. 2:529–539. [Google Scholar]
- Popot, J.-L., and D.M. Engleman. 2000. Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 69:881–922. [DOI] [PubMed] [Google Scholar]
- Ravindran, A., and E. Moczydlowski. 1989. Influence of negative surface charge on toxin binding to canine heart Na channels in planar bilayers. Biophys. J. 55:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, A., L. Schild, and E. Moczydlowski. 1991. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin: Zn2+ induces discrete substates in cardiac Na+ channels. J. Gen. Physiol. 97:89–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta. 1376:467–479. [DOI] [PubMed] [Google Scholar]
- Rohács, T., C.M.B. Lopes, T. Jin, P.P. Ramdya, Z. Molnar, and D.E. Logothetis. 2003. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. USA. 100:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva, T.K., V.M. Aguilella, I. Vodoyany, S. Bezrukov, and V.A. Parsegian. 1998. Membrane surface-charge titration probed by gramicidin A channel conductance. Biophys. J. 75:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng, S.-L., and C.G. Nichols. 1998. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 282:1138–1141. [DOI] [PubMed] [Google Scholar]
- Sohma, Y., A. Harris, C.J.C. Wardle, B.E. Argent, and M.A. Gray. 1996. Two barium binding sites on a maxi K+ channel from human vas deferens epithelial cells. Biophys. J. 70:1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, M., and C. Miller. 1994. Electrostatic distance geometry in a K+ channel vestibule. Proc. Natl. Acad. Sci. USA. 91:9509–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara, I. 1998. Activation and two modes of blockade by strontium of Ca2+-activated K+ channels in goldfish saccular hair cells. J. Gen. Physiol. 111:363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnheim, K., J. Gruber, C. Wachter, and V. Ruiz-Gutierrez. 1999. Membrane phospholipid composition affects function of potassium channels from rabbit colon epithelium. Am. J. Physiol. 277:C83–C90. [DOI] [PubMed] [Google Scholar]
- Valiyaveetil, F.I., Y. Zhou, and R. MacKinnon. 2002. Lipids in the structure, folding, and function of the KcsA channel. Biochemistry. 41:10771–10777. [DOI] [PubMed] [Google Scholar]
- Van Dalen, A., S. Hegger, J.A. Killian, and B. de Kruijff. 2002. Influence of lipids on assembly and stability of the potassium channel, KcsA. FEBS Lett. 525:33–38. [DOI] [PubMed] [Google Scholar]
- Vergara, C., and R. Latorre. 1983. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers: evidence for a Ca2+ and Ba2+ blockade. J. Gen. Physiol. 82:543–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel, A., O. Alvarez, A. Oberhauser, and R. Latorre. 1988. Probing a Ca2+-activated K+ channel with quaternary ammonium ions. Pflugers Arch. 413:118–126. [DOI] [PubMed] [Google Scholar]
- Walters, F.S., M. Covarrubias, and J.S. Ellingson. 2000. Potent inhibition of the aortic smooth muscle maxi-K channel by clinical doses of ethanol. Am. J. Physiol. Cell Physiol. 279:C1107–C1115. [DOI] [PubMed] [Google Scholar]
- Williamson, I.M., S.J. Alvis, J.M. East, and A.G. Lee. 2002. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 83:2026–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf, T.B., and B. Roux. 1996. Structure, energetics, and dynamics of lipid-protein interactions: a molecular dynamics study of the gramicidin A channel in a DMPC bilayer. Proteins. 24:92–114. [DOI] [PubMed] [Google Scholar]
- Worley, J.F., R.J. French, and B.K. Krueger. 1986. Trimethyloxonium modification of single batrachotoxin-activated sodium channels in planar bilayers: changes in unit conductance and in block by saxitoxin and calcium. J. Gen. Physiol. 87:327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley, J.F., R.J. French, B.A. Pailthorpe, and B.K. Krueger. 1992. Lipid surface charge does not influence conductance or calcium block of single sodium channels in planar bilayers. Biophys. J. 61:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yifrach, O., and R. MacKinnon. 2002. Energetics of pore opening in a voltage-gated K+ channel. Cell. 111:231–239. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]