Abstract
The InsP3R proteins have three recognized domains, the InsP3-binding, regulatory/coupling, and channel domains (Mignery, G.A., and T.C. Südhof. 1990. EMBO J. 9:3893–3898). The InsP3 binding domain and the channel-forming domain are at opposite ends of the protein. Ligand regulation of the channel must involve communication between these different regions of the protein. This communication likely involves the interceding sequence (i.e., the regulatory/coupling domain). The single channel functional attributes of the full-length recombinant type-1, -2, and -3 InsP3R channels have been defined. Here, two type-1/type-2 InsP3R regulatory/coupling domain chimeras were created and their single channel function defined. One chimera (1-2-1) contained the type-2 regulatory/coupling domain in a type-1 backbone. The other chimera (2-1-2) contained the type-1 regulatory/coupling domain in a type-2 backbone. These chimeric proteins were expressed in COS cells, isolated, and then reconstituted in proteoliposomes. The proteoliposomes were incorporated into artificial planar lipid bilayers and the single-channel function of the chimeras defined. The chimeras had permeation properties like that of wild-type channels. The ligand regulatory properties of the chimeras were altered. The InsP3 and Ca2+ regulation had some unique features but also had features in common with wild-type channels. These results suggest that different independent structural determinants govern InsP3R permeation and ligand regulation. It also suggests that ligand regulation is a multideterminant process that involves several different regions of the protein. This study also demonstrates that a chimera approach can be applied to define InsP3R structure-function.
Keywords: inositol trisphosphate receptor, Ca2+ release channel, intracellular Ca2+ signaling
INTRODUCTION
The inositol 1,4,5-trisphosphate receptor (InsP3R)* genes encode intracellular Ca2+ release channels that are fundamental to many intracellular Ca2+-signaling processes in mammalian cells. The InsP3R Ca2+-release channels are found in intracellular membranes, predominately the ER. These channels mobilize intracellular Ca2+ in response to InsP3 generated by receptor-activated hydrolysis of phosphatidyl-inositol 4,5 bisphosphate in the inner leaflet of the surface membrane.
The InsP3Rs are part of a family of three homologous proteins. The InsP3R proteins have three recognized domains: the InsP3-binding, regulatory/coupling, and channel domains (Mignery and Südhof, 1990, 1993; Südhof et al., 1991; Blondel et al., 1993). The ligand binding and channel domains are relatively well conserved between the different InsP3R family members. The least conserved domain is the regulatory/coupling domain that contains several potential regulatory sites (including the putative Ca2+ binding region; Mignery et al., 1992; Sienaert et al., 1996, 1997). The regulatory/coupling domain physically links the amino-terminal InsP3-binding domain to the carboxyl-terminal channel domain.
One consequence of this structural heterogeneity is thought to be the isoform-specific functional differences between the different InsP3R family members observed in single channel studies (Watras et al., 1991; Perez et al., 1997; Hagar et al., 1998; Mak et al., 1998; Ramos-Franco et al., 1998a). A prominent isoform-specific functional attribute is how InsP3 and cytosolic Ca2+ regulate these channels. For example, the Ca2+ sensitivity of the type-1 and -2 InsP3R channels are quite different (Bezprozvanny et al., 1991; Ramos-Franco et al., 1998a). Under identical experimental conditions, one is sharply bell-shaped (type-1) while other is not (type-2). The type-1 and -2 channels are also regulated by InsP3 differently. The type-2 InsP3R has the higher InsP3 affinity (Südhof et al., 1991; Newton et al., 1994; Perez et al., 1997; Miyakawa et al., 2001) and higher InsP3 efficacy (Ramos-Franco et al., 1998a).
The function of single recombinant type-1 and -2 InsP3R channels has been defined and this function matches that of their native counterparts (Kaznacheyeva, et al., 1998; Ramos-Franco et al., 1998b, 2000; Mak et al., 2000; Boehning et al., 2001). The InsP3 binding site and the channel pore are in distinct regions at opposite ends of the protein. The cytosolic region encompassing the InsP3 binding domain region apparently undergoes a significant conformational change upon ligand binding (Mignery and Südhof, 1990). Despite the large linear separation of the InsP3 binding and channel domains, Boehning and Joseph (2000) reported that there may be a direct association of these two domains. Thus, ligand regulation of the pore may involve interactions with distant regions of the protein (considering the linear protein sequence). Intuitively, these interactions may depend on properties of the interceding sequence (i.e., the regulatory/coupling domain). Single channel data suggest that these interactions may be different in the different InsP3R isoforms.
To test this possibility, the single channel function of type-1 and type-2 InsP3R regulatory/coupling domain chimeras was defined here. Two domain-swap InsP3R chimeras were tested. One chimera contained the type-2 regulatory/coupling domain in a type-1 backbone (i.e., the 1-2-1 chimera). The other chimera contained the type-1 regulatory/coupling domain in a type-2 backbone (i.e., the 2-1-2 chimera). The ligand regulatory properties of the chimeric channels were analyzed and compared with their full-length progenitors.
MATERIALS AND METHODS
Materials
Lipids (phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine) were obtained from Avanti Polar Lipids, Inc. D-myo inositol 1,4,5-trisphosphate was purchased from LC Laboratories. Ryanodine was purchased from Calbiochem. All reagents and enzymes were of molecular biology grade.
Construction and Transfection of InsP3R Expression Vectors
The chimeras were constructed using the strategy described below. The type-1 ligand binding, type-2 coupling, and type-1 channel domain chimera (1-2-1) were constructed exploiting a unique Bst BI restriction site located at the beginning of the channel domain. The type-1 ligand-binding region (residues 1–607, nucleotides 236–2148) was PCR amplified (CGGAATTCAGATCGTCACCAAGGAGCTG and CCGGACGCGTGTGCTTCTCGAGGAGCTTTCGG) to introduce a unique XhoI restriction site without altering the amino acid sequence. This product was digested with EcoRI and MluI and ligated into similarly digested pCMV5 to generate the first intermediate plasmid (pA). Next nucleotides 2066–3418 of the InsP3R type-2 cDNA were PCR amplified to introduce a similar unique XhoI site using the following primers (CGCAAACTCCTCGAGAAACACATC and GCCTGAAGCACTTCTGC) and digested with XhoI and AflII. The full-length type-2 InsP3R cDNA (Südhof et al., 1991; Ramos-Franco et al., 2000) was digested with AflII-Sal I (vector site) and the 5.13-kbp fragment was gel purified. Intermediate plasmid A was digested with XhoI-SalI and ligated with the XhoI-AflII–digested PCR product and the AflII-Sal I fragment of pInsP3R-T2 to generate a second foundational plasmid (pB) consisting of the type-1 ligand binding domain and the type-2 coupling and channel domains (1-2-2).
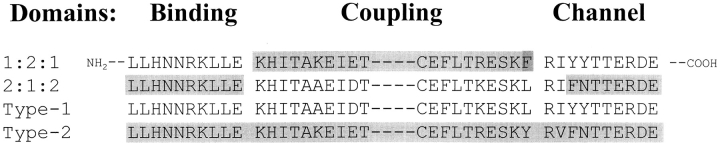
A third intermediate (pC) exploiting the unique Bst BI restriction site located at the beginning of the channel domain in the type 1 receptor was prepared. Specifically, the ligand-binding and regulatory/coupling type-2 sequence (residues 1–2,177) was inserted into the type-1 cDNA at residue 2225. This was accomplished by combining the ∼6.4 kbp EcoRI-HindIII (nucleotides 1–6383) fragment from the full-length type-2 cDNA (pInsP3R-T2) with a PCR product spanning the Hind III site at nucleotide 6383 and extending 3′ through nucleotide 6792 that had a BstBI site introduced at nucleotide 6779. The primers for this amplification were CGCCTCCAAGCTTCTGCTGGCC and CCGATTCTAGAGTTGAACATTCGAAATTTGGATTCCC. This product was digested with HindIII-XbaI and the two fragments were ligated to EcoRI-Xba I cut pCMV5. Intermediate pC was digested with Bst BI-Xba I and the 2.47 kbp BstBI-XbaI fragment of the type-1 receptor (pInsP3R-T1) was inserted to form a type-2 ligand binding/coupling/type-1 channel domain chimera (2–2-1, pD). The addition of the BstBI site at the 3′ end of the type-2 sequence resulted in the substitution of Y2178 to an F. The inter-domain linkages in the chimeras and native receptors are shown in Fig. 1.
Figure 1.
Amino acid sequences across the chimera junctional boundaries. Sequences of the type-1 and -2 wild-type sequences are shown for comparison. The heavily shaded residue (F) in the 1:2:1 chimera represents a spurious residue substituting the L residue normally in the type-1 receptor sequence that was introduced during the construction of this chimera.
The final assembly of the 1-2-1 chimera was accomplished by digesting pB with Bam HI and inserting the 2.85 kbp BamHI fragment of pD containing nucleotides 6630–6780 of the type-2 coupling domain and the type-1 channel domain (nucleotides 7000–9465).
The type-2 ligand binding and channel domains interspersed with the type 1 coupling/regulatory domain was constructed as follows. The type-1 InsP3R (pInsP3R-T1) was digested with BstBI-Nhe I and the resulting 11.33 kbp fragment was isolated. To this the Nhe I-BstBI fragment (nucleotides 7637–8535) of the type-2 receptor was inserted generating intermediate pE. The type-2 InsP3R cDNA piII-2 (Südhof et al., 1991) was linearized with BamHI and sequences spanning residues 6781–7637 were PCR amplified introducing a BstBI site at nucleotide 6781 (primers: CCAAATTTCGAATCTTCAACACAACGG and GGTCCTCTCGATGCCG). This fragment was digested with BstBI and inserted into BstBI-digested pE resulting in a 1-1-2 chimera (intermediate pF). Note, that in addition to introducing the Bst BI site in this PCR product, V2180 was changed to an I. This allows the type-2 channel domain to be introduced into intermediate pE such that there are no spurious residues introduced and the actual type-1/-2 junction begins between I2226 of the type-1 receptor and F2181 of the type-2 receptor. The full-length type-1 cDNA (pInsP3R-T1) EcoRI-Xba I fragment was inserted into similar sites of pGEM3Z in which the SphI site was abolished. Residues 236–4733 were excised by digestion with Eco RI-Sph I and the resulting vector fragment was ligated with a PCR product spanning nucleotides 2142–4746 that introduces an XhoI site spanning residues 606 and 607 of the type-1 receptor to generate intermediate pG. Next, nucleotides 1–2081 of the type-2 InsP3R cDNA were PCR amplified with the following oligonucleotides: GGAATTCGGGACGCAGAGGGAGCGC and CCGTGATGTGTTTCTCGAGGAGTTTGCG, which introduced a unique XhoI, site spanning residues 606 and 607. This PCR product was ligated into Eco RI-XhoI–digested intermediate pG at similar sites and then the entire EcoR I-Xba I fragment was inserted into pCMV5 to generate a 2-1-1 chimera (intermediate pH). The final assembly of the 2-1-2 chimera was achieved by digesting the 1-1-2 plasmid (pF) with EcoRI-XhoI and inserting the EcoRI-XhoI fragment of the 2-1-1 chimera (pH).
PCR products and junctional sequences in the chimeric constructs were determined by DNA sequence analysis using either a Sequenase 2.0 sequencing kit (Amersham Biosciences) or out-sourced to the Loyola University sequencing facility using an ABI Model 310 sequencer.
Equivalent numbers of COS-1 cells were transiently transfected with the chimeric cDNA using the DEAE-dextran method. Cells were incubated at 37°C (5% CO2). Typical transfection efficiencies were generally ≥60% as determined by indirect immunofluoresence or via a green fluorescent reporter.
CHAPS Solubilization and Gradient Sedimentation
The transfected COS-1 cells were harvested 48–72 h posttransfection and microsomes were prepared following established procedures (Mignery et al., 1990; Ramos-Franco et al., 1998b, 2000). Briefly, cells were washed with PBS and scrapped into 50 mM Tris-HCl pH 8.3, 1 mM EDTA, 1 mM 2-mercaptoethanol, 1 mM PMSF, and lysed by 40 passages through a 27-gauge needle. Membranes were pelleted by a 20-min centrifugation (289,000 g), resuspended in buffer, and frozen at −80°C. Microsomal fractions were solubilized in 50 mM Tris-HCl pH 8.3, 1 mM EDTA, 1 mM 2-mercaptoethanol, 1 mM PMSF, 1.8% CHAPS on ice for 1 h. Insoluble fractions were eliminated by a 10-min centrifugation at 289,000 g. The supernatant containing solubilized channel complex were fractionated through 5–20% continuous sucrose (wt/vol) gradients. Gradient fractions containing the chimeric InsP3R channel complex were then identified by immunoblotting and analyzed by 5% sodium dodecyl-sulfate PAGE (SDS-PAGE) as described previously (Mignery et al., 1990). Gradient fractions containing recombinant receptor protein were reconstituted into proteoliposomes as described previously (Perez et al., 1997; Ramos-Franco et al., 1998a).
SDS-PAGE and Immunoblotting
SDS-PAGE and immunoblotting were performed as described (Mignery et al., 1990; Ramos-Franco et al., 1998b, 2000) using 5% discontinuous polyacrylamide gels and antipeptide antibodies directed against the amino and carboxyl termini of both the type-1 and -2 InsP3 receptors. The antibodies were generated in rabbits against the following sequences: T1NH = CLATGHYLAAEVDPDQDASR; T1C = CRIGLLGHPPHMNVNPQQPA; T2NH = CPDYRDAQNEGKTVRDGELP; T2C = CNKQRLGFLGSNTPHENHHMPPH and were affinity purified against immunogenic peptide.
Single Channel Assay
Planar lipid bilayers were formed across a 150-μm diameter aperture in the wall of a Delrin partition as described previously (Ramos-Franco et al., 1998b, 2000). Lipid bilayer–forming solution contained a 7:3 mixture of phosphatidylethanolamine and phosphatidylcholine dissolved in decane (50 mg/ml). Proteoliposomes were added to the solution on one side of the bilayer (defined as cis-chamber). The other side was defined as the transchamber and was held at virtual ground. Standard solutions contained 220 mM CsHEPES cis (20 mM trans), pH 7.4, and 1 mM EGTA ([Ca2+]FREE = 200 nM). The [Ca2+]FREE was verified using a Ca2+ electrode. The Ca2+ electrodes contained the Ca-ligand ETH 129 in a polyvinylchloride membrane at the end of small (2 mm) polyethylene tube. These Ca2+ minielectrodes were made and used as described previously (Baudet et al., 1994). A standard patch clamp amplifier and D/A system were used for single-channel recording. The method used to estimate the number of reconstituted channels relied on the principles of stochastic interpretation of ion channel gating as outlined by Colquhoun and Hawkes (1981). In other words, it relied on the probability of multichannel opening events in long recordings (a classic approach).
The proteoliposome preparations consist of overexpressed InsP3R protein that has been CHAPS-solubilized and sedimented on sucrose gradients before its reconstitution into proteoliposomes. Consequently, most potential contaminating channel proteins are lost and the contribution of endogenous InsP3R dramatically diluted. The channels tested had permeation properties identical to those of native and recombinant InsP3R channels (Ramos-Franco et al., 1998a,b, 2000).
Acquisition and analysis software were obtained from Axon Instruments, Inc. Single channel data were digitized at 5–10 kHz and filtered at 2 kHz. Ligands (Ca2+ and InsP3) were applied in the cis-chamber at the concentrations specified in the text. Ryanodine was added in control experiments (unpublished data) to test that reconstituted channels were not ryanodine receptor channels. Chimeric InsP3R channel sidedness was determined by InsP3 sensitivity. The InsP3-sensitive side of the reconstituted channels was consistently in the cis-chamber compartment. Open probability and unitary current amplitude of the chimeric InsP3R channels was defined from Gaussian fitting of total amplitude histograms. Long duration (>2 min) single channel recordings were idealized using a conventional 50% detection criteria to identify open-closed transitions. Open and closed dwell time histograms were fit by two exponential components. Time constants and proportions of these components were pooled from several different channels and averaged. Data was statistically evaluated using a two-tailed T test.
RESULTS
Expression and Isolation of Chimeric InsP3R Channels
The amino-terminal end of the receptor forms the ligand binding domain and the carboxyl terminus, which harbors the multiple membrane spanning sequences responsible for subunit assembly (Galvan et al., 1999; Galvan and Mignery, 2002), forms the permeation pathway (Ramos-Franco et al., 1999). Interspersed between these two domains is the coupling or modulatory domain (nearly 1,700 amino acids) that undergoes an apparent conformational change upon InsP3 binding. This change may couple ligand occupancy to channel gating (Mignery and Südhof, 1990). In addition, this region contains several sequence motifs that may regulate the channel and include PKA-phosphorylation sites, ATP, calmodulin, and Ca2+ binding sites as well as alternative splicing events. Recently, electron microscopy of negatively stained InsP3R revealed that the protein also undergoes a conformational change in response to elevated Ca2+ and that this change may also be important to regulation of InsP3R channel activity (Hamada et al., 2002).
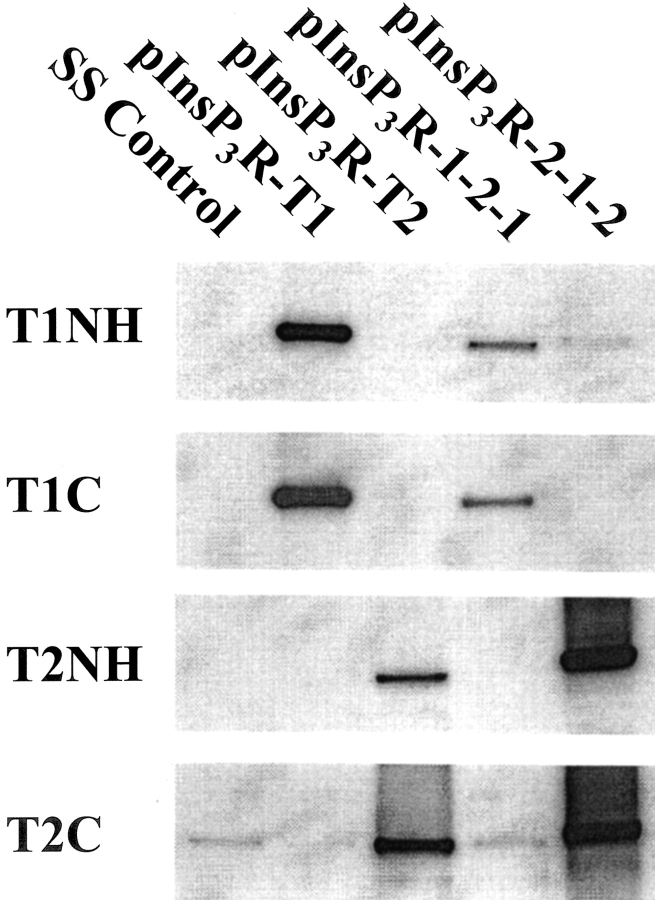
Two chimeric InsP3Rs were used in this study. The 1-2-1 chimera contains the type-2 coupling domain inserted in the type-1 backbone (i.e., type-1 binding and channel domains). The 2-1-2 chimera contains the type-1 coupling domain inserted in the type-2 backbone (i.e., type-2 binding and channel domains). The chimeric receptors were constructed such that the junctional sequences between heterologous domains were relatively conserved (see Fig. 1) . COS-1 cells were transiently transfected with the chimeric 1:2:1 and 2:1:2 expression constructs (Gorman, 1985). Cells were harvested 48–72 h posttransfection. Microsomal protein (10 μg) from the transfected cells was immunoblotted using type-1– and 2–specific amino- and carboxyl-terminal antibodies to confirm expression (Fig. 2) . The antibodies directed against the type-1 isoform (T1NH and T1C) reacted with only the 1-2-1 chimera and type-1 control, pInsP3R-T1 (panels T1NH and T1C). The type-2 antibodies (T2NH and T2C) only reacted to the 2-1-2 chimera or the full-length type-2 control, pInsP3R-T2 (panels T2NH and T2C). Both chimeric proteins expressed at significant levels. There was, however, some weak cross-reactivity observed in all lanes apparently due to endogenous type-2 or perhaps type-3 isoforms in the COS cells using T2C (bottom). The type-2 and -3 receptor isoforms share some sequence homology over the carboxyl terminus. Additionally, the chimeras integrity was evaluated for the ability to bind InsP3. The two chimeras bound similar levels of 3H InsP3 as their wild type progenitors (unpublished data) and together with the recognition of the recombinant proteins by the appropriate carboxyl-terminal antibodies, we concluded that the constructs were faithfully expressed as full-length products.
Figure 2.
Immunoblot analysis of chimera expression products from transiently transfected COS-1 cells. Microsomal protein (10 μg) from COS-1 cells transiently transfected with control (SS), the full-length type-1 and -2 (pInsP3R-T1, pInsP3R-T2 respectively), or the two chimeras pInsP3R-1:2:1 and pInsP3R-2:1:2 were electrophoresed on 5% SDS-PAGE and immunoblotted. The antibodies were directed against the type-1 and -2 receptor amino and carboxyl termini (T1NH, T1C, T2NH, and T2C).
The transfected COS-1 microsomal proteins were CHAPS solubilized and enriched on 5–20% sucrose linear gradients for incorporation into proteoliposomes. In all cases the chimeric proteins sedimented to positions on the sucrose gradients consistent with those of tetramers (unpublished data).
Permeation Properties of the Chimeric InsP3R Channels
Proteoliposomes containing the chimeric InsP3R channels were fused into artificial plannar lipid bilayers. The method used was identical to that applied previously to define the single channel function of the native and recombinant InsP3R channels (Perez et al., 1997; Ramos-Franco et al., 1998a,b). The single channel function the full-length type-1 and -2 recombinant InsP3R channels were previously compared by Ramos-Franco et al. (2000). The permeation properties of the type-1 and -2 recombinant channels were indistinguishable. This is consistent with the highly conserved nature of the InsP3R channel domain.
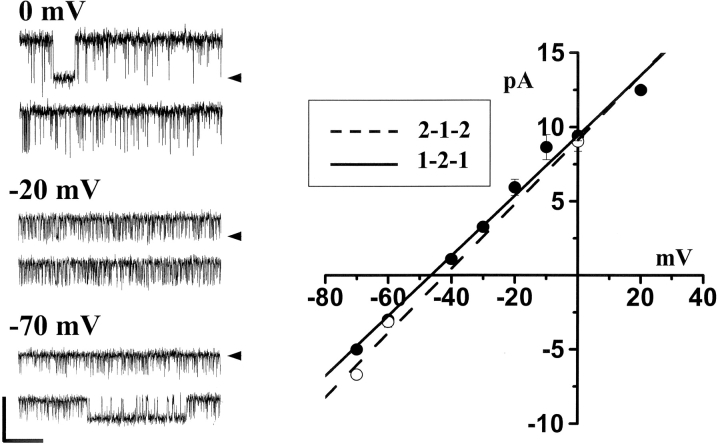
The permeation properties of the two chimeric channels were defined in solutions containing 220 mM CsHEPES cis (20 mM trans), pH 7.4, and 1 mM EGTA ([Ca2+]FREE = 200 nM). The cis-chamber solution also contained 1 μM InsP3. The channel was spontaneously active exhibiting frequent and rapid opening events. Sample single channel recordings obtained from the 1-2-1 chimeric channel at three different steady-state membrane potentials are presented in Fig. 3 (left). Open events at −70 mV are shown as downward deflections (zero current level marked). Open events at −20 and 0 mV are shown as upward deflections. Averaged unit current values obtained from both the 1-2-1 chimera (filled symbols; n = 9) and 2-1-2 chimera (open symbols; n = 8) are plotted as a function of membrane potential (Fig. 3, right). The slope conductances of the chimeric channels were nearly identical (∼220 pS). Unitary current reversed near −45 mV, indicating the chimeric channels were cation selective (i.e., permeating Cs+). These data are very similar to those obtained in similar studies on the recombinant full-length type-1 and -2 channels (Ramos-Franco et al., 1998b, 2000). The data is also similar to that obtained from native full-length type-1 and -2 channels (Ramos-Franco et al., 1998a). Thus, the permeation pathway of the chimeric channels functions quite normal retaining features common to their native and full-length recombinant progenitors.
Figure 3.
Unitary current-voltage plots. Solutions contained 220 mM CsHEPES cis (20 mM trans), pH 7.4, and 1 mM EGTA ([Ca2+]FREE = 200nM). The cis-chamber solution also contained 1 μM InsP3. Sample single 1-2-1 chimeric channel recordings at three different steady-state membrane potentials are presented. Calibration bar represents 10 pA and 200 ms. Zero current levels are marked. Mean unit current for 1-2-1 (filled symbols) and 2-1-2 (open symbols) chimeric channels are plotted. Error bars represent standard errors. The conductance of both channels was ∼220 pS.
InsP3 Sensitivity of the Chimeric InsP3R Channels
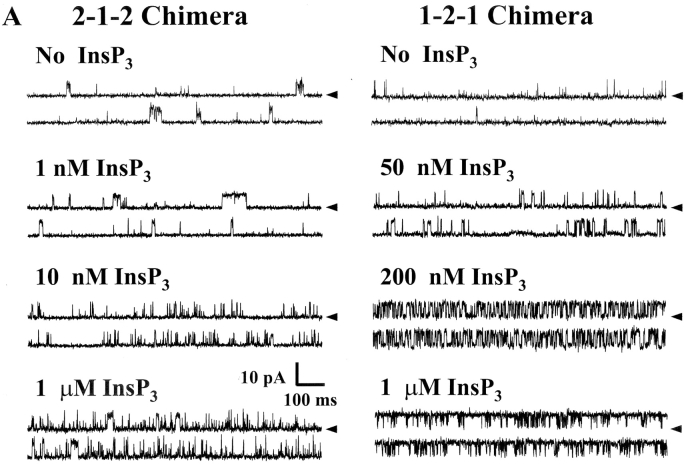
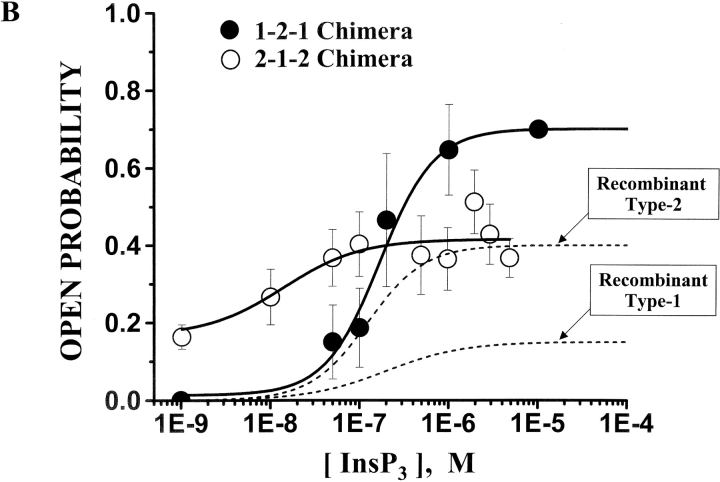
The InsP3 sensitivity of the chimeric channels was defined. Sample single channel recordings of both chimeras in the absence of InsP3 and three different InsP3 concentrations are shown in Fig. 4 A. These recordings were made at 0 mV and open events are shown as upward deflections. The current carrier was Cs+ and solutions contained 200 nM free Ca2+. The addition of InsP3 (cis-chamber) activated both chimeric channels. Application 1 μM InsP3 robustly activated the 1-2-1 chimera to high Po levels but activated the 2-1-2 chimera to a lesser extent. The relatively short single channel recordings shown (Fig. 4 A) were selected to illustrate relative changes in open probability (Po).
Figure 4.
InsP3 sensitivity of the chimeric channels. Solutions contained 200 nM free Ca2+ (1 mM total EGTA). (A) Sample single channel recordings from 2-1-2 and 1-2-1 chimeras in the absence of InsP3 and in three different InsP3 concentrations (marked). The holding potential was 0 mV and open events are upward deflections. The current carrier was Cs+ (220/20 mM) and InsP3 was added to the cis-chamber. (B) Mean Po of two chimeric channels are plotted as a function of InsP3 concentration. Data represent 11 different 2-1-2 channels and 8 different 1-2-1 channels. The EC50 of the 2-1-2 chimeric channel was 13.9 ± 38 nM (Hill coefficient = 1.27). The EC50 of the 1-2-1 chimeric channel was 261 ± 114 nM (Hill coefficient = 1.43). The InsP3 dose response curves of the full-length type-1 and -2 InsP3R channels are also shown (dashed lines; Ramos-Franco et al., 2000).
Mean steady-state Po of the two chimeric channels at different InsP3 concentrations is plotted in Fig. 4 B. These data represent the behavior of 11 different 2-1-2 channels and 8 different 1-2-1 channels. The dose of InsP3 required for half maximal activation (EC50) of the 2-1-2 chimeric channel was 13.9 ± 38 nM (Hill coefficient = 1.27). The EC50 of the 1-2-1 chimeric channel was 261 ± 114 nM (Hill coefficient = 1.43). These values are statistically different (P < 0.05). Previously published data collected on full-length recombinant type-1 (Ramos-Franco et al., 1998b) and type-2 (Ramos-Franco et al., 2000) InsP3R channels are presented as dashed lines. The EC50 of InsP3 activation of the full-length recombinant type-1 channel was 97 ± 66 nM. The EC50 full-length type-2 recombinant channel was 122 ± 100 nM. The InsP3 EC50 for the 1-2-1 chimera was in a range similar to that of the full-length recombinant channels. The InsP3 EC50 for the 2-1-2 chimera was shifted substantially to lower InsP3 concentrations.
Different efficacies of InsP3 in the activation of the chimeric channels were also noted. At very low InsP3 concentration (i.e., 1 nM, the lowest dose applied here), the 2-1-2 chimera had a Po of near 0.18. This was similar to the Po of the 2-1-2 chimera channel when no InsP3 was added. In contrast, the Po of the 1-2-1 chimera was near 0 at 1 nM InsP3 (or when no InsP3 was added). At high InsP3 concentrations (e.g., 1–10 μM InsP3), the 2-1-2 chimera had a maximal Po close to that of the full-length recombinant type-2 channel (Fig. 4 B, dashed line). The 1-2-1 chimera had a substantially higher maximal Po ∼2–4 fold higher than either of the full-length channels. Thus, InsP3 regulation of the pores of the chimeric channels is not identical to that of their full-length counterparts. The fact that InsP3 regulates their pores and that this regulation has certain similarities with wild-type InsP3 regulation implies that, (a) the ligand-binding domains in the chimeric channels modulate the interaction of InsP3 binding and channel domains and, (b) this interaction depends on the identity of the interceding coupling or modulatory sequence.
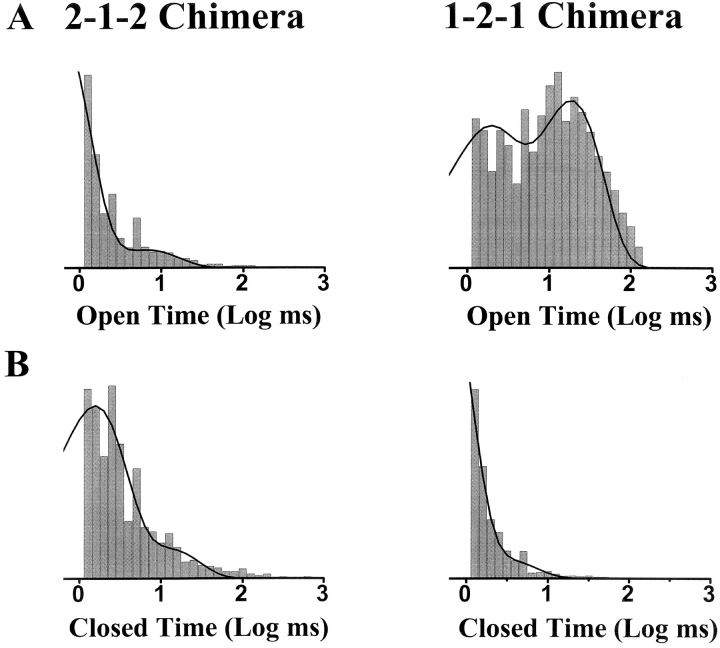
The gating kinetics (open and closed dwell times) of the two chimeric channels in the presence of 1 μM InsP3 are shown in Fig. 5 . Under these conditions, the 1-2-1 chimera was characterized by frequent long open events and short closed events. This was not the case for the 2-1-2 chimera. The gating kinetics of the two chimeras in the absence of InsP3 and at different InsP3 concentrations are summarized in Table I .
Figure 5.
Open and closed dwell time histograms. Sample open (A) and closed (B) logarithmic dwell time histograms (10 bins per decade) are shown. These histograms were generated from more than 15,000 events in the presence of 1 μM InsP3. The 2-1-2 chimera data were best fit by two exponential components with open time constants of 0.52 and 6.67 ms. The 2-1-2 chimera closed time constants were 1.49 and 11.65 ms. The 1-2-1 chimera data were best fit by two exponential components with open time constants of 1.40 and 18.2 ms. The 1-2-1 chimera closed time constants were 0.44 and 2.88 ms.
TABLE I.
Dwell Times of the 2-1-2 and 1-2-1 Chimeras
| 2-1-2 Chimera data1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| [InsP3] | τo1 | τo2 | τc1 | τc2 | ||||
| ms | ms | ms | ms | |||||
| 0 | 1.01 ± 0.04 (0.89) | 16.31 ± 7.24 (0.11) | 2.02 ± 0.09 (0.70) | 50.6 ± 19.51 (0.30) | ||||
| 50 nM | 1.43 ± 0.21 (0.86) | 21.49 ± 1.51 (0.14) | 3.05 ± 0.48 (0.71) | 57.78 ± 10.65 (0.29) | ||||
| 1 μM | 1.69 ± 0.50 (0.79) | 22.13 ± 11.79 (0.21) | 1.63 ± 0.56 (0.87) | 19.98 ± 7.75 (0.13) | ||||
| 1-2-1 Chimera data1 | ||||||||
| [InsP3] | τo1 | τo2 | τc1 | τc2 | ||||
| ms | ms | ms | ms | |||||
| 0 | 1.49 ± 0.31 (0.62) | 22.55 ± 14.03 (0.38) | 2.99 ± 1.22 (0.70) | 52.25 ± 14.62 (0.30) | ||||
| 50 nm | 1.33 ± 0.34 (0.64) | 11.45 ± 2.8 (0.36) | 2.74 ± 0.82 (0.43) | 47.95 ± 13.24 (0.57) | ||||
| 1 μM | 4.70 ± 1.25 (0.60) | 16.14 ± 3.05 (0.40) | 1.19 ± 0.22 (0.47) | 42.29 ± 41.51 (0.53) | ||||
Values represent means and standard deviations of three to five different channels.
The Ca2+ Sensitivity of the Chimeric InsP3R Channels
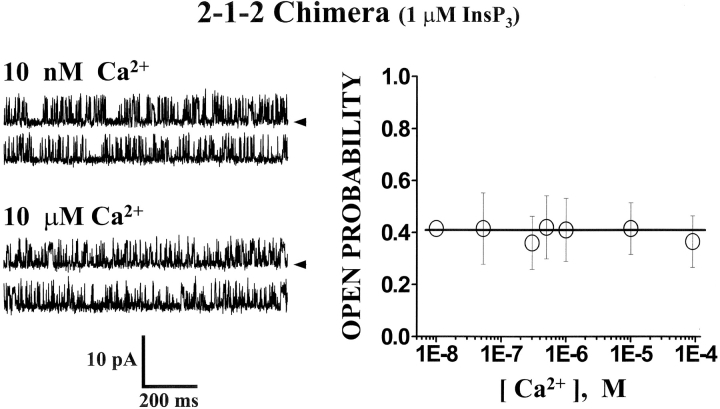
The Ca2+ sensitivity of the 2-1-2 chimeric channel is shown in Fig. 6 . These data were collected in the presence of 1 μM InsP3 (cis-chamber). Sample single channel recordings at two different free Ca2+ concentrations are presented (Fig. 6, left). The free Ca2+ concentration was adjusted by altering the Ca2+-EGTA mixture and verified using a Ca2+ electrode. Channel activity (Po) of the 2-1-2 chimeric channel was similar at 10 nM and 10 μM free Ca2+ (cis-chamber). Mean Po (± SE, n = 4) of the 2-1-2 chimeric channel at different free Ca2+ concentrations is plotted in Fig. 6 (right). These data show that the activity of the 2-1-2 chimera was not Ca2+ dependent over a wide free Ca2+ range (10 nM to 100 μM). This implies that the interceding type-1 regulatory/coupling domain (i.e., containing the putative Ca2+ regulatory sites) does not confer Ca2+ binding information to the surrounding type-2 backbone.
Figure 6.
Ca2+ sensitivity of the 2-1-2 chimeric channel. Sample single channel recordings from the 2-1-2 chimera are shown at two different Ca2+ concentrations (left) in the presence of 1 μM InsP3. The holding potential was 0 mV and open events are upward deflections. The current carrier was Cs+. Mean Po of the 2-1-2 chimeric channel is plotted as a function of Ca2+ concentration (right). Data points represent means (± SE). Data represent four different 2-1-2 chimeric channels.
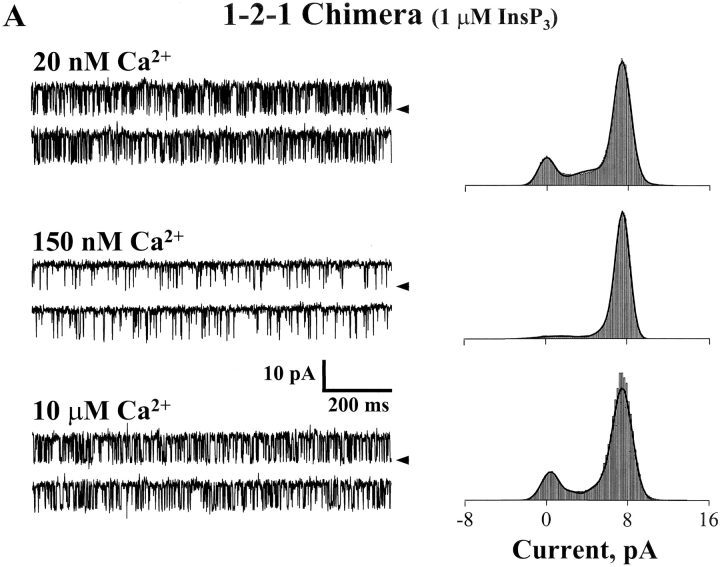
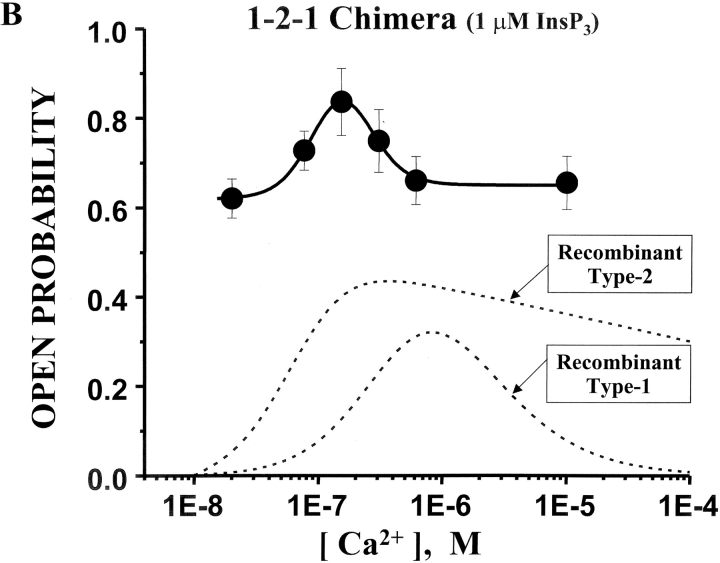
The Ca2+ sensitivity of the 1-2-1 chimeric channel is shown in Fig. 7 . These data were also collected in the presence of 1 μM InsP3. Sample single channel recordings at three different free Ca2+ concentrations are presented (Fig. 7 A, left). Channel activity (Po) of the 1-2-1 chimera at a low free Ca2+ concentration (20 nM) was relatively high (∼0.6). Compared with the either of the recombinant full-length channels (type-1 or -2; Ramos-Franco et al., 1998b, 2000), this is a usually high activity level under these conditions. From this apparently basal activity level (at 1 μM InsP3), the Po of the 1-2-1 chimera increased to ∼0.85 at intermediate free Ca2+ concentrations (150 nM) and then decreased again to ∼0.6 at higher Ca2+ concentrations (e.g., 10 μM). Mean Po (± SE, n = 7) of the 1-2-1 chimeric channel is plotted (solid symbols) as a function of free Ca2+ concentrations in Fig. 7 B. For comparison, the Ca2+ dependencies of the full-length recombinant type-1 and -2 InsP3R channels are also shown (Fig. 7 B, dashed lines). The full-length recombinant type-1 channel has the classical bell-shaped Ca2+ dependency (Ramos-Franco et al., 1998b). The full-length type-2 recombinant channel has a more sigmoidal Ca2+ dependency (Ramos-Franco et al., 2000). The Ca2+ dependency of the 1-2-1 chimera has a bell-shaped component, like that of the full-length type-1 channel, but this bell-shaped Ca2+ dependency arises from a substantially elevated basal Po level. Further, the peak of the bell-shaped component is shifted to lower Ca2+ concentrations compared with the full-length type-1 channel. Activation at such low Ca2+ levels is more reminiscent of the full-length type-2 than the type-1 channel. Thus, the interceding type-2 regulatory/coupling domain (i.e., containing the putative Ca2+ regulatory sites) does confer Ca2+ binding information to the surrounding type-1 backbone but in an atypical way. The Ca2+ dependency of the chimera has features associated with both type-1 (i.e., bell-shaped) and type-2 (i.e., high affinity) but also has features unlike either (i.e., high basal Po).
Figure 7.
Ca2+ sensitivity of the 1-2-1 chimeric channel. (A) Sample single channel recordings from the 1-2-1 chimera are shown at three different Ca2+ concentrations in the presence of 1 μM InsP3. The holding potential was 0 mV and open events are upward deflections. The current carrier was Cs+. Corresponding total amplitude histograms (3–5 min of recording) are shown for each Ca2+ concentrations. (B) Mean Po of the 1-2-1 chimeric channel is plotted as a function of Ca2+ concentration (filled circles). Data points represent means (± SE). Data represent seven different 1-2-1 chimeric channels. The Ca2+ dose response curves of the full-length type-1 and -2 InsP3R channels are also shown (dashed lines; Ramos-Franco et al., 2000).
Interdependency of InsP3 and Ca2+ Sensitivity of the 1-2-1 Chimeric Channel
The pore of the 1-2-1 chimera is regulated by both InsP3 and Ca2+. This implies that the InsP3 and Ca2+ binding regions of the protein (i.e., the binding and regulatory/coupling domain, respectively) both interact with the channel domain. It has been suggested that InsP3 and Ca2+ may interact to regulate the InsP3R pore (Kaftan et al., 1997; Mak et al., 1998). Here, possible interdependency of InsP3 and Ca2+ in regulation of the 1-2-1 chimera pore was evaluated by comparing its Ca2+ sensitivity at two InsP3 concentrations.
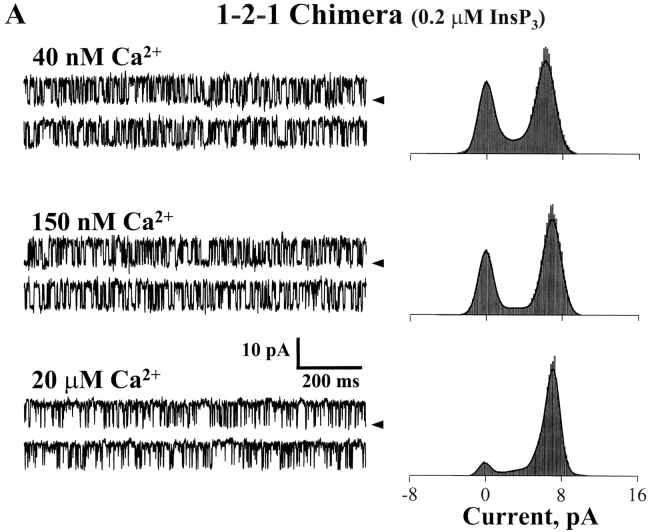
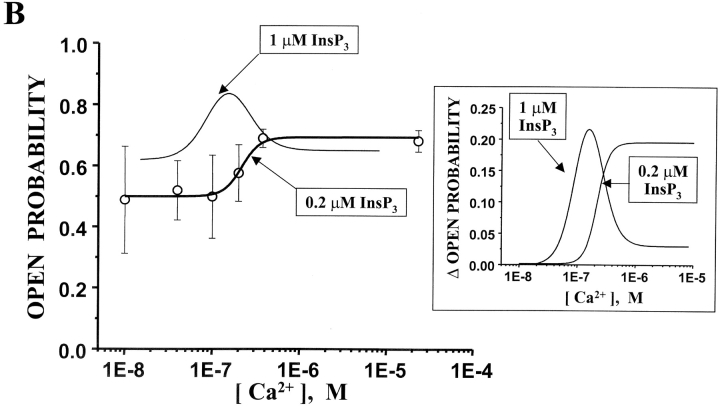
In Fig. 8 A, sample single channel recordings illustrate the Ca2+ sensitivity of the 1-2-1 chimera in the presence of 0.2 μM InsP3 (cis-chamber). The Po of the 1-2-1 chimera at 40 nM free Ca2+ was relatively high (∼0.5). The Po increased at higher Ca2+ concentrations (150 nM and 20 μM). The corresponding total amplitude histograms (Fig. 8 A, right) also illustrate the Ca2+-dependent changes in Po (unit current remained constant). The mean Po of the 1-2-1 chimera (n = 9) in the presence of 0.2 μM InsP3 is plotted (open circles) as a function of free Ca2+ concentrations in Fig. 8 B. The Ca2+ dependency of this channel in the presence of 1 μM InsP3 is represented by the thin bell-shaped line (reproduced here from Fig. 7 B). To compare the two curves, the Po at 10 nM was subtracted from each. These offset-subtracted curves are superimposed in the inset (Fig. 8 B). The general shape of the 1-2-1 chimera's Ca2+ dependency was different at the two InsP3 concentrations. At one InsP3 level (1.0 μM), it was bell-shaped (EC50 = 62.8 ± 13.1; IC50 = 159.5 ± 35.9). The Hill coefficients associated with these values (EC50 and IC50 at 1 μM InsP3) were about three. At the other InsP3 level (0.2 μM), the channel's Ca2+ dependency was sigmoidal (EC50 = 216.6 ± 43.9; Hill coefficient = 3.2). The EC50 values of Ca2+ activation at the two different InsP3 concentrations were statistically different (P < 0.05). These data suggest that the Ca2+ regulation of the 1-2-1 chimera pore is modulated by InsP3. Thus, the interceding type-2 regulatory/coupling domain (i.e., containing putative Ca2+ regulatory sites) appears to exchange Ca2+ binding information in both directions (i.e., to the amino-terminal InsP3-binding domain and the carboxyl-terminal channel domain).
Figure 8.
(A) Ca2+-dependent gating of 1-2-1 chimeric channels at low InsP3 concentrations. Sample single channel recordings from the 1-2-1 chimera are shown at three different Ca2+ concentrations in the presence of 0.2 μM InsP3. The holding potential was 0 mV and open events are upward deflections. The current carrier was Cs+. Corresponding total amplitude histograms (3–5 min of recording) are shown for each Ca2+ concentrations. (B) InsP3 sensitivity of 1-2-1 chimeric channel Ca2+ dependence. Mean Po of the 1-2-1 chimeric channel is plotted as a function of Ca2+ concentration (open circles) in the presence of 0.2 μM InsP3. Data points represent means (± SE). Data represent 5–11 single channels. The Ca2+ dose response curve of the 1-2-1 chimeric channel in the presence of 1 μM InsP3 is shown as the bell-shaped curve. The Po at 1 nM free Ca2+ was subtracted from both curve fits and the resulting curves are plotted on an expanded scale in the inset.
DISCUSSION
Several labs have defined the single-channel function of InsP3R channels by reconstituting these channels into planar lipid bilayers (Bezprozvanny et al., 1991; Watras et al., 1991; Ramos-Franco et al., 1998a). There has been particular attention lately on defining the isoform-specific attributes of the InsP3R channels. Some studies have focused on the type-3 InsP3R channel (Hagar et al., 1998; Mak et al., 2000). Others have focused on the type-1 InsP3R channel (Bezprozvanny et al., 1991; Kaznacheyeva et al., 1998). Our group has focused on the type-2 InsP3R channel (Perez et al., 1997; Ramos-Franco et al., 1998a, 2000).
All three InsP3R channels have similar permeation properties. However, their regulation by Ca2+ and InsP3 is isoform specific. All three InsP3R channel have been cloned and are routinely expressed in heterologous systems (Kaznacheyeva et al., 1998; Ramos-Franco et al., 1998b, 2000; Mak et al., 2000). Thus, the stage is set to use molecular genetic manipulations to explore the molecular determinants of InsP3R channel structure and function.
The InsP3R Permeation Pathway
The single-channel properties of the two chimeras were similar to each other and to their full-length wild-type counterparts (Ramos-Franco et al., 1998a,b, 2000). Specifically, their conductance (∼220 pS in a 220/20 Cs+ gradient) and selectivity (reversal potential of −45 mV in Cs-HEPES salts) were preserved. This indicates that the genetic manipulations required to create these domain-swap chimeras did not interrupt the structural integrity of the permeation pathway. However, the Ca2+ and InsP3 regulation of the pores formed by the chimeric proteins was not identical to that of the wild-type channels. The pores were ligand regulated (in most cases) but the molecular manipulations clearly altered the nature of this regulation. These results are consistent with a previous study involving gross TMR-deletion mutants (Ramos-Franco et al., 1999). There a gross deletion of the four amino-terminal TMRs generated mutant proteins that formed channels when reconstituted in bilayers. The permeation pathway of these channels had the well-defined permeation properties of the InsP3R channel but was not ligand regulated. Deletion of the four TMRs effectively disrupted the information flow from the binding and the regulatory/coupling domain to the channel domain. Thus, it appears that the InsP3R pore structure is a robust structure that is relatively resistant to manipulations around it. This is not true for the regulation of the InsP3R pore (i.e., how often it opens and closes). The ligand regulation of the pore is clearly impacted by the presence and identity of the structures surrounding it. The salient point here is that the permeation and ligand regulatory properties of the InsP3R channel appear to be governed independently by different structural determinants of the protein.
InsP3 Regulation
The type-1 and -2 InsP3R proteins contain InsP3 binding sites with different InsP3 affinities (Südhof et al., 1991; Newton et al., 1994; Perez et al., 1997). The InsP3 binding affinity of the soluble binding domain of the type-2 InsP3R is ∼3 times higher than that of the type-1 receptor (Südhof et al., 1991). A similar relationship is evident in the InsP3 sensitivity of the native and recombinant InsP3R channels (Ramos-Franco et al., 1998a,b, 2000). Together, these data imply that the relative InsP3 affinity of the type-1 and -2 channels is defined by structural determinants that lie within the binding domain of the protein.
Here, the InsP3 sensitivity of two chimeric InsP3R channels with different binding domains was defined. The InsP3 modulation of the chimeric channels was different than that of either of the full-length progenitors. The type-2 binding domain did impart high InsP3 sensitivity. The type-1 domain did impart lower InsP3 sensitivity. This confirms that the identity of the binding domain does indeed play a role in establishing InsP3 affinity. Efficacy of InsP3 activation of the 2-1-2 chimera was similar to that of the full-length recombinant type-2 channel (Po's 0.42 vs. 0.40, respectively). Efficacy of InsP3 activation of the 1-2-1 chimera was about fourfold higher than that of the full-length recombinant type-1 channel (Po's 0.70 vs. 0.17, respectively). Interestingly, there was also substantial activity of the 2-1-2 chimera (Po ∼0.2) at very low InsP3 concentrations (or when no InsP3 was added). Such sustained channel activity is not observed in the full-length progenitor channels. Thus, the transduction of InsP3 binding to channel opening is altered in these chimeric channels. These alterations could be due to the identity of the introduced regulatory/coupling domains or abnormal interdomain crosstalk. In either case, the implication is that structural determinants that define InsP3 binding affinity in the binding domain are not the only factors that define InsP3 regulation of the channel.
Ca2+ Regulation
Several Ca2+ binding sites and residues key to the calcium sensor (E2100 of the rat type-1 isoform) that regulate the InsP3R channel have been shown to reside in the regulatory/coupling domain of the receptor (Mignery and Südhof, 1990; Sienaert et al., 1996, 1997; Miyakawa et al., 2001; Nadif-Kasari et al., 2002). Additionally, all three isoforms contain Ca2+ binding sites in the ligand binding as well as channel domains (Sienaert et al., 1996, 1997, 2002; Sipma et al., 1999). The details of calcium's role in activation and inhibition are beginning to emerge, yet much more information is required to elucidate which critical residues are involved in the complex modulation of the receptor by this ion. The 2-1-2 chimera has the type-1 regulatory/coupling domain and the 1-2-1 chimera the type-2 regulatory/coupling domain. The activity of the 2-1-2 chimera was not Ca2+ sensitive. One possible interpretation is that the 2-1-2 chimera does not bind Ca2+. Considerable care was taken to craft smooth junctional transitions between the different receptor domains and these transitions are relatively distant from the putative Ca2+ binding sites. However, a failure to bind Ca2+ may involve coordination of different regions of the protein and this coordination may not be available in the 2-1-2 chimera structure. Another possibility, and perhaps more likely, is a process that transduces Ca2+ binding into channel regulation is disrupted in the 2-1-2 chimera. In this case, the data would suggest that the type-1 regulatory/coupling domain does not communicate well with the type-2 channel (and/or binding) domain. This result is not entirely surprising in that the type-1 and -2 coupling/regulatory domains are the least conserved (having only ∼60% identity). These domains contain large interspersed stretches of heterologous sequence. This provides multiple putative modulatory binding sites that may contribute to the observed isoform-specific Ca2+ signaling of these channels. The implication is that the type-1 and -2 coupling domains are not interchangeable.
The function of the 1-2-1 chimera was Ca2+ dependent. Its Ca2+ dependence was bell shaped (like that of the full-length type-1), but this bell shape was substantially offset from 0 Po. This Po offset may be related to the unusually high InsP3 efficacy of this chimera. Its bell-shaped Ca2+ dependency was centered at lower Ca2+ concentrations compared with that of the full-length type-1 receptor. Activation at such low Ca2+ levels is typical of the full-length type-2. Thus, chimera has features common to its progenitors. It is interesting that a channel containing the type-2 regulatory/coupling domain has a bell-shaped component of Ca2+ sensitivity reminiscent of that generally associated with the full-length type-1 channel. This could imply that some of the structural determinants of the bell-shaped Ca2+ modulation may lie outside the regulatory/coupling domain. Alternatively, it could mean that structural determinants outside the regulatory/coupling domains alter the bell-shaped Ca2+ modulation in the native full-length type-2 context. Numerous Ca2+ binding motifs have been detected in other regions of these receptors, including their ligand binding and channel domains. Further, it has been suggested that InsP3R Ca2+ inactivation may be modulated (or “tuned”) by InsP3 binding (Hagar et al., 1998; Mak et al., 1998). In any event, these data support the notion that InsP3R ligand (InsP3 and Ca2+) regulation is a multideterminant and isoform-specific process.
Potential InsP3 and Ca2+ Interaction
There is a consensus that the function of the single InsP3R channels is isoform specific (Hagar et al., 1998; Mak et al., 1998; Ramos-Franco et al., 1998a, 2000). Studies of type-1 and -3 InsP3R channel regulation indicate that InsP3 binding regulates Ca2+ inactivation of the channel (Hagar et al., 1998; Mak et al., 1998). The underlying mechanism of the Ca2+–InsP3 interaction is debated. Kaftan et al. (1997) has proposed that two InsP3 binding sites regulate InsP3R channels. A high affinity InsP3 binding site activates the channel while a lower affinity InsP3 binding site modulates the Ca2+ inactivation of the channel. Occupancy of the low affinity site reduces the degree of Ca2+ inactivation. In contrast, Mak et al. (1998) has proposed that a single high affinity InsP3 binding site “tunes” the Ca2+ sensitivity of the InsP3R channel. Increased InsP3 occupancy of this site reduces the affinity of the Ca2+ inactivation process (i.e., inactivation occurs at higher Ca2+ levels).
There are certain considerations that must be taken into account if our results are to be interpreted in the type-1/type-3 regulatory context outlined above. First, the InsP3 binding affinities of the type-2 and -3 isoforms are very different relative to the type-1 channel (type-2 < type-1 << type-3; Newton et al., 1994). Second, all three isoforms share at least one potential Ca2+ regulatory site (E2100; Miyakawa et al., 2001) but likely have others. Third, it has been suggested that calmodulin binding may be critical in InsP3 and Ca2+ regulatory interaction (Michikawa et al., 1999) and the calmodulin–InsP3R interaction may be isoform-specific (for review see Nadif-Kasari et al., 2002). For example, the type-3 does not contain the calmodulin-binding motif as the type-1 or -2 receptors. Recently, the complex role of calmodulin in the modulation of the type-1 receptor has been examined by Nosyreva et al. (2002), in which a mutant (W1577A) abolished Ca2+-dependent CaM binding of the rat type-1 receptor. Despite the absence of CaM binding, the channel activity showed a similar biphasic response to Ca2+ as the wild-type receptor and similar sensitivity to exogenously applied CaM. At the present, the role of CaM in the regulation of the receptor family is a confounding and elusive property of the receptor that remains to be elucidated. Fourth, the same InsP3 and Ca2+ regulatory interaction scheme may simply not apply to all three InsP3R channel isoforms. It may be misleading to interpret the ligand regulation of the three channels in a single conceptual framework.
The possibility that InsP3 alters the Ca2+ dependence of the 1-2-1 chimeric channel was examined here. In the presence of 1 μM InsP3, the Ca2+ dependence of the 1-2-1 chimera had a bell-shaped component. In the presence of a lower InsP3 concentration (0.2 μM), the Ca2+ dependence of the 1-2-1 chimera was sigmoidal (i.e., lost its bell-shaped component). This data is difficult to explain in the context of either the Kaftan et al. (1997) or Mak et al. (1998) Ca2+/InsP3 regulatory schemes. For example, the apparent Ca2+ inactivation was lost at lower InsP3 concentrations in the chimeric channels. Also, the EC50 of Ca2+ activation was InsP3 dependent (i.e., it was more sensitive to Ca2+ activation at high InsP3 concentrations). This is not observed in the previously published Ca2+/InsP3 regulatory results on full-length channels (Kaftan et al., 1997; Hagar et al., 1998; Mak et al., 1998, 2001). In any event, chimeric channel behavior was defined over a wide range of [Ca2+]s (10−8 to 2 × 10−5 M) at two [InsP3]s (1 and 0.2 μM; see Fig. 8 B). The Ca2+ sensitivity of the 1-2-1 chimera was different at the two [InsP3]s tested (i.e., its Ca2+ sensitivity was InsP3 dependent). This suggests that this channel does not behave as a solely Ca2+-gated or InsP3-gated channel. Thus, the chimera results clearly show that there is an InsP3–Ca2+ regulatory interaction. This interaction has three components. One involves InsP3 modulation of the EC50 of Ca2+ activation. A second involves InsP3 modulation of high Ca2+ inactivation. The third involves InsP3 modulation of the Po offset at low Ca2+ concentrations.
These initial chimera studies demonstrate that it is possible to use a chimera strategy to define InsP3R structure-function. The large domain-swap chimeras created here formed functional channels, but these structural manipulations introduced unique ligand regulatory attributes. Application of a more conservative chimera strategy that exchanges certain nonconserved motifs and/or regions of heterologous sequence, in tandem with these bulk domain exchanges, will likely lead to important information regarding isoform-specific structure and function.
Acknowledgments
The authors thank Dr. Thomas C. Südhof for the generous gift of the type-1 and type-2 InsP3R cDNAs. We also thank Dr. Dan Bare and Sean Caenepeel for expert technical assistance.
This work was supported by National Institutes of Health grants R01-HL58851, R01-MH53367 (G. Mignery), and R01-HL64210 (M. Fill).
Olaf S. Andersen served as editor.
Footnotes
Abbreviation used in this paper: InsP3R, inositol 1,4,5-trisphosphate receptor.
References
- Baudet, S., L. Hove-Madsen, and D.M. Bers. 1994. How to make and use calcium-specific mini- and microelectrodes. Methods Cell Biol. 40:93-113. [PubMed] [Google Scholar]
- Bezprozvanny, I., J. Watras, and B.E. Ehrlich. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3 and calcium gated channels from endoplasmic reticulum of cerebellum. Nature. 351:751–754. [DOI] [PubMed] [Google Scholar]
- Blondel, O., J. Takeda, H. Janssen, S. Seino, and G.I. Bell. 1993. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 268:11356–11363. [PubMed] [Google Scholar]
- Boehning, D., and S.K. Joseph. 2000. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 19:5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning, D., S.K. Joseph, D.O. Mak, and J.K. Foskett. 2001. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys. J. 81:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, D., and A.G. Hawkes. 1981. On the stochastic properties of single ion channels. Proc. R. Soc. Lond. B. Biol. Sci. 211:205–235. [DOI] [PubMed] [Google Scholar]
- Galvan, D., E. Borrego-Diaz, P.J. Perez, and G.A. Mignery. 1999. Subunit oligomerization and topology of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 274:29483–29492. [DOI] [PubMed] [Google Scholar]
- Galvan, D., and G.A. Mignery. 2002. Carboxyl-terminal sequences critical for inositol 1,4,5-trisphosphate receptor subunit assembly. J. Biol. Chem. 277:48248–48260. [DOI] [PubMed] [Google Scholar]
- Gorman, C. 1985. High efficiency gene transfer into mammalian cells. DNA Cloning. Vol. I. D.M. Glover, editor. IRL Press, Oxford. 143–190.
- Hagar, R.E., A.D. Burgstahler, M.H. Nathanson, and B.E. Ehrlich. 1998. Type-III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 396:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, K., T. Miyata, K. Mayanagi, J. Hirota, and K. Mikoshiba. 2002. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J. Biol. Chem. 277:21115–21118. [DOI] [PubMed] [Google Scholar]
- Kaftan, E.J., B.E. Ehrlich, and J. Watras. 1997. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J. Gen. Physiol. 110:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva, E., V.D. Lupu, and I. Bezprozvanny. 1998. Single channel properties of inositol trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol. 111:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D.O., S. McBride, and J.K. Foskett. 1998. Inositol-1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 95:15821–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D.O., S. McBride, V. Raghuram, Y. Yue, S.K. Joseph, and J.K. Foskett. 2000. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 115:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D.O., S. McBride, and J.K. Foskett. 2001. Regulations by Ca2+ and inositol (1,4,5) trisphosphate (InsP3) of InsP3 receptor channels. J. Gen. Physiol. 117:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa, T., J. Hirota, S. Kawano, M. Hiraoka, M. Yamada, T. Furuichi, and K. Mikoshiba. 1999. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 23:799–808. [DOI] [PubMed] [Google Scholar]
- Mignery, G.A., and T.C. Südhof. 1990. The ligand-binding site and transduction mechanism in the inositol-1,4,5-trisphosphate receptor. EMBO J. 9:3893–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery, G.A., C.L. Newton, B.T. Archer III, and T.C. Südhof. 1990. Structure and expression of the rat inositol-1,4,5-trisphosphate receptor. J. Biol. Chem. 265:12679–12685. [PubMed] [Google Scholar]
- Mignery, G.A., P.A. Johnston, and T.C. Südhof. 1992. Mechanism of Ca2+ inhibition of inositol 1,4,5-trisphosphate (InsP3) binding to the cerebellar InsP3 receptor. J. Biol. Chem. 267:7450–7455. [PubMed] [Google Scholar]
- Mignery, G.A., and T.C. Südhof. 1993. Molecular analysis of inositol 1,4,5-trisphosphate receptors. Methods Neurosci. 18:247–265. [Google Scholar]
- Miyakawa, T., A. Mizushima, K. Hirose, T. Yamazawa, I. Bezprozvanny, T. Kurosaki, and M. Iino. 2001. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J. 20:1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif-Kasari, N., G. Bultynck, I. Sienaert, G. Callewaert, C. Erneux, L. Missiaen, J.B. Parys, and H. De Smedt. 2002. The role of calmodulin for inositol 1,4,5-trisphosphate receptor function. Biochim. Biophys. Acta. 1600:19–31. [DOI] [PubMed] [Google Scholar]
- Nosyreva, E., T. Miyakawa, Z. Wang, L. Glouchankova, A. Mizushima, M. Iino, and I. Bezprozvanny. 2002. The high-affinity calcium-calmodulin-binding site does not play a role in the modulation of type 1 inositol 1,4,5-trisphosphate receptor function by calcium and calmodulin. Biochem. J. 365:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, C.L., G.A. Mignery, and T.C. Südhof. 1994. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem. 269:28613–28619. [PubMed] [Google Scholar]
- Perez, P.J., J. Ramos-Franco, M. Fill, and G.A. Mignery. 1997. Identification and functional reconstitution of the type-2 InsP3 receptor from ventricular cardiac myocytes. J. Biol. Chem. 272:23961–23969. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco, J., M. Fill, and G.A. Mignery. 1998. a. Isoform specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys. J. 75:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco, J., M. Fill, and G.A. Mignery. 1998. b. Single channel function of recombinant type-1 inositol 1,4,5-trisphosphate receptor ligand binding domain splice variants. Biophys. J. 75:2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco, J., D. Galvan, G.A. Mignery, and M. Fill. 1999. Location of the permeation pathway in the recombinant type-1 inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol. 114:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco, J., D. Bare, S. Caenepeel, A. Nani, M. Fill, and G.A. Mignery. 2000. Single-channel function of recombinant type 2 inositol 1,4, 5-trisphosphate receptor. Biophys. J. 79:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienaert, I., H. De Smedt, J.B. Parys, L. Missiaen, S. Vanlingen, H. Sipma, and R. Casteels. 1996. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 271:27005–27012. [DOI] [PubMed] [Google Scholar]
- Sienaert, I., L. Missiaen, H. De Smedt, J.B. Parys, H. Sipma, and R. Casteels. 1997. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 272:25899–25906. [DOI] [PubMed] [Google Scholar]
- Sienaert, I., N. Nadif Kasri, S. Vanlingen, J.B. Parys, G. Callewaert, L. Missiaen, and H. de Smedt. 2002. Localization and function of a calmodulin-apocalmodulin-binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 365:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipma, H., P. De Smet, I. Sienaert, S. Vanlingen, L. Missiaen, J.B. Parys, and H. De Smedt. 1999. Modulation of inositol 1,4,5-trisphosphate binding to the recombinant ligand-binding site of the type-1 inositol 1,4, 5-trisphosphate receptor by Ca and calmodulin. J. Biol. Chem. 274:12157–12162. [DOI] [PubMed] [Google Scholar]
- Südhof, T.C., C.L. Newton, B.T. Archer, Y.A. Ushkaryov, and G.A. Mignery. 1991. The structure of a novel InsP3 receptor. EMBO J. 10:3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras, J., I. Bezprozvanny, and B.E. Ehrlich. 1991. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductances states. J. Neurosci. 11:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]