Abstract
The interaction of biotin-binding proteins with biotinylated gramicidin (gA5XB) was studied by monitoring single-channel activity and sensitized photoinactivation kinetics. It was discovered that the addition of streptavidin or avidin to the bathing solutions of a bilayer lipid membrane (BLM) with incorporated gA5XB induced the opening of a channel characterized by approximately doubled single-channel conductance and extremely long open-state duration. We believe that the deceleration of the photoinactivation kinetics observed here with streptavidin and previously (Rokitskaya, T.I., Y.N. Antonenko, E.A. Kotova, A. Anastasiadis, and F. Separovic. 2000. Biochemistry. 39:13053–13058) with avidin reflects the formation of long-lived channels of this type. Both opening and closing of the double-conductance channels occurred via a transient sub-state of the conductance coinciding with that of the usual single-channel transition. The appearance of the double-conductance channels after the addition of streptavidin was preceded by bursts of fast fluctuations of the current with the open state duration of the individual events of 60 ms. The streptavidin-induced double-conductance channels appeared to be inherent only to the gramicidin analogue with a biotin group linked to the COOH terminus through a long linker arm. Including biotinylated phosphatidylethanolamine into the BLM prevented the formation of the double-conductance channels even with the excess streptavidin. In view of the results obtained here, it is suggested that the double-conductance channel represents a tandem of two neighboring gA5XB channels with their COOH termini being cross-linked by the bound streptavidin at both sides of the BLM. The finding that streptavidin induces the formation of the tandem gramicidin channel comprising two channels functioning in concert is considered to be relevant to the physiologically important phenomenon of ligand-induced receptor oligomerization.
Keywords: bilayer lipid membrane, sensitized photoinactivation, ligand, clustering
INTRODUCTION
Receptor–ligand interactions play a crucial role in most physiological processes. In particular, chemical signals are converted into electrical ones through ligand gating of ionic channels in cellular membranes (Breitinger, 2001). Clustering of channels is accepted to be the basic mechanism of signal transduction and amplification (Laver and Gage, 1997; Colledge and Froehner, 1998; Swillens et al., 1999; Garner et al., 2000; Neumann, 2000; Breitinger, 2001). Colocalization of PDZ domain–containing or cytoskeletal proteins, on one hand, and receptor channel proteins, on the other hand, leading to formation of their clusters has been visualized for acetylcholine and glutamine receptors as well as for potassium, sodium, calcium, and chloride channels. This coclustering has been reported to activate and immobilize the specific ionic channels (Westenbroek et al., 1990; Joe and Angelides, 1992; Issa and Hudspeth, 1994; Kim et al., 1995; Shieh and Zhu, 1996; Horio et al., 1997; Kurschner et al., 1998; Wood and Slater, 1998; Zhou et al., 1998; Burke et al., 1999; Caldwell, 2000; Chen et al., 2000; El-Husseini et al., 2000; Nehring et al., 2000; Sheng and Pak, 2000; Tiffany et al., 2000; Wang et al., 2000; Bezprozvanny and Maximov, 2001; Kaplan et al., 2001; Raghuram et al., 2001; Ratcliffe et al., 2001; Anzai et al., 2002; Duggan et al., 2002; Imamura et al., 2002; Tanemoto et al., 2002). Clustering is also one of the main principles of molecular organization of the gap-junction channels (Bukauskas et al., 2000; Falk, 2000).
Synchronous opening and closing of ensembles of ion channels have been observed upon incorporation of channel-forming proteins and peptides into planar bilayer lipid membranes (Volkova et al., 1980; Schindler and Rosenbusch, 1981; Schindler et al., 1984; Hymel et al., 1988; Watras et al., 1991; Mironova et al., 1994; Delcour, 1997; Kaulin et al., 1998; Marx et al., 1998; Ternovsky and Berestovsky, 1998; Dargan et al., 2002; Kullman et al., 2002) and by the patch-clamp method (Kazachenko and Geletyuk, 1984; Geletyuk and Kazachenko, 1989; Meves and Nagy, 1989; Schreibmayer et al., 1989; Honore et al., 1992; Larsen et al., 1996a,b; Neumann et al., 1996; Eghbali et al., 1997; Laver and Gage, 1997). In spite of the fact that studying channel clusters has attracted much attention, the molecular mechanisms of their functioning remain unclear.
Here we demonstrate that one of the simplest and best studied ionic channels formed by a transmembrane dimer of the pentadecapeptide gramicidin A (Koeppe and Andersen, 1996; Andersen et al., 1999; Cross et al., 1999) can be rendered ligand gated by attaching a biotin group to the COOH terminus of the channel former. The interaction of biotinylated gramicidin with avidin or streptavidin, having four biotin-binding sites of a remarkably high affinity (Hendrickson et al., 1989; Weber et al., 1989; Green, 1990; Livnah et al., 1993; Pugliese et al., 1993; Sano and Cantor, 1995; Stayton et al., 1999) results in opening of channels with unusual characteristics, namely, extremely long duration and double conductance as compared with the control. Thus, the concerted ligand-gated opening of a couple of gramicidin channels is shown for the first time. This model system can be used to simulate a broad class of the ligand-induced receptor oligomerization phenomena (Metzger, 1992; Lemmon and Schlessinger, 1994; Heldin, 1995; Wells, 1996; Lemmon et al., 1997; Reich et al., 1997; Guo and Levine, 1999; Bouvier, 2001; Cochran et al., 2001), including ion channel regulation via dimerization (Liu et al., 1998; Richards and Gordon, 2000; Yellen, 2001; Schumacher et al., 2001), as well as the formation of coordinated arrays of signaling proteins (Xu et al., 1998; Fanning and Anderson, 1999; Hung and Sheng, 2002). In the accompanying article by Goforth et al. (2003)(this issue) the problem is addressed using an independent experimental approach.
Some of these results have appeared in preliminary form (Rokitskaya et al., 2002).
MATERIALS AND METHODS
Bilayer lipid membranes (BLMs)* were formed from a 2% solution of diphytanoylphosphatidylcholine (DPhPC; Avanti Polar Lipids) or its mixture (if otherwise stated) with N-(biotinoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (biotin-PE; Molecular Probes, Inc.) in n-decane (Merck) by the brush technique on a hole in a Teflon partition separating two compartments of a cell containing aqueous buffer solutions. The cell with the 0.15-mm diameter hole (unless otherwise stated) was used in single-channel experiments, and that with the 0.55-mm diameter hole was used in photoinactivation experiments. The biotinylated analogs of gramicidin A (gifts of F. Separovic, University of Melbourne, Australia) with a biotin group attached to the COOH terminus of gramicidin A through a linker arm comprising five (gA5XB) or two (gA2XB) aminocaproyl groups were added from stock solutions in ethanol to the bathing solutions at both sides of the BLM and routinely incubated for 15 min with constant stirring. The structure and synthesis of biotinylated gramicidins were described in (Separovic et al., 1999; Anastasiadis et al., 2001). Streptavidin and avidin were from Fluka. In all the experiments the solution was 1 M KCl, 10 mM Tris, 10 mM MES, 10 mM β-alanine, pH = 7.0. All the experiments were performed at room temperature (22–24°C). In photoinactivation experiments, aluminum trisulfophthalocyanine (AlPcS3) from Porphyrin Products was added to the bathing solution at the trans-side (the cis-side is the front side with respect to the flash lamp).
The electric currents (I) were recorded under voltage-clamp conditions. Voltages were applied to BLMs with Ag-AgCl electrodes placed directly into the cell. The currents, measured by means of a patch-clamp amplifier (OES-2; OPUS) in single-channel experiments and by a U5–11 amplifier in photoinactivation experiments, were digitized by using a LabPC 1200 (National Instruments) and analyzed using a personal computer with the help of WinWCP Strathclyde Electrophysiology Software designed by J. Dempster (University of Strathclyde, UK). Single-channel currents were low-pass filtered with a cutoff frequency of 100 Hz, sampled at 1 kHz and stored directly to the hard disk.
In photoinactivation experiments, BLMs were illuminated by single flashes produced by a xenon lamp with flash energy of ∼400 mJ/cm2 and flash duration <2 ms.
RESULTS
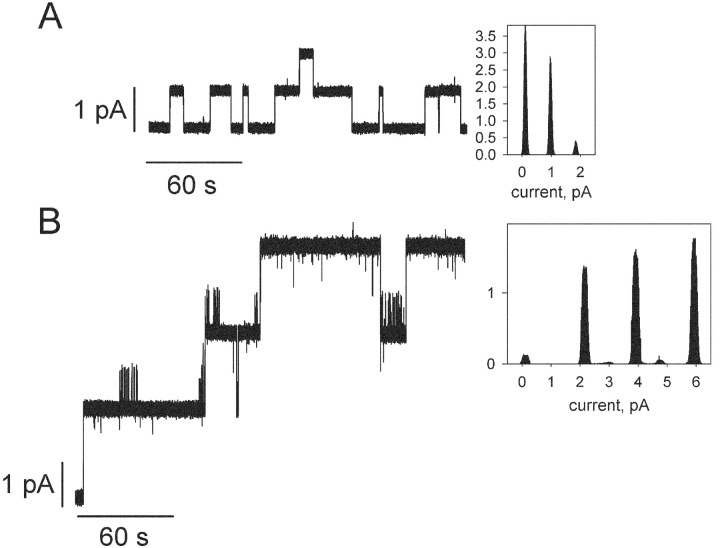
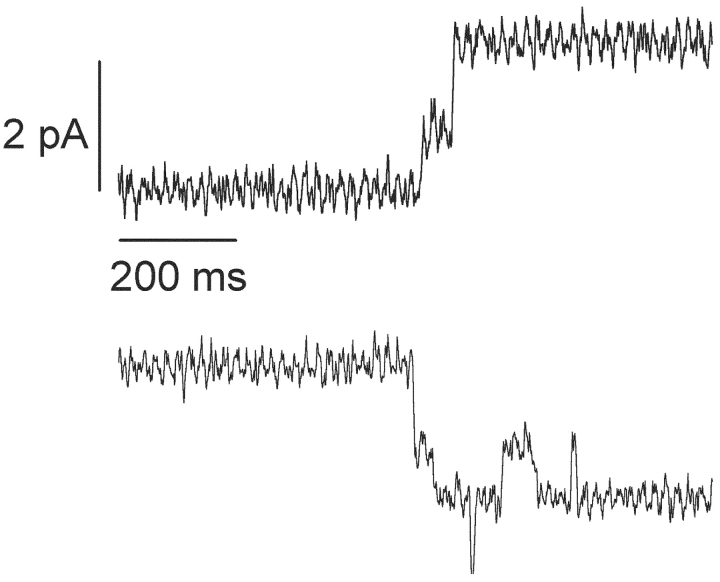
Fig. 1 presents single-channel recordings and current amplitude histograms of gA5XB (the biotinylated gramicidin with a linker arm comprising five aminocaproyl groups) in the control (A) and after 10-min incubation with 1 μg/ml streptavidin added to both sides of BLM (B). Remarkably, it is seen that the usual current fluctuations of gA5XB with the open state duration comparable to that of gramicidin A and the single-channel conductance of 17.6 ± 1 pS (recording A) were replaced after the incubation with streptavidin by current fluctuations with a very long-lived open state and the single-channel conductance exceeding that of the control more than twice, 39.5 ± 3 pS (recording B). If presented in the expanded time scale (Fig. 2) , the recordings made in the presence of streptavidin revealed that both opening and closing of the double-conductance channels occurred via an intermediate substate of 50 ± 25-ms duration (mean ± SD, n = 17). The conductance of this transient substate was equal to the single-channel conductance of gA5XB, whereas the conductance of the subsequent step was 25% higher.
Figure 1.
(A) Single-channel traces of gA5XB and the corresponding current amplitude histogram. (B) Single-channel traces of gA5XB after incubation with 1 μg/ml streptavidin added to both sides of a BLM and the corresponding current amplitude histogram. The BLM voltage was 50 mV. The solution was 1 M KCl, 10 mM Tris, 10 mM MES, 10 mM β-alanine, pH = 7.0. Planar bilayers were from DPhPC. The low-pass digital filter with a cutoff frequency of 53 Hz was used in these recordings.
Figure 2.
Selected parts of the recordings similar to that shown in Fig. 1 B revealing a transient substate in both opening and closing of the double-conductance channel. Here the digital filter was not used.
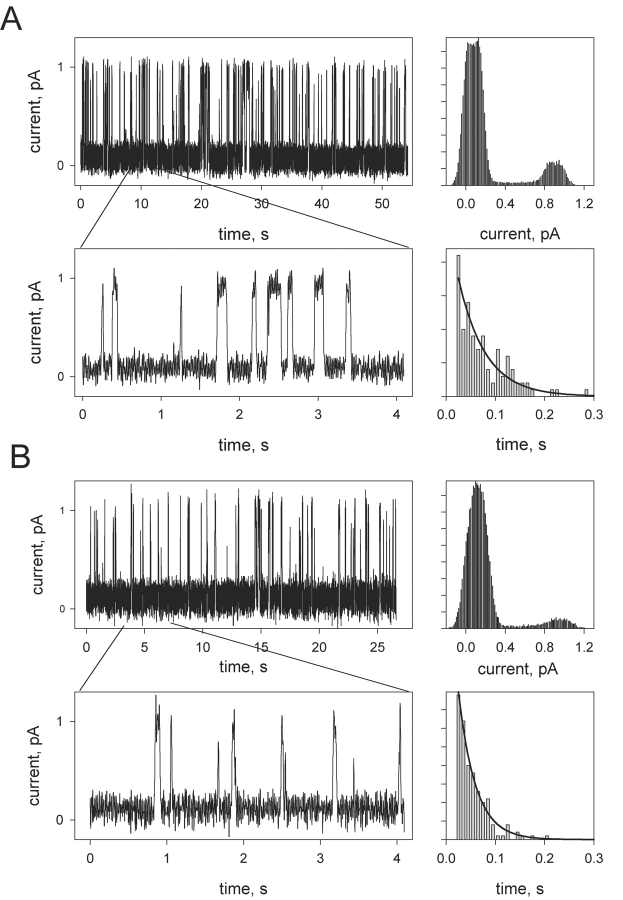
It is worth noting that the opening of the long-lived double-conductance channels after the addition of streptavidin was preceded by the appearance of bursts of very short-lived channel events (Fig. 3 A) having the transition amplitude coinciding with that of the control single channels of gA5XB. The open state duration of the individual events in these bursts displayed the exponential distribution with the characteristic time of 60 ± 10 ms. The duration of the closed state varied from 60 to 400 ms in different series of bursts. The duration of the bursts also varied substantially (from 10 to 140 s). After the opening of the double-conductance channels the occurrence of the bursts decreased markedly and their duration on the average became shorter.
Figure 3.
(A) Fast fluctuations of the gA5XB single-channel current after the addition of 1 μg/ml streptavidin to both sides of a BLM observed before the appearance of the events shown in Fig. 1 B. (Top right) The corresponding current amplitude histogram. (Bottom right) The open state duration histogram with the exponential fit (the characteristic time is 55 ms). (B) Fast fluctuations of the gA2XB single-channel current after the addition of 1 μg/ml streptavidin to both sides of a BLM. (Top right) The corresponding current amplitude histogram. (Bottom right) The open state duration histogram with the exponential fit (the characteristic time is 35 ms). The BLM voltage was 50 mV. The solution was the same as in Fig. 1. Planar bilayers were from DPhPC. The low-pass digital filter with a cutoff frequency of 53 Hz was used in these recordings.
In contrast to gA5XB channels, gA2XB (the biotinylated gramicidin having a shorter linker arm) channels never exhibited the double-conductance state in the presence of streptavidin (unpublished data). Nevertheless, the incubation with 1 μg/ml streptavidin led to a substantial reduction of the number of open gA2XB channels in agreement with the data of (Suarez et al., 1998; Futaki et al., 2001) and the appearance of fast fluctuations of the current with the open state duration of 33 ± 4 ms (Fig. 3 B).
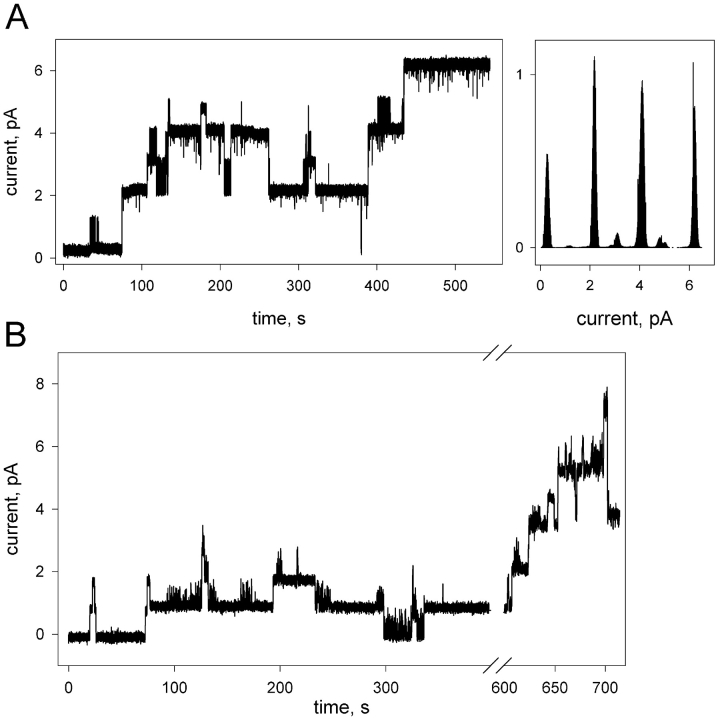
Fig. 4 displays single-channel recordings and the current-amplitude histogram of gA5XB obtained after incubation with 4 μg/ml avidin. The experiments using membranes of different areas (A, 0.15-mm diameter hole; B, 0.55-mm diameter hole) permitted us to compare the avidin effect on the parameters of gA5XB single channels at a high (A) and a low (B) surface density of the channel-former. As seen from recording A, the incubation with avidin led to the opening of very long-lived channels with the single-channel conductance of 36.4 ± 2 pS (see the histogram). Thus, avidin induced the formation of double-conductance channels similarly to streptavidin. The transient substate of 50-ms duration was also discernible in this case, with the conductance of the subsequent step exceeding that of the initial step of the channel opening by 7%. Recording B shows, however, that at low surface density of gA5XB the addition of avidin caused the appearance of long-lived channels with the single-channel conductance equal to that of the control channels, as observed previously (Rokitskaya et al., 2000a). The prolonged incubation with avidin resulted ultimately in the appearance of high-conductance channels also with the large-diameter membrane, though the amplitude of these channels was somewhat less than those observed with the small-diameter membrane.
Figure 4.
Single-channel traces of gA5XB after the addition of 4 μg/ml avidin to both sides of BLMs formed from DPhPC on the 0.15-mm diameter hole (A) and on the 0.55-mm diameter hole (B), and the current amplitude histogram corresponding to A. The solution was the same as in Fig. 1. The low-pass digital filter with a cutoff frequency of 53 Hz was used in these recordings.
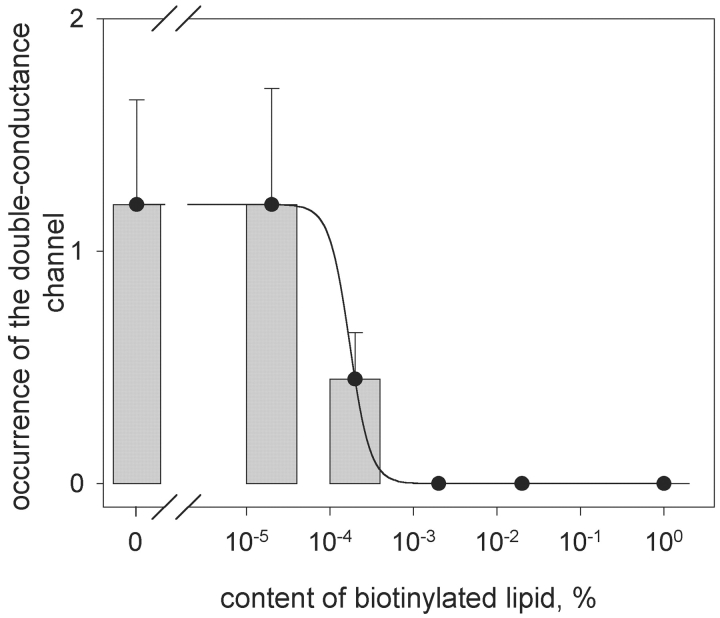
It was reasonable to expect that biotinylated lipid if included in the membrane would compete with biotinylated gramicidin for binding sites on streptavidin and thereby would alter the probability of the formation of the double-conductance channels. In further experiments we studied the effect of including biotinylated phosphatidylethanolamine (biotin-PE) in the BLM on the single-channel activity of gA5XB in the presence of streptavidin. It appeared that in the whole range of the biotin-PE content of the membrane studied here the interaction of streptavidin with gA5XB resulted in the reduction of the number of open channels having the standard single-channel conductance of 17 pS (unpublished data), as it was observed in the absence of biotin-PE. Besides, fast fluctuations of the current (bursts of very short-lived channel events having the transition amplitude equal to that of the control single channels) with the same open state duration, as in pure DPhPC membranes, were observed with all membranes containing biotin-PE. On the contrary, the occurrence of the double-conductance channels of gA5XB after incubation with streptavidin was extremely sensitive to the presence of biotin-PE (Fig. 5) . At the biotin-PE content amounting to 2 × 10−5% of the membrane lipid (the rest being DPhPC) the double-conductance channels opened with the same frequency as in the absence of biotin-PE. At the biotin-PE content of 2 × 10−4% the double-conductance channels rarely formed. Beginning from the biotin-PE content of 0.002% the double-conductance channels were not observed at all.
Figure 5.
The bar chart of the double-conductance channel occurrence plotted versus the content of biotinylated lipid (biotin-PE) in the membrane-forming DPhPC solution. The line was drawn by eye.
In accord with the streptavidin-induced decrease in the number of open gA5XB channels, the suppression of gA5XB-mediated current across BLM after the addition of streptavidin was also observed at the multichannel level (Fig. 6 A). This effect of streptavidin that had been found earlier (Cornell et al., 1997) appeared to be independent of the presence of the biotinylated lipid; in particular, it was seen at least up to the biotin-PE of 1% (Fig. 6 A). At 0.02% biotin-PE, the time course of the macroscopic current was biphasic (the increase in the current followed its decrease) that can be explained by slow formation of the long-lived double-conductance channels leading to the growth of the macroscopic current. Actually, the magnitude of the gA5XB-mediated current depends not only on the channel lifetime, i.e., on the dissociation rate constant of gA5XB dimers, but also on the frequency of channel openings related to the gA5XB dimerization rate constant, and on the stationary concentration of gA5XB molecules in the membrane.
Figure 6.
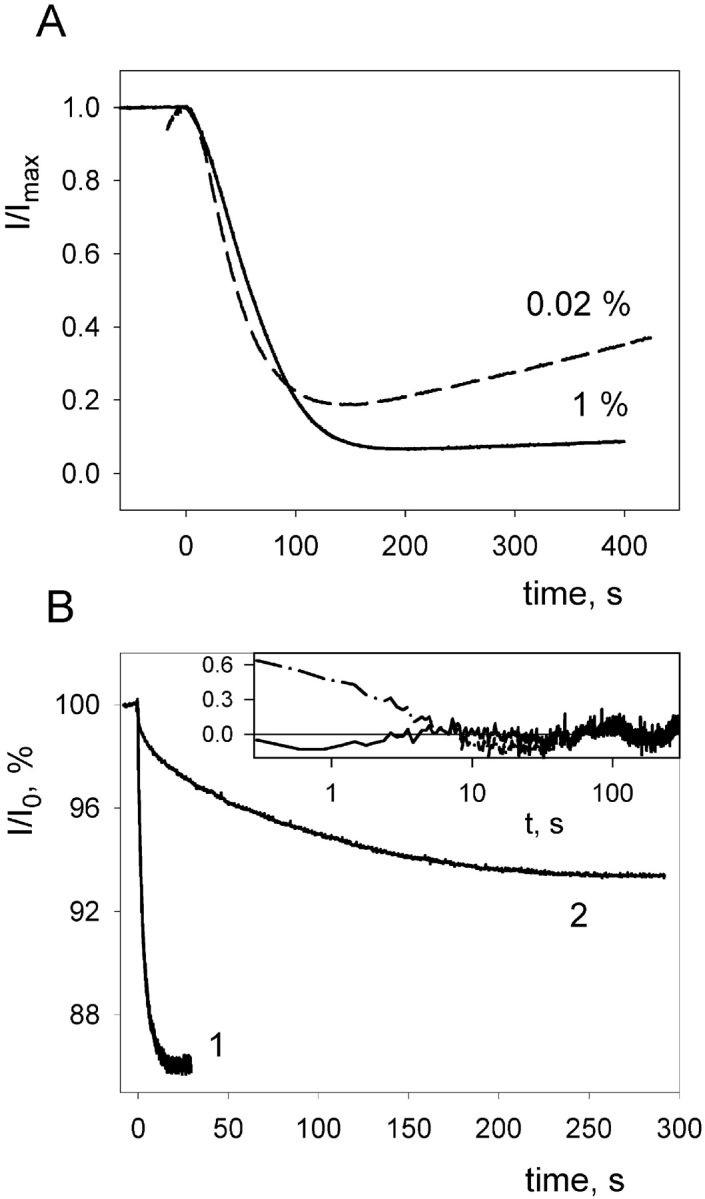
(A) Effect of the addition (at t = 0 s) of 0.5 μg/ml streptavidin on the gA5XB-mediated current across BLMs formed from the DPhPC solution with different contents of biotinylated lipid (0.02%, dashed line; 1%, solid line). The normalized values of the current (I/I max) are plotted versus the time (the I amx was ∼1 μA). (B) The time courses of the decrease in the gA5XB-mediated current across a BLM after a flash of visible light (at t = 0 s) in the presence of 1 μM AlPcS3 before (curve 1) and after (curve 2) the addition of 0.5 μg/ml streptavidin to both sides of the BLM. The normalized values of the current (I/I0) are plotted versus the time. The initial current (I 0) was ∼1 μA. The BLM voltage was 65 mV. The solution was the same as in Fig. 1. (Inset) Deviations of the curve 2 from monoexponential (dashed-dotted curve) and biexponential (solid curve) fits.
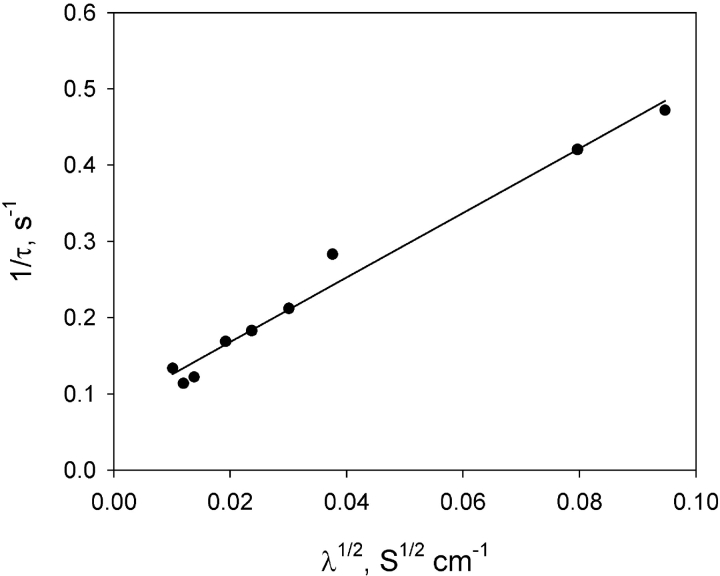
The interaction of streptavidin with gA5XB channels was studied in the present work also by the method of sensitized photoinactivation (Rokitskaya et al., 1996, 2000b) based on measuring the time course of a flash-induced decrease in the multichannel current across BLM in the presence of a photosensitizer (e.g., aluminum phthalocyanine, AlPcS3). The sensitized photoinactivation is thought to result from a damage to tryptophan residues in gramicidin, caused by light-induced generation of reactive oxygen species (Strassle and Stark, 1992; Rokitskaya et al., 1993; Kunz et al., 1995). As shown previously (Rokitskaya et al., 1996, 1997, 2000a), this method makes it possible to get information on channel kinetics from the current measurements at the multichannel level. Fig. 6 B demonstrates the kinetic curves of the flash-induced decrease (below called the kinetics of photoinactivation) in the gA5XB-mediated current in the presence of AlPcS3 measured before (curve 1) and after (curve 2) the addition of 0.5 μg/ml streptavidin. It is seen that streptavidin provoked a tremendous deceleration of the gA5XB photoinactivation kinetics that is similar to the effect of avidin described earlier (Rokitskaya et al., 2000a). The control time course of photoinactivation in the absence of streptavidin was well fitted by a monoexponential curve, which allowed us to determine the rate constants of formation (k R) and dissociation (k D) of gA5XB channels by plotting the reciprocal of the characteristic time constant, τ, of the kinetic curves versus the square root of the steady-state multichannel conductance (Fig. 7) according to Bamberg and Lauger (1973) and Rokitskaya et al. (1996) (see ). From the approximation of this plot by a linear function we calculated the following values of the rate constants (k R and k D) and the equilibrium constant K: k D = 0.083 s−1, k R = 1.4 × 1014 s−1 mole−1cm2, K = k R/k D = 1.7 × 1015 mole−1cm2.
Figure 7.
The dependence of the reciprocal of the characteristic time constant (τ) of the exponential curve fitting the photoinactivation kinetics (i.e., the time course of the flash-induced decrease in the gA5XB-mediated current in the presence of 1 μM AlPcS3) on the square root of the steady-state multichannel conductance (λ). A solid line represents the linear approximation of the dependence. The example of the photoinactivation kinetics is shown in Fig. 6 B (curve 1). The temperature was 22°C.
The time course of gA5XB photoinactivation recorded after the addition of streptavidin was poorly fitted by a monoexponential curve, but was well described by a sum of two exponentials (Fig. 6 B): I/I 0 = α1exp(−t/τ1) + α2exp(−t/τ2) + C, where α1 = 1.5%, τ1 = 3.2 s, α2 = 5.2%, τ2 = 90 s, C = 93.3%. As seen from the Fig. 8, A and B , the contribution (α2) of the slow phase (the component with a longer characteristic time, τ2) to the overall kinetics decreased upon including biotinylated lipid in the BLM and became negligible at the biotin-PE content of 1% and higher. It should be noted, however, that in contrast to the decrease in the occurrence of the streptavidin-induced double-conductance channels of gA5XB observed at very low biotin-PE content (2 × 10−4%), the reduction of the slow phase contribution to the photoinactivation kinetics manifested itself noticeably only at the biotin-PE content >0.02%. Thus, the comparison of the effect of biotin-PE on the gA5XB single-channel activity with that on the kinetics of the sensitized photoinactivation of the gA5XB multichannel currents in the presence of streptavidin revealed the pronounced difference between the dependencies of these effects on the biotin-PE content. Namely, the threshold content of biotin-PE, at which its inhibiting effect on the double-conductance channels became noticeable, differed from the minimum content of biotin-PE giving rise to the inhibition of the streptavidin-induced deceleration of the gA5XB photoinactivation kinetics by two orders of magnitude (compare Figs. 5 and 8). However, this difference became insignificant, if both dependencies were plotted versus the ratio between the surface concentrations of biotin-PE and gA5XB in the membrane (Fig. 8 B, inset). This ratio was calculated as described in the . It is seen that the inflection point of both dependencies corresponded to the [biotin-PE]/[gA5XB] ratio of ∼100.
Figure 8.
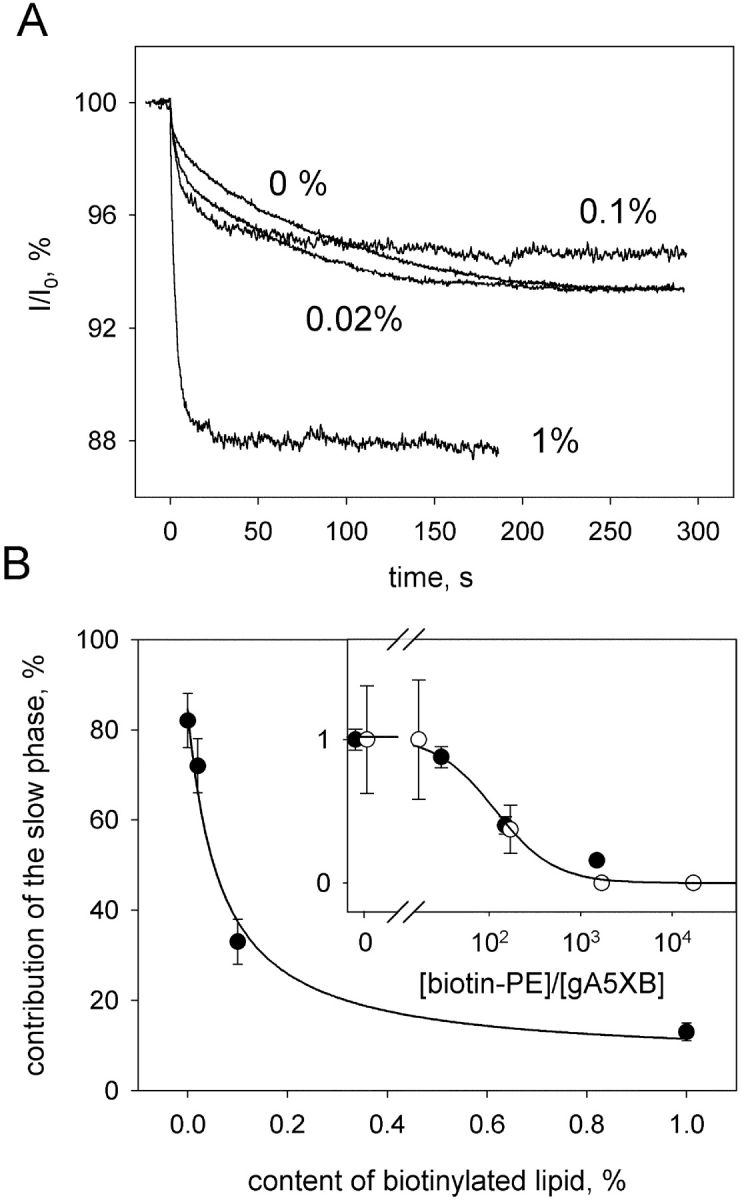
Effect of biotinylated lipid (biotin-PE) on the gA5XB photoinactivation kinetics in the presence of streptavidin. (A) The time courses of the decrease in the gA5XB-mediated current across a BLM after a flash of visible light (at t = 0 s) in the presence of 1 μM AlPcS3 after the addition of 0.5 μg/ml streptavidin to both sides of the BLM formed from the DPhPC solution with different contents of biotinylated lipid (0%, 0.02%, 0.1%, 1%). The normalized values of the current (I/I 0) are plotted versus the time. The initial current (I 0) was ∼1 μA. The BLM voltage was 65 mV. The solution was the same as in Fig. 1. (B) The contribution of the slow phase to the photoinactivation kinetics plotted versus the content of biotinylated lipid in the membrane-forming solution. (Inset) The normalized occurrence of the double-conductance channels (open circles) and the normalized contribution of the slow phase (filled circles) are replotted versus the ratio of the surface concentration of biotin-PE to that of gA5XB. The line was drawn by eye.
It should be pointed out that in all our experiments streptavidin was present in the excess providing infinite stock of biotin-binding sites for gA5XB even at high biotin-PE content. In particular, streptavidin was added at 1 μg/ml concentration corresponding to 70 nM, whereas gA5XB was added at 10 pM and 5 nM in single-channel and multichannel experiments, respectively. The volume concentration of biotin-PE in the experimental cell even at the high content of 1% in the BLM was <10 pM. It can be easily estimated by dividing the number of biotin-PE molecules in the BLM, calculated as described in the , by the cell volume (3 ml).
DISCUSSION
The most striking finding of this work consists in the observation of an unusual state of gramicidin channels that is characterized by the extraordinary long open state duration and the predominant conductance approximately twice as high as the usual single-channel conductance of gA5XB channels (Figs. 1 and 4). This state observed in the presence of the biotin-binding proteins functionally corresponds to the synchronous opening of two gA5XB channels. Similar long-lived open channel state is described in the accompanying article (Goforth et al., 2003) using covalently linked gramicidin analogues.
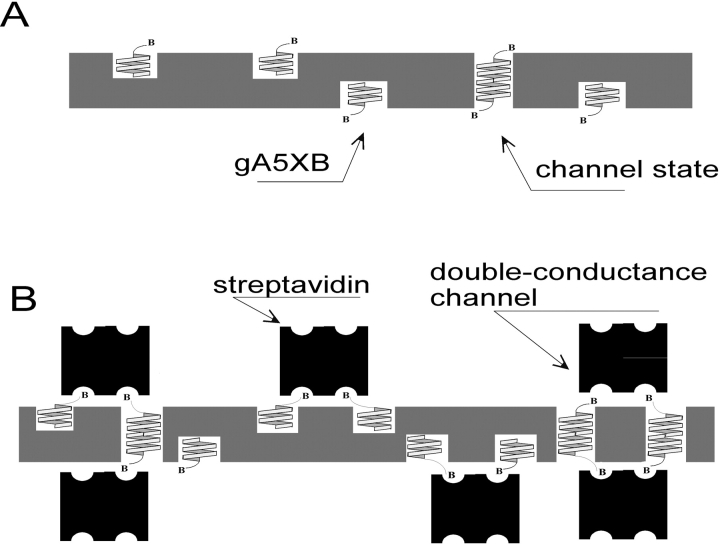
Taking into account the properties of the double-conductance channels elicited by the interaction of gA5XB with streptavidin and avidin (the value of the predominant conductance level approximately doubled as compared with that of the usual gramicidin single channels and the occurrence of the transient substate [Fig. 2] at the level corresponding to the usual single-channel conductance), it is reasonable to suggest that the double-conductance channels are formed as a result of bivalent binding of two (strept)avidin molecules located at the opposite sides of the BLM to the COOH termini of two transmembrane dimers of gA5XB at both water-membrane interfaces (Fig. 9 B, right). Here the bivalent binding means that two (of the four) biotin-binding sites on (strept)avidin are occupied by biotin groups belonging to two gA5XB molecules. Most likely, the formation of such a complex is preceded by cross-linking of pairs of gA5XB monomers through binding to streptavidin at each side of the BLM. The opening of the double-conductance channel apparently corresponds to the moment when the pairs of gA5XB monomers bound to streptavidin at opposite sides of the BLM associate through hydrogen bonds between their NH2 termini, thereby forming two neighboring transmembrane dimers. This couple of gA5XB channels appears to be highly stable, obviously due to the increased number of hydrogen bonds stabilizing the complex and/or to a reduction of the elastic force originating from a local thinning of the bilayer that acts on each transmembrane dimer in the couple (see the accompanying paper by Goforth et al. [2003][this issue] and Miloshevsky et al. [2002]). The alternative explanation of the complex formation, assuming that streptavidin binds to two preformed transmembrane dimers of gA5XB, seems to be unlikely because in this case it is difficult to explain the suppression of the gA5XB-mediated current observed after the addition of streptavidin both at the single-channel and at the multichannel level (Fig. 6 A).
Figure 9.
Scheme of formation of the tandem channel comprising two gA5XB channels cross-linked by streptavidin.
The transient substate of ∼50-ms duration (Fig. 2) may be tentatively ascribed to the process of mutual reorientation of the cross-linked pairs of gA5XB monomers, so as the proper conformation for linking into two neighboring head-to-head dimers is achieved. We note that the difference in the two current steps leading to the opening of the double-conductance gA5XB channel is in good agreement with the corresponding property of the double-barreled channels observed by Goforth et al. (2003) with covalently linked gramicidin analogues (the accompanying paper).
We believe that the fast fluctuations of the current (Fig. 3 A) preceding the emergence of the double-conductance channels also reflect certain steps of the mutual reorientation of streptavidin-bound gA5XB pairs that are accompanied by transient formation of transmembrane dimers (but not pairs of dimers) manifesting itself in short-lived openings of channels of the usual conductance. Remarkably, the similar bursting channel activity followed by a two-step transition to the higher current level was observed by Goforth et al. (2003) with covalently linked gramicidin analogues.
If the bivalent binding of streptavidin to a pair of gA5XB channels takes place at only one side of the BLM, whereas at the opposite side of the BLM the monovalent binding of streptavidin to gA5XB (which means that streptavidin has a single biotin-binding site occupied) occurs (Fig. 9 B, left), then another long-lived state of gA5XB channels may be expected to arise. We surmise that due to the reduced gA5XB surface density this state of gA5XB channels was observed with the large-diameter membranes both in this work (Fig. 4) and in the previous study (Rokitskaya et al., 2000a). The prolonged duration of this state having the standard single-channel conductance may be associated with such factors as lateral interaction of peptide helices in the membrane and an increased molecular weight of the channel former bound to streptavidin.
The fact that gA2XB proved to be unable to form the double-conductance channels in the presence of streptavidin despite the unambiguous evidence in favor of streptavidin binding to gA2XB shows that the long linker arm, obviously providing better access to biotin-binding pockets of streptavidin due to higher flexibility of a biotin group in gA5XB, is needed for the streptavidin interaction with biotinylated gramicidin to result in the formation of the double-conductance channels. Obviously, the cross-linking of two transmembrane dimers of biotinylated gramicidin requires that two binding sites should be located within the reach of biotin groups linked to two gramicidin molecules. The role of linker flexibility and the local monomer concentration is also discussed in the accompanying article by Goforth et al. (2003).
The model of the double-conductance channel representing a tandem of two neighboring gA5XB channels with their COOH termini being cross-linked by the bound streptavidin at both sides of the BLM is supported by the fact that the one-side addition of streptavidin to the bathing solution of the BLM containing gA5XB led neither to the formation of the double-conductance channels, nor to the appearance of the fast current fluctuations (unpublished data). We believe that the formation of the double-conductance channels represents the reason of the deceleration of the gA5XB photoinactivation kinetics observed at the multichannel level here with streptavidin and previously (Rokitskaya et al., 2000a) with avidin. In principle, the double-conductance channels might be formed upon addition of streptavidin at one side of the membrane. However, the probability of the formation of a transmembrane complex from cross-linked gA5XB in one monolayer and a pair of free gA5XB in the opposite monolayer should be substantially lower than that from cross-linked peptides located at both sides of the membrane. In fact, the concentration of gA5XB is very low under the conditions of single-channel measurements and the probability of the simultaneous appearance of two free peptides in close vicinity of the cross-linked gA5XB should be negligible. It is worth noting that at the multichannel level a noticeable deceleration of gA5XB kinetics was observed (Rokitskaya et al., 2000a), even in the case of avidin addition at one side of the BLM.
The data on the prevention of the opening of the double-conductance channels (Fig. 5) and on the inhibition of the deceleration of the photoinactivation kinetics of gA5XB (Fig. 8) by including biotinylated lipids (biotin-PE) into the DPhPC membrane are also in good agreement with the proposed model. Apparently, biotin-PE competing with gA5XB for the binding sites on streptavidin should prevent the bivalent but not the monovalent binding of the protein to gA5XB, if streptavidin is present in the excess with respect to gA5XB and biotin-PE. This statement was substantiated by a theory (see ) that considered explicitly the competition between gA5XB and biotin-PE for two binding sites on streptavidin at the membrane surface under the conditions of the excess streptavidin in the bulk phase. The system of equations led to a four-power equation that was solved numerically for a wide range of parameters. This theoretical model allowed us to calculate that the Stv binding constant for gA5XB exceeds that for biotin-PE by a factor of 40.
The conclusions based on the theoretical consideration strongly support the bivalent character of the (strept)avidin interaction with gA5XB. In particular, it was demonstrated that the bivalent binding model readily explained the inhibiting effect of biotin-PE on the formation of the long-lived channel state of gA5XB, whereas the monovalent binding model failed to explain this inhibition.
In summary, it can be concluded that the interaction with streptavidin turns two independent gramicidin channels into a couple of conducting units operating in concert. In other words, streptavidin plays a role of a multivalent ligand, binding of which to membrane receptors (gA5XB) activates the ionic channels by converting them into a new highly stable open state formed by a tandem of the transmembrane dimers. This kind of streptavidin interaction with gA5XB channels models the known phenomenon of ligand-induced receptor dimerization wherein membrane proteins containing single transmembrane α-helices associate to form dimers (or larger oligomers) in the membrane, either as stable complexes or as transient species, in response to the binding of a suitable ligand. Such dimerization or oligomerization have been shown to occur after binding of several polypeptide hormones, cytokines, growth factors, or growth inhibitors to their receptors (Lemmon and Schlessinger, 1994; Heldin, 1995).
Acknowledgments
We are grateful to Professor F. Separovic and A. Anastasiadis (University of Melbourne) for the gifts of gA5XB and gA2XB. The valuable discussions with Professors O.S. Andersen (Cornell University) and R.E. Koeppe (University of Arkansas) are greatly appreciated.
This work was supported in part by grants from the RFBR: 03–04–48905 (Y.N. Antonenko), 03–04–06151 (T.I. Rokitskaya), and Fogarty Award 510–1392–4220.
Edward Moczydlowski served as guest editor.
APPENDIX I
Assuming that gA5XB monomers (A) and dimers (A 2) floating in the membrane are in equilibrium with each other,
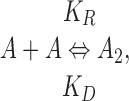 |
(A1) |
we estimated monomer [N 1] and dimer [N 2] equilibrium concentrations in the membrane from the measured values of gA5XB-mediated current, that is proportional to the number of gA5XB open channels, and the equilibrium constant (K). The equilibrium concentrations of monomers and dimers established as a result of the reversible dimerization reaction (Eq. A1) obey the following equation:
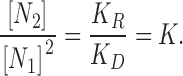 |
(A2) |
As shown in (Rokitskaya et al., 1996), the values of K R, K D, and K can be calculated from the plot of 1/τ versus (λ∞)0.5, obtained by measuring the flash-induced gramicidin-mediated current transients following exponential kinetics with a characteristic time (τ):
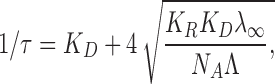 |
(A3) |
where N A is Avogadro's number, λ∞ is the steady-state BLM conductance after the light flash, and Λ is the single-channel conductance.
The number of gA5XB open channels, N 2, is proportional to the concentration of dimers [N 2] according to the equation:
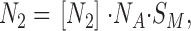 |
(A4) |
where S m is the membrane area.
From Eqs. A2 and A4 it can be derived that,
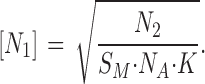 |
(A5) |
The number of gA5XB monomers in the membrane, N 1, is proportional to the concentration of monomers [N 1] according to the equation:
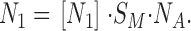 |
(A6) |
Thus, we obtain:
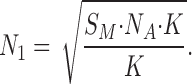 |
(A7) |
Bearing in mind that in single-channel experiments the average number of open channels N 2 is equal to 1, we substituted the values of S m ≃ 10−4 cm2 for the small-diameter membrane and K = 1.7 × 1015 mole−1 cm2 into Eq. A7, and calculated that N1 ≃ 200 under single-channel conditions. Based on the known value of lateral area per lipid molecule in a BLM, 0.6 nm2 (Cornell et al., 1980; Koenig et al., 1997; Nagle and Tristram-Nagle, 2000; Balgavy et al., 2001), we estimated the total number of lipid molecules in the membrane to be 1.7 × 1010 by dividing the value of S m ≃ 10−4 cm2 by 0.6 nm2.
Under multichannel conditions, the number of gA5XB dimers, N 2, can be estimated from the equation based on Ohm's law:
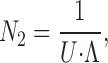 |
(A8) |
where I is the value of the gA5XB-mediated current, U is the voltage applied to the BLM, and Λ is the single-channel conductance. By substituting the average value of I in multichannel experiments (1 μA), U = 65 mV and Λ = 17.6 pS into Eq. A8, we calculated N 2 to be ∼106. Taking into account the different value of S m for the membranes used in multichannel experiments, 2 × 10−3 cm2, we used Eq. A6 to calculate the number of monomers N 1 under these conditions and obtained N 1 ≃ 106. Thus, the overall number of gA5XB molecules (monomers and dimers) was ∼2 × 106. The total number of lipid molecules in the membrane of this area was estimated to be 3 × 1011 by dividing the value of S m ≃ 2 × 10−3 cm2 by 0.6 nm2.
Given the total number of lipid molecules and the number of gA5XB molecules in the BLMs in single-channel and in multichannel experiments, we calculated the ratios of biotin-PE and gA5XB surface concentrations corresponding to different values of the biotin-PE content in the BLM for both conditions.
APPENDIX II
Let us consider a system comprising streptavidin (Stv) molecules floating in the bathing solution and those adsorbed on BLM, on one hand, and biotinylated gramicidin (gA5XB) molecules incorporated in BLM, on the other hand. The membrane also contains molecules of biotinylated phosphatidylethanolamine (biotin-PE). We consider here the interaction that takes place at one side of the membrane neglecting the influence of the formation of transmembrane complexes (channels) on the inhibiting effect of biotin-PE. Let's assume that the total concentration of gA5XB and biotin-PE in BLM and the concentration of Stv in the aqueous solution are constant and equal to N, M, and [Stv], respectively. The latter condition means that the bathing solution represents the streptavidin stock of the infinite capacity which is valid for planar BLM under our experimental conditions (see results). The interaction of streptavidin with biotinylated gramicidin and biotinylated lipid at one side of the membrane can be described by a system of reversible reactions:
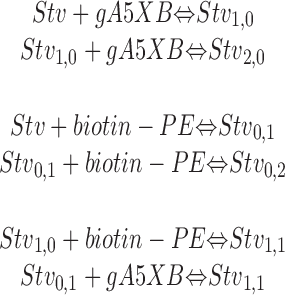 |
(A9) |
Here it is assumed that two binding sites on streptavidin are effective in the interaction with biotin groups exposed at the membrane surface, because it is known that the biotin-binding pockets are located in pairs at opposite faces of the streptavidin tetramer (Weber et al., 1989). This is consistent with a recent conclusion (Niemeyer et al., 1999) that this tetravalent protein displays a high preference for acting as a bivalent linker molecule. The above equations imply that the interaction of gA5XB and biotin-PE with Stv is weak enough to let these reactions be reversible. The interaction of biotin with streptavidin in solution is hardly reversible and is known as one of the strongest noncovalent bonds (Weber et al., 1989). However, as shown in (Zhao and Reichert, 1992; Perez-Luna et al., 1999), the interaction of Stv with biotinylated lipids at the membrane–water interface is not so strong and is reversible. Obviously, the interaction of (strept)avidin with gA5XB at the interface is also reversible, because the addition of biotin to the bathing solution removed the avidin-induced deceleration of the gA5XB photoinactivation kinetics (Rokitskaya et al., 2000a).
The first and third equations in Eq. A9 are characterized by three-dimensional binding constants K 1 (in M−1) and K 2 (in M−1), whereas the other equations are characterized by two-dimensional binding constants. For the sake of simplicity we assume that all these two-dimensional constants are equal to each other and amount to K X (in cm2/mole). Because the total concentration of gA5XB is equal to N and that of biotin-PE is equal to M, it can be written:
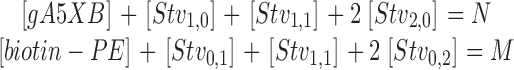 |
(A10) |
The above system of equations can be converted to the following equations by using dimensionless parameters (Perelson, 1981; Stone et al., 2001):
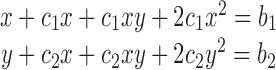 |
(A11) |
where x = [gA5XB]KX; c 1 = [Stv]K 1; b 1 = NKX; y = [biotin-PE]K X; c 2 = [Stv]K 2; b 2 = MKX.
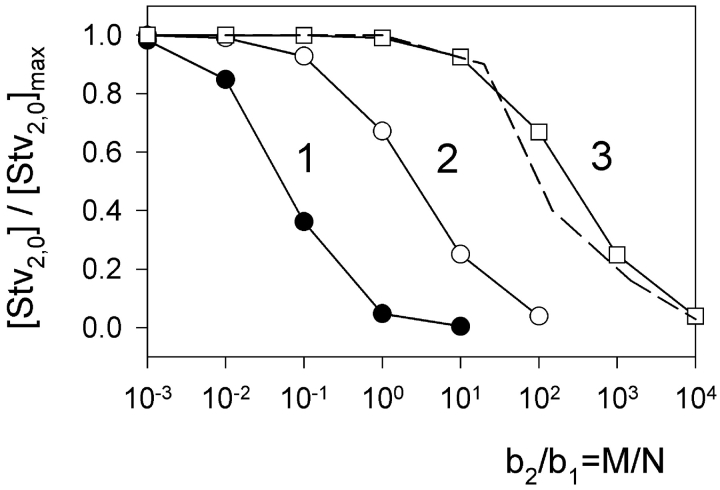
This system of two equations was solved numerically. The solution enabled us to calculate the effect of increasing concentration of biotin-PE on the number of Stv2,0 complexes that are formed at one side of the membrane and are thought to be the precursors of the double-conductance gA5XB channels. The dependence of [Stv2,0] on c 1 has a bell-like shape with a maximum at c 1 ≃ 1. So we performed further calculations at c 1 = 1, because the Stv-induced deceleration of gA5XB channel kinetics depended weakly on [Stv] under our experimental conditions (unpublished data). Fig. 10 shows the dependence of [Stv2,0] on b 2/b1 (which is equal to M/N) at b 1 = 103 and different values of c 2, namely c 2 = 102 (curve 1), c 2 = 1 (curve 2), and c 2 = 10−2 (curve 3). It means that the ratio of two constants K 1 and K 2 is varied. It is seen from Fig. 10 that the variation of K 1/K 2 is accompanied by a shift of theoretical dependencies of [Stv2,0] on the ratio of b 2/b1, so that the value of the b 2/b1 ratio corresponding to the inflection point of the dependence correlates with the value of the K 1/K 2 ratio. Further calculations have shown that this correlation exists in a wide range of parameters provided that b 1 >> 1, and the b 2/b1 ratio at the inflection point appears to be independent of b 1 under these conditions. The data presented in Fig. 8 B (inset) proved that the latter statement is valid for our experimental conditions, as the dependence of the occurrence of Stv-induced double-conductance channels recorded at very low gA5XB concentration (low b 1 value) on the ratio of biotin-PE and gA5XB concentrations coincided with the corresponding dependence of the contribution of the slow phase to the photoinactivation kinetics measured at high gA5XB concentration (high b 1 value). Thus, it may be concluded that b 1 >> 1, i.e., N >> 1/K X, under all our experimental conditions including single-channel recordings, obviously due to extremely high (strept)avidin-biotin binding constant (Weber et al., 1989). The theoretical calculations have shown that the b 2/b1 ratio at the inflection point essentially depends on b 1, if b 1 < 1.
Figure 10.
Theoretical dependence of the equilibrium concentration of a complex of streptavidin with two gA5XB molecules at the membrane surface ([Stv2,0]) on the concentration of biotin-PE in the membrane. The calculations were performed by solving the system of equations (A11) numerically with the following parameters: c 1 = 1, b 1 = 103, c 2 = 102 (curve 1), c 2 = 1 (curve 2), and c 2 = 10−2 (curve 3). The dashed line is an averaged experimental curve from the data presented in the inset to Fig. 8 B.
As seen from Fig. 8, inset, the inflection point of experimental dependencies corresponds to the [biotin-PE]/[gA5XB] ratio, i.e., the b 2/b1 ratio, of ∼100. As follows from Fig. 10, it means that K 1/K 2 is of the order of 100 (curve 3). The precise calculations have given the value of K 1/K 2 = 40. Therefore, we have come to an important conclusion that our theoretical model makes it possible to estimate the ratio of Stv binding constants for gA5XB and biotin-PE. The calculated difference between these binding constants can be explained by taking into account that in both gA5XB and biotin-PE biotin groups are attached to species embedded in the lipid membrane hindering their interaction with water-soluble streptavidin, but a long linker arm present in gA5XB and not in biotin-PE provides higher accessibility of biotin in gA5XB as compared with biotin-PE.
Footnotes
Abbreviations used in this paper: AlPcS3, aluminum trisulfophthalocyanine; BLM, bilayer lipid membrane; DPhPC, diphytanoylphosphatidylcholine.
References
- Anastasiadis, A., F. Separovic, and J. White. 2001. Synthesis of deuterated aminocaproyl linkers. Aust. J. Chem. 54:747–750. [Google Scholar]
- Andersen, O.S., H.J. Apell, E. Bamberg, D.D. Busath, R.E. Koeppe, F.J. Sigworth, G. Szabo, D.W. Urry, and G.A. Woolley. 1999. Gramicidin channel controversy–the structure in a lipid environment. Nat. Struct. Biol. 6:609–612. [DOI] [PubMed] [Google Scholar]
- Anzai, N., E. Deval, L. Schaefer, V. Friend, M. Lazdunski, and E. Lingueglia. 2002. The multivalent PDZ domain-containing protein CIPP is a partner of acid sensing ion channel 3 (ASIC3) in sensory neurons. J. Biol. Chem. 277:16655–16661. [DOI] [PubMed] [Google Scholar]
- Balgavy, P., M. Dubnickova, N. Kucerka, M.A. Kiselev, S.P. Yaradaikin, and D. Uhrikova. 2001. Bilayer thickness and lipid interface area in unilamellar extruded 1,2-diacylphosphatidylcholine liposomes: a small-angle neutron scattering study. Biochim. Biophys. Acta. 1512:40–52. [DOI] [PubMed] [Google Scholar]
- Bamberg, E., and P. Lauger. 1973. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J. Membr. Biol. 11:177–194. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., and A. Maximov. 2001. PDZ domains: More than just a glue. Proc. Natl. Acad. Sci. USA. 98:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier, M. 2001. Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2:274–286. [DOI] [PubMed] [Google Scholar]
- Breitinger, H.G. 2001. Fast kinetic analysis of ligand-gated ion channels. Neuroscientist. 7:95–103. [DOI] [PubMed] [Google Scholar]
- Bukauskas, F.F., K. Jordan, A. Bukauskiene, M.V. Bennett, P.D. Lampe, D.W. Laird, and V.K. Verselis. 2000. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. USA. 97:2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, N.A., K. Takimoto, D. Li, W. Han, S.C. Watkins, and E.S. Levitan. 1999. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J. Gen. Physiol. 113:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell, J.H. 2000. Clustering of sodium channels at the neuromuscular junction. Microsc. Res. Tech. 49:84–89. [DOI] [PubMed] [Google Scholar]
- Chen, L., H. Wang, S. Vicini, and R.W. Olsen. 2000. The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl. Acad. Sci. USA. 97:11557–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran, J.R., D. Aivazian, T.O. Cameron, and L.J. Stern. 2001. Receptor clustering and transmembrane signaling in T cells. Trends Biochem. Sci. 26:304–310. [DOI] [PubMed] [Google Scholar]
- Colledge, M., and S.C. Froehner. 1998. Signals mediating ion channel clustering at the neuromuscular junction. Curr. Opin. Neurobiol. 8:357–363. [DOI] [PubMed] [Google Scholar]
- Cornell, B.A., V.L. Braach-Maksvytis, L.G. King, P.D. Osman, B. Raguse, L. Wieczorek, and R.J. Pace. 1997. A biosensor that uses ion-channel switches. Nature. 387:580–583. [DOI] [PubMed] [Google Scholar]
- Cornell, B.A., J. Middlehurst, and F. Separovic. 1980. The molecular packing and stability within highly curved phospholipid bilayers. Biochim. Biophys. Acta. 598:405–410. [DOI] [PubMed] [Google Scholar]
- Cross, T.A., A. Arseniev, B.A. Cornell, J.H. Davis, J.A. Killian, R.E. Koeppe, L.K. Nicholson, F. Separovic, and B.A. Wallace. 1999. Gramicidin channel controversy–revisited. Nat. Struct. Biol. 6:610–612. [DOI] [PubMed] [Google Scholar]
- Dargan, S.L., E.J. Lea, and A.P. Dawson. 2002. Modulation of type-1 Ins(1,4,5)P3 receptor channels by the FK506-binding protein, FKBP12. Biochem. J. 361:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour, A.H. 1997. Function and modulation of bacterial porins: insights from electrophysiology. FEMS Microbiol. Lett. 151:115–123. [DOI] [PubMed] [Google Scholar]
- Duggan, A., J. Garcia-Anoveros, and D.P. Corey. 2002. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J. Biol. Chem. 277:5203–5208. [DOI] [PubMed] [Google Scholar]
- Eghbali, M., J.P. Curmi, B. Birnir, and P.W. Gage. 1997. Hippocampal GABA(A) channel conductance increased by diazepam. Nature. 388:71–75. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E., S.E. Craven, D.M. Chetkovich, B.L. Firestein, E. Schnell, C. Aoki, and D.S. Bredt. 2000. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 148:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, M.M. 2000. Connexin-specific distribution within gap junctions revealed in living cells. J. Cell Sci. 113:4109–4120. [DOI] [PubMed] [Google Scholar]
- Fanning, A.S., and J.M. Anderson. 1999. Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol. 11:432–439. [DOI] [PubMed] [Google Scholar]
- Futaki, S., Z. Youjun, and Y. Sugiura. 2001. Detecting a tag on a channel opening: blockage of the biotinylated channels by streptavidin. Tetrahedron Lett. 42:1563–1565. [Google Scholar]
- Garner, C.C., J. Nash, and R.L. Huganir. 2000. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 10:274–280. [DOI] [PubMed] [Google Scholar]
- Geletyuk, V.I., and V.N. Kazachenko. 1989. Single potential-dependent K+ channels and their oligomers in molluscan glial cells. Biochim. Biophys. Acta. 981:343–350. [DOI] [PubMed] [Google Scholar]
- Goforth, R.L., A.K. Chi, L.L. Providence, R.E. Koeppe, II, O.S. Andersen, and D.V. Greathouse. 2003. Hydrophobic coupling of lipid bilayer energetics to channel function. J. Gen. Physiol. 121:477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, N.M. 1990. Avidin and streptavidin. Methods Enzymol. 184:51–67. [DOI] [PubMed] [Google Scholar]
- Guo, C., and H. Levine. 1999. A thermodynamic model for receptor clustering. Biophys. J. 77:2358–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin, C.H. 1995. Dimerization of cell surface receptors in signal transduction. Cell. 80:213–223. [DOI] [PubMed] [Google Scholar]
- Hendrickson, W.A., A. Pahler, J.L. Smith, Y. Satow, E.A. Merritt, and R.P. Phizackerley. 1989. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc. Natl. Acad. Sci. USA. 86:2190–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore, E., B. Attali, G. Romey, F. Lesage, J. Barhanin, and M. Lazdunski. 1992. Different types of K+ channel current are generated by different levels of a single mRNA. EMBO J. 11:2465–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio, Y., H. Hibino, A. Inanobe, M. Yamada, M. Ishii, Y. Tada, E. Satoh, Y. Hata, Y. Takai, and Y. Kurachi. 1997. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J. Biol. Chem. 272:12885–12888. [DOI] [PubMed] [Google Scholar]
- Hung, A.Y., and M. Sheng. 2002. PDZ domains: structural modules for protein complex assembly. J. Biol. Chem. 277:5699–5702. [DOI] [PubMed] [Google Scholar]
- Hymel, L., J. Striessnig, H. Glossmann, and H. Schindler. 1988. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc. Natl. Acad. Sci. USA. 85:4290–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, F., S. Maeda, T. Doi, and Y. Fujiyoshi. 2002. Ligand binding of the second PDZ domain regulates clustering of PSD-95 with the Kv1.4 potassium channel. J. Biol. Chem. 277:3640–3646. [DOI] [PubMed] [Google Scholar]
- Issa, N.P., and A.J. Hudspeth. 1994. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc. Natl. Acad. Sci. USA. 91:7578–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe, E.H., and K. Angelides. 1992. Clustering of voltage-dependent sodium channels on axons depends on Schwann cell contact. Nature. 356:333–335. [DOI] [PubMed] [Google Scholar]
- Kaplan, M.R., M.H. Cho, E.M. Ullian, L.L. Isom, S.R. Levinson, and B.A. Barres. 2001. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 30:105–119. [DOI] [PubMed] [Google Scholar]
- Kaulin, Y.A., L.V. Schagina, S.M. Bezrukov, V.V. Malev, A.M. Feigin, J.Y. Takemoto, J.H. Teeter, and J.G. Brand. 1998. Cluster organization of ion channels formed by the antibiotic syringomycin E in bilayer lipid membranes. Biophys. J. 74:2918–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazachenko, V.N., and V.I. Geletyuk. 1984. The potential-dependent K+ channel in molluscan neurones is organized in a cluster of elementary channels. Biochim. Biophys. Acta. 773:132–142. [DOI] [PubMed] [Google Scholar]
- Kim, E., M. Niethammer, A. Rothschild, Y.N. Jan, and M. Sheng. 1995. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 378:85–88. [DOI] [PubMed] [Google Scholar]
- Koenig, B.W., H.H. Strey, and K. Gawrisch. 1997. Membrane lateral compressibility determined by NMR and x-ray diffraction: effect of acyl chain polyunsaturation. Biophys. J. 73:1954–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe, R.E., and O.S. Andersen. 1996. Engineering the gramicidin channel. Annu. Rev. Biophys. Biomol. Struct. 25:231–258. [DOI] [PubMed] [Google Scholar]
- Kullman, L., M. Winterhalter, and S.M. Bezrukov. 2002. Transport of maltodextrins through maltoporin: a single-channel study. Biophys. J. 82:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, L., U. Zeidler, K. Haegele, M. Przybylski, and G. Stark. 1995. Photodynamic and radiolytic inactivation of ion channels formed by gramicidin A: oxidation and fragmentation. Biochemistry. 34:11895–11903. [DOI] [PubMed] [Google Scholar]
- Kurschner, C., P.G. Mermelstein, W.T. Holden, and D.J. Surmeier. 1998. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol. Cell. Neurosci. 11:161–172. [DOI] [PubMed] [Google Scholar]
- Larsen, E.H., S.E. Gabriei, M.J. Stutts, J. Fullton, E.M. Price, and R.C. Boucher. 1996. a. Endogenous chloride channels of insect sf9 cells. Evidence for coordinated activity of small elementary channel units. J. Gen. Physiol. 107:695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, E.H., E.M. Price, S.E. Gabriel, M.J. Stutts, and R.C. Boucher. 1996. b. Clusters of Cl− channels in CFTR-expressing Sf9 cells switch spontaneously between slow and fast gating modes. Pflugers Arch. 432:528–537. [DOI] [PubMed] [Google Scholar]
- Laver, D.R., and P.W. Gage. 1997. Interpretation of substates in ion channels: unipores or multipores? Prog. Biophys. Mol. Biol. 67:99–140. [DOI] [PubMed] [Google Scholar]
- Lemmon, M.A., Z. Bu, J.E. Ladbury, M. Zhou, D. Pinchasi, I. Lax, D.M. Engelman, and J. Schlessinger. 1997. Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 16:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M.A., and J. Schlessinger. 1994. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem. Sci. 19:459–463. [DOI] [PubMed] [Google Scholar]
- Liu, D.T., G.R. Tibbs, P. Paoletti, and S.A. Siegelbaum. 1998. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 21:235-248. [DOI] [PubMed] [Google Scholar]
- Livnah, O., E.A. Bayer, M. Wilchek, and J.L. Sussman. 1993. Three-dimensional structures of avidin and the avidin-biotin complex. Proc. Natl. Acad. Sci. USA. 90:5076–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, S.O., K. Ondrias, and A.R. Marks. 1998. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science. 281:818–821. [DOI] [PubMed] [Google Scholar]
- Metzger, H. 1992. Transmembrane signaling: the joy of aggregation. J. Immunol. 149:1477–1487. [PubMed] [Google Scholar]
- Meves, H., and K. Nagy. 1989. Multiple conductance states of the sodium channel and of other ion channels. Biochim. Biophys. Acta. 988:99–105. [DOI] [PubMed] [Google Scholar]
- Miloshevsky, G.V., M.B. Partenskii, and P.C. Jordan. 2002. Membrane mediated interactions between peptides. 2. Many-body effects. Biophys. J. 82:146a. [Google Scholar]
- Mironova, G.D., M. Baumann, O. Kolomytkin, Z. Krasichkova, A. Berdimuratov, T. Sirota, I. Virtanen, and N.E.L. Saris. 1994. Purification of the channel component of the mitochondrial calcium uniporter and its reconstitution into planar lipid bilayers. J. Bioenerg. Biomembr. 26:231–238. [DOI] [PubMed] [Google Scholar]
- Nagle, J.F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehring, R.B., E. Wischmeyer, F. Doring, R.W. Veh, M. Sheng, and A. Karschin. 2000. Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J. Neurosci. 20:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, E. 2000. Digression on chemical electromagnetic field effects in membrane signaltransduction–cooperativity paradigm of the acetylcholine receptor. Bioelectrochemistry. 52:43–49. [DOI] [PubMed] [Google Scholar]
- Neumann, E., J. Weber, and T. Schurholz. 1996. The initiation of the muscle action potential. Arch. Physiol. Biochem. 104:731–744. [DOI] [PubMed] [Google Scholar]
- Niemeyer, C.M., M. Adler, B. Pignataro, S. Lenhert, S. Gao, L. Chi, H. Fuchs, and D. Blohm. 1999. Self-assembly of DNA-streptavidin nanostructures and their use as reagents in immuno-PCR. Nucleic Acids Res. 27:4553–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson, A.S. 1981. Receptor clustering on a cell surface. III. Theory of receptor cross-linking by multivalent ligands: description by ligand states. Math. Biosci. 53:1–39. [Google Scholar]
- Perez-Luna, V.H., M.J. O'Brien, K.A. Opperman, P.D. Hampton, G.P. Lopez, L.A. Klumb, and P.S. Stayton. 1999. Molecular recognition between genetically engineered streptavidin and surface bound biotin. J. Am. Chem. Soc. 121:6469–6478. [Google Scholar]
- Pugliese, L., A. Coda, M. Malcovati, and M. Bolognesi. 1993. Three-dimensional structure of the tetragonal crystal form of egg-white avidin in its functional complex with biotin at 2.7 A resolution. J. Mol. Biol. 231:698–710. [DOI] [PubMed] [Google Scholar]
- Raghuram, V., D.D. Mak, and J.K. Foskett. 2001. Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction. Proc. Natl. Acad. Sci. USA. 98:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe, C.F., R.E. Westenbroek, R. Curtis, and W.A. Catterall. 2001. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, Z., J.J. Boniface, D.S. Lyons, N. Borochov, E.J. Wachtel, and M.M. Davis. 1997. Ligand-specific oligomerization of T-cell receptor molecules. Nature. 387:617–620. [DOI] [PubMed] [Google Scholar]
- Richards, M.J., and S.E. Gordon. 2000. Cooperativity and cooperation in cyclic nucleotide-gated ion channels. Biochemistry. 39:14003–14011. [DOI] [PubMed] [Google Scholar]
- Rokitskaya, T.I., Y.N. Antonenko, and E.A. Kotova. 1993. The interaction of phthalocyanine with planar lipid bilayers - photodynamic inactivation of gramicidin channels. FEBS Lett. 329:332–335. 7689977 [Google Scholar]
- Rokitskaya, T.I., Y.N. Antonenko, and E.A. Kotova. 1996. Photodynamic inactivation of gramicidin channels: a flash-photolysis study. Biochim. Biophys. Acta. 1275:221–226. [DOI] [PubMed] [Google Scholar]
- Rokitskaya, T.I., Y.N. Antonenko, and E.A. Kotova. 1997. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophys. J. 73:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokitskaya, T.I., Y.N. Antonenko, E.A. Kotova, A. Anastasiadis, and F. Separovic. 2000. a. Effect of avidin on channel kinetics of biotinylated gramicidin. Biochemistry. 39:13053–13058. [DOI] [PubMed] [Google Scholar]
- Rokitskaya, T.I., M. Block, Y.N. Antonenko, E.A. Kotova, and P. Pohl. 2000. b. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys. J. 78:2572–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokitskaya, T.I., E.A. Kotova, and Y.N. Antonenko. 2002. Monovalent and multivalent binding of streptavidin to biotinylated gramicidin determines kinetic properties of the ion channel. Biophys. J. 82:556a. [DOI] [PubMed] [Google Scholar]
- Sano, T., and C.R. Cantor. 1995. Intersubunit contacts made by tryptophan 120 with biotin are essential for both strong biotin binding and biotin-induced tighter subunit association of streptavidin. Proc. Natl. Acad. Sci. USA. 92:3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, H., and J.P. Rosenbusch. 1981. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc. Natl. Acad. Sci. USA. 78:2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, H., F. Spillecke, and E. Neumann. 1984. Different channel properties of Torpedo acetylcholine receptor monomers and dimers reconstituted in planar membranes. Proc. Natl. Acad. Sci. USA. 81:6222–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer, W., H.A. Tritthart, and H. Schindler. 1989. The cardiac sodium channel shows a regular substate pattern indicating synchronized activity of several ion pathways instead of one. Biochim. Biophys. Acta. 986:172–186. [DOI] [PubMed] [Google Scholar]
- Schumacher, M.A., A.F. Rivard, H.P. Bachinger, and J.P. Adelman. 2001. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 410:1120–1124. [DOI] [PubMed] [Google Scholar]
- Separovic, F., S. Barker, M. Delahunty, and R. Smith. 1999. NMR structure of C-terminally tagged gramicidin channels. Biochim. Biophys. Acta. 1416:48–56. [DOI] [PubMed] [Google Scholar]
- Sheng, M., and D.T. Pak. 2000. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu. Rev. Physiol. 62:755–778. [DOI] [PubMed] [Google Scholar]
- Shieh, B.H., and M.Y. Zhu. 1996. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 16:991–998. [DOI] [PubMed] [Google Scholar]
- Stayton, P.S., S. Freitag, L.A. Klumb, A. Chilkoti, V. Chu, J.E. Penzotti, R. To, D. Hyre, I. Le Trong, T.P. Lybrand, and R.E. Stenkamp. 1999. Streptavidin-biotin binding energetics. Biomol. Eng. 16:39–44. [DOI] [PubMed] [Google Scholar]
- Stone, J.D., J.R. Cochran, and L.J. Stern. 2001. T-cell activation by soluble MHC oligomers can be described by a two- parameter binding model. Biophys. J. 81:2547–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassle, M., and G. Stark. 1992. Photodynamic inactivation of an ion channel: gramicidin A. Photochem. Photobiol. 55:461–463. [DOI] [PubMed] [Google Scholar]
- Suarez, E., E.D. Emmanuelle, G. Molle, R. Lazaro, and P. Viallefont. 1998. Synthesis and characterization of a new biotinylated gramicidin. J. Pept. Sci. 4:371–377. [DOI] [PubMed] [Google Scholar]
- Swillens, S., G. Dupont, L. Combettes, and P. Champeil. 1999. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA. 96:13750–13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemoto, M., A. Fujita, K. Higashi, and Y. Kurachi. 2002. PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron. 34:387–397. [DOI] [PubMed] [Google Scholar]
- Ternovsky, V.I., and G.N. Berestovsky. 1998. Effective diameter and structural organization of reconstituted calcium channels from the Characeae algae Nitellopsis. Membr. Cell Biol. 12:79–88. [PubMed] [Google Scholar]
- Tiffany, A.M., L.N. Manganas, E. Kim, Y.P. Hsueh, M. Sheng, and J.S. Trimmer. 2000. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J. Cell Biol. 148:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova, S.P., V.Z. Lunevskii, N.A. Spiridonov, M.G. Vinokurov, and G.N. Berestovskii. 1980. Chemical composition of calcium channels of characean algal cells. Biofizika. 25:537–542. [PubMed] [Google Scholar]
- Wang, S., H. Yue, R.B. Derin, W.B. Guggino, and M. Li. 2000. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 103:169–179. [DOI] [PubMed] [Google Scholar]
- Watras, J., I. Bezprozvanny, and B.E. Ehrlich. 1991. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J. Neurosci. 11:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, P.C., D.H. Ohlendorf, J.J. Wendoloski, and F.R. Salemme. 1989. Structural origins of high-affinity biotin binding to streptavidin. Science. 243:85–88. [DOI] [PubMed] [Google Scholar]
- Wells, J.A. 1996. Binding in the growth hormone receptor complex. Proc. Natl. Acad. Sci. USA. 93:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek, R.E., M.K. Ahlijanian, and W.A. Catterall. 1990. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 347:281–284. [DOI] [PubMed] [Google Scholar]
- Wood, S.J., and C.R. Slater. 1998. beta-Spectrin is colocalized with both voltage-gated sodium channels and ankyrin G at the adult rat neuromuscular junction. J. Cell Biol. 140:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X.Z., A. Choudhury, X. Li, and C. Montell. 1998. Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J. Cell Biol. 142:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G. 2001. Dimers among friends: ion channel regulation by dimerization of tail domains. Trends Pharmacol. Sci. 22:439–441. [DOI] [PubMed] [Google Scholar]
- Zhao, S., and W.M. Reichert. 1992. Influence of biotin lipid surface density and accessibility on avidin binding to the tip of an optical fiber sensor. Langmuir. 8:2785–2791. [Google Scholar]
- Zhou, D., S. Lambert, P.L. Malen, S. Carpenter, L.M. Boland, and V. Bennett. 1998. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]