Abstract
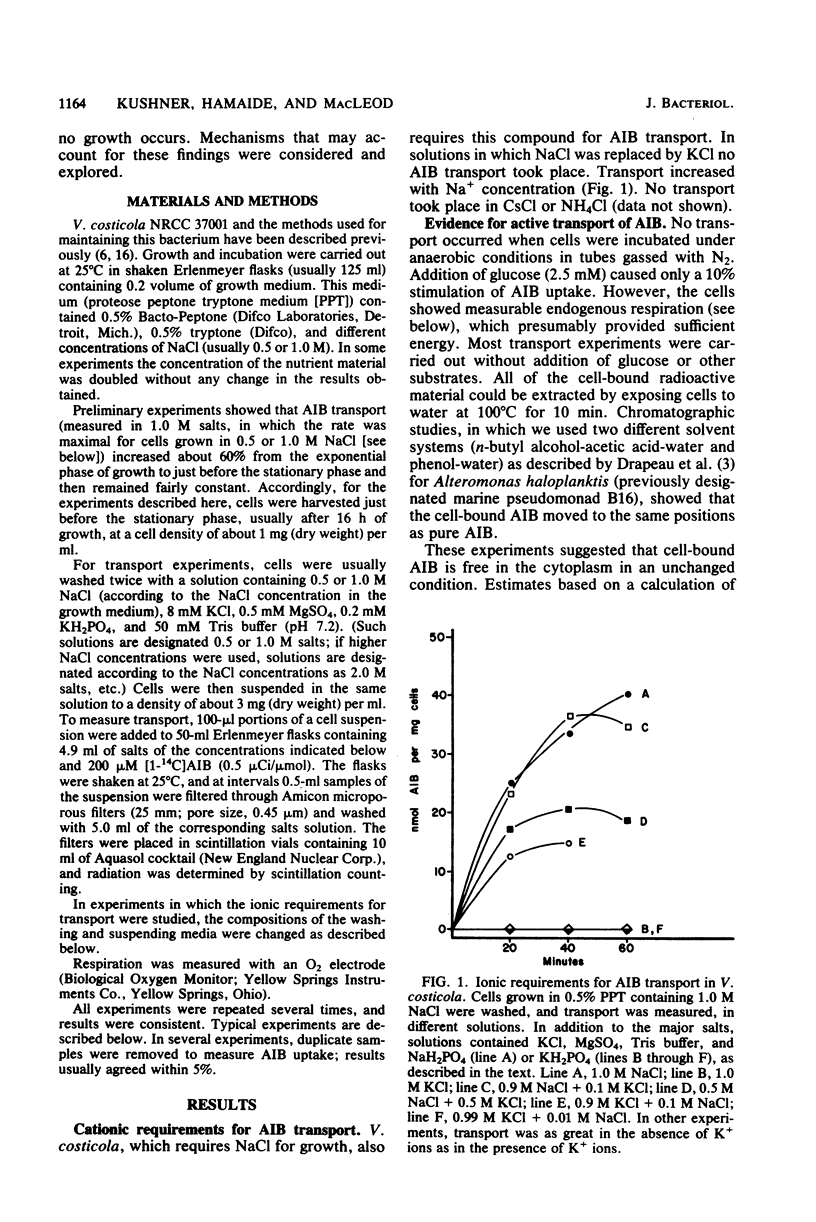
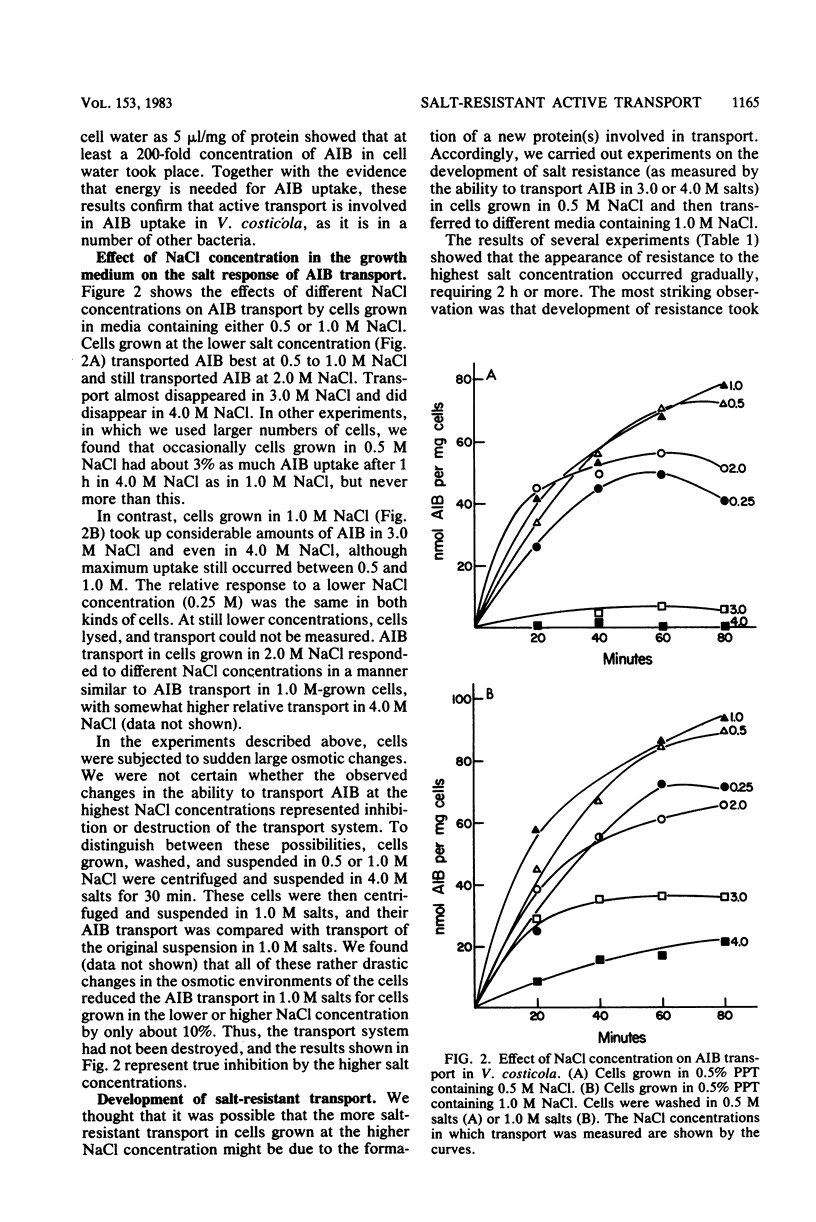
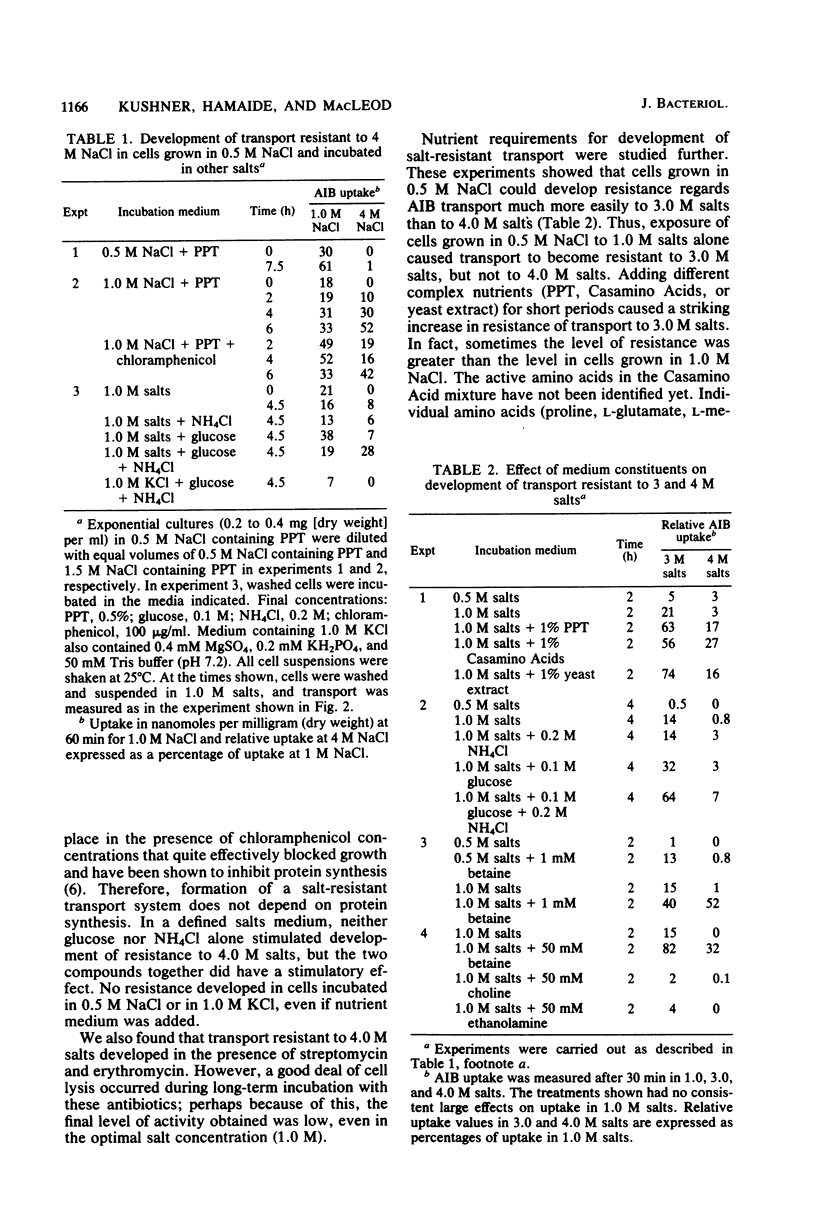
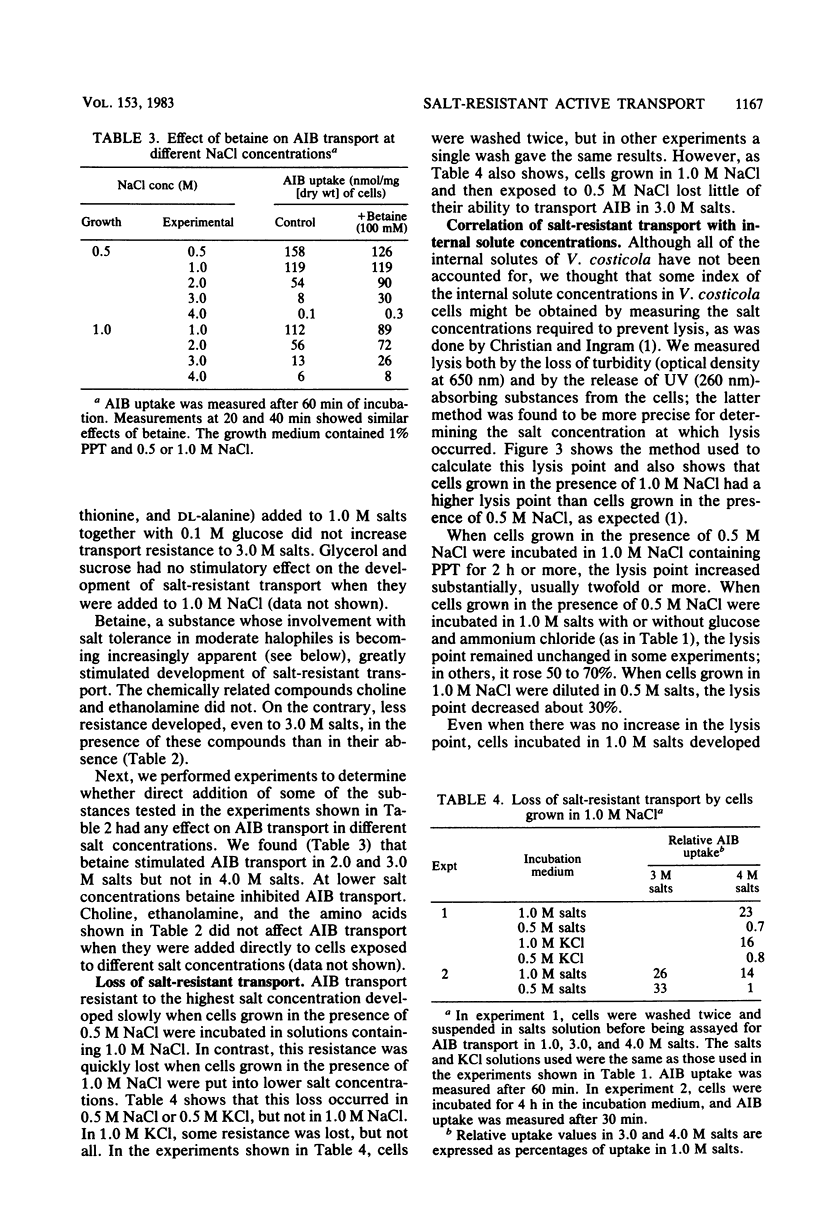
The moderately halophilic bacterium Vibrio costicola accumulates α-aminoisobutyric acid (AIB) by active transport. Substantial amounts of Na+ ions are needed for this transport. This is not due to an ionic requirement for respiration; cells respire as well as KCl as in NaCl but do not transport AIB in KCl. In cells grown in the presence of 1.0 or 2.0 M NaCl, AIB transport took place in higher NaCl concentrations than in cells grown in the presence of 0.5 M NaCl. The latter cells developed salt-resistant transport when they were exposed to 1.0 M NaCl in the presence of chloramphenicol and other antibiotics that inhibit protein synthesis. Two levels of salt-resistant transport were observed. One level (resistance to 3.0 M NaCl) developed in 1.0 M NaCl without the addition of nutrients, did not seem to require an increase in internal solute concentration, and was not lost when cells grown in 1.0 M NaCl were suspended in 0.5 M NaCl. The second level (resistance to 4.0 M NaCl) developed in 1.0 M NaCl only when nutrients were added, may have required an increased internal solute concentration, and was lost when 1.0 M NaCl-grown cells were suspended in 0.5 M NaCl or KCl. Among the substances that stimulated the development of salt-resistant AIB transport, betaine was especially active. Furthermore, direct addition of betaine permitted cells to transport AIB at higher NaCl concentrations. High salt concentrations inhibited endogenous respiration to a lesser extent than AIB transport, especially in 0.5 M NaCl-grown cells. Thus, these concentrations of salt did not inhibit AIB transport by inhibiting respiration. However, oxidation of glucose and oxidation of succinate were at least as sensitive to high salt concentrations as AIB transport, suggesting that a salt-sensitive transport step(s) is involved in the oxidation of these substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISTIAN J. H., INGRAM M. Lysis of Vibrio costicolus by osmotic shock. J Gen Microbiol. 1959 Feb;20(1):32–42. doi: 10.1099/00221287-20-1-32. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth M. P., Shindler D. B., Gochnauer M. B., Kushner D. J. Salt tolerance of intertidal marine bacteria. Can J Microbiol. 1971 Jun;17(6):825–828. doi: 10.1139/m71-133. [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R., Armstrong D. W., Kushner D. J. Protein turnover in a moderately halophilic bacterium. Can J Microbiol. 1980 Feb;26(2):196–203. doi: 10.1139/m80-030. [DOI] [PubMed] [Google Scholar]
- Kanemasa Y., Yoshioka T., Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureus cultured in medium containing NaCl. Biochim Biophys Acta. 1972 Nov 30;280(3):444–450. [PubMed] [Google Scholar]
- Komaratat P., Kates M. The lipid composition of a halotolerant species of Staphylococcus epidermidis. Biochim Biophys Acta. 1975 Sep 19;398(3):464–484. doi: 10.1016/0005-2760(75)90197-6. [DOI] [PubMed] [Google Scholar]
- Lindman B. Effect of NaCl on kinetics of D-glucosamine uptake in yeasts differing in halotolerance. Antonie Van Leeuwenhoek. 1981;47(4):297–306. doi: 10.1007/BF02350780. [DOI] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Niven D. F., MacLeod R. A. Sodium ion-proton antiport in a marine bacterium. J Bacteriol. 1978 Jun;134(3):737–743. doi: 10.1128/jb.134.3.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg E., Tietz A., Friedberg I. Effects of salts and ionophores on proline transport in a moderately halopholic halotolerant bacterium. Biochim Biophys Acta. 1980 Feb 15;596(1):118–128. doi: 10.1016/0005-2736(80)90175-3. [DOI] [PubMed] [Google Scholar]
- Shindler D. B., Wydro R. M., Kushner D. J. Cell-bound cations of the moderately halophilic bacterium Vibrio costicola. J Bacteriol. 1977 May;130(2):698–703. doi: 10.1128/jb.130.2.698-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemoto T., Hayashi M. Regulation of internal solute concentrations of marine Vibrio alginolyticus in response to external NaCl concentration. Can J Microbiol. 1979 Aug;25(8):922–926. doi: 10.1139/m79-137. [DOI] [PubMed] [Google Scholar]
- Wydro R. M., Madira W., Hiramatsu T., Kogut M., Kushner D. J. Salt-sensitive in vitro protein synthesis by a moderately halophilic bacterium. Nature. 1977 Oct 27;269(5631):824–825. doi: 10.1038/269824a0. [DOI] [PubMed] [Google Scholar]
- Wydro R., Kogut M., Kushner D. J. Salt response of ribosomes of a moderately halophilic bacterium. FEBS Lett. 1975 Dec 1;60(1):210–215. doi: 10.1016/0014-5793(75)80453-4. [DOI] [PubMed] [Google Scholar]


