Abstract
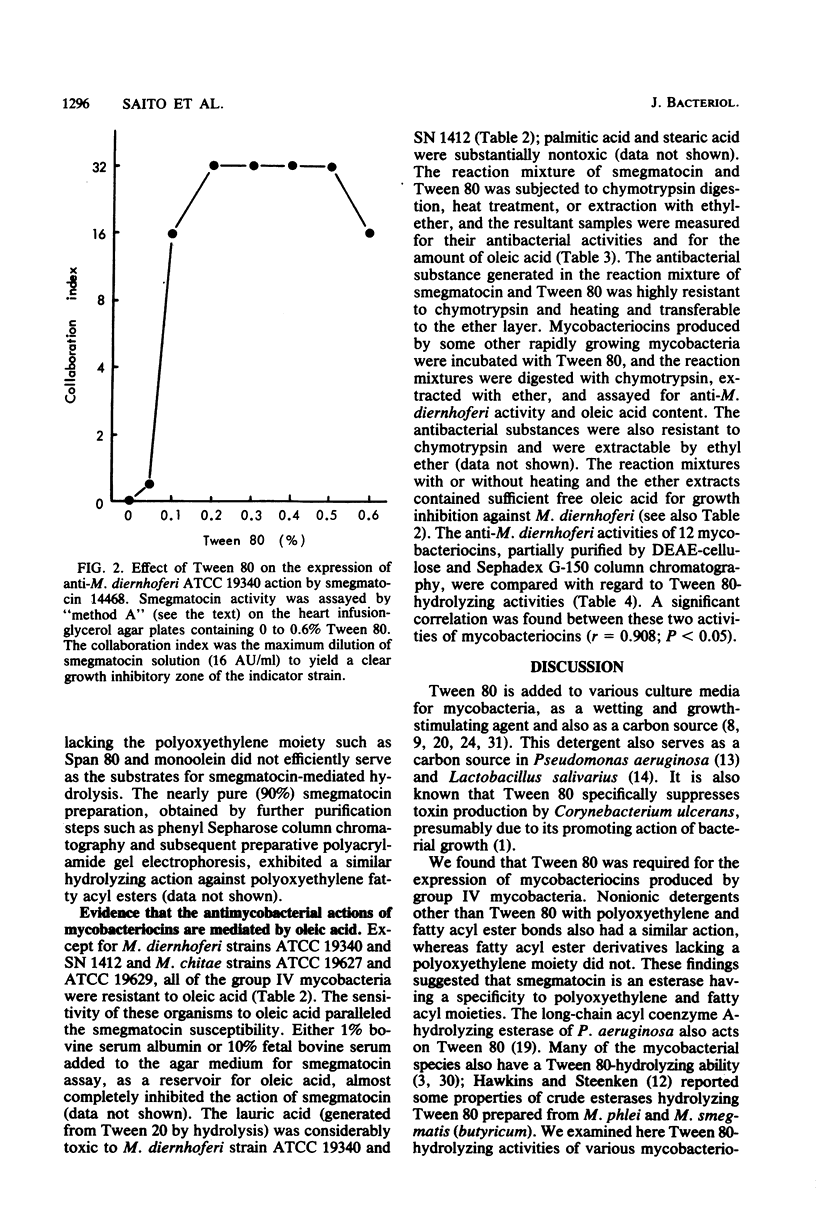
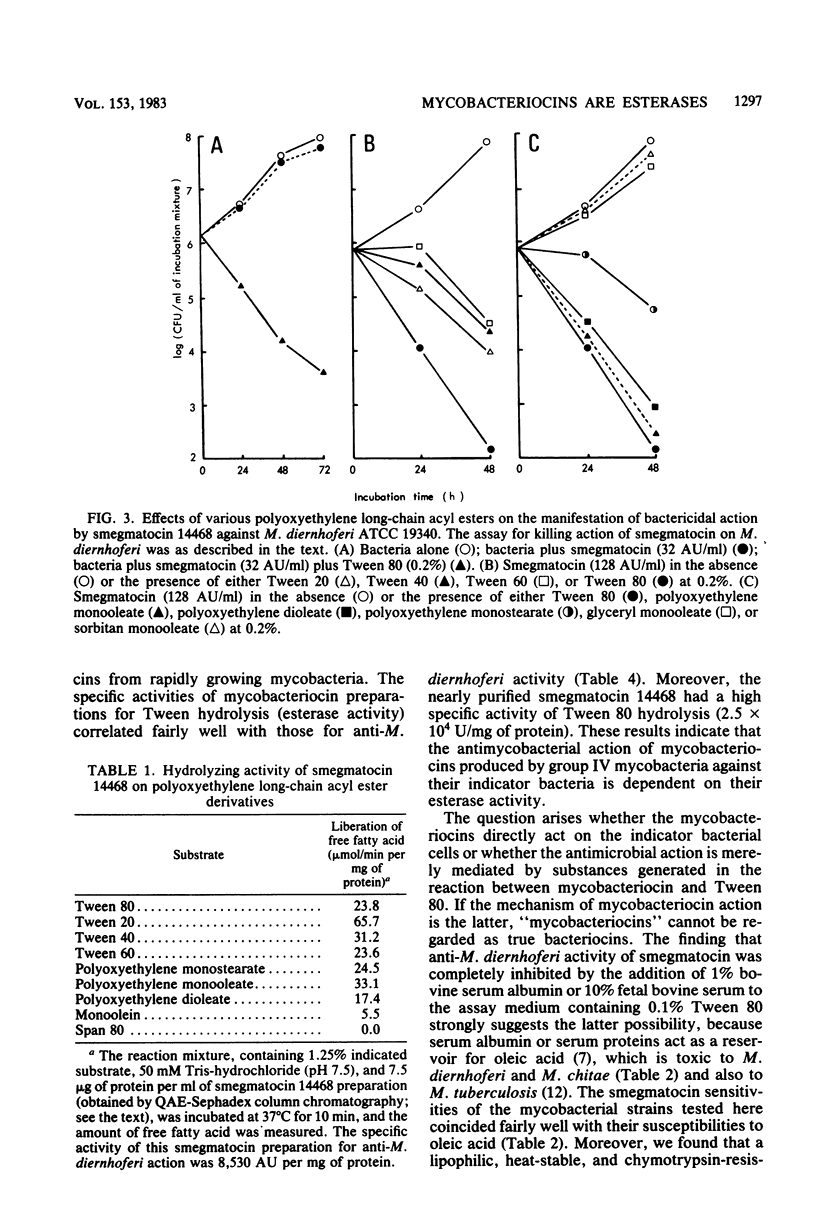
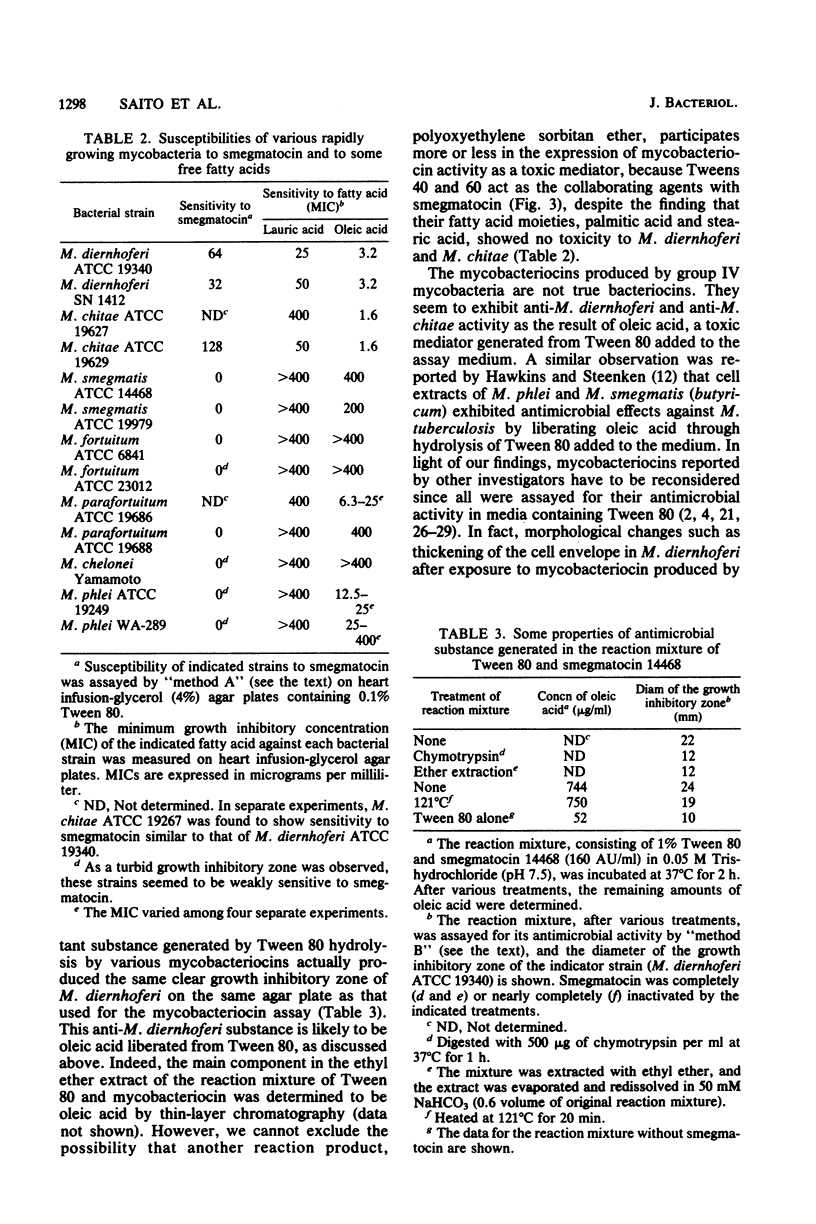
Smegmatocin, a protein produced by Mycobacterium smegmatis ATCC 14468, was found to have an esterase activity, hydrolyzing Tween 80, polyoxyethylene sorbitan monooleate, added to the assay medium for various "bacteriocins" from mycobacteria. Because M. diernhoferi ATCC 19340 (indicator strain for smegmatocin) is highly susceptible to oleic acid and smegmatocin requires Tween 80 for manifestation of its anti-M. diernhoferi activity, it is likely that smegmatocin-mediated antimicrobial action is caused by oleic acid generated by hydrolysis of Tween 80 by the inherent esterase action of smegmatocin. Other mycobacteriocins from rapidly growing mycobacteria also have inherent esterase activity against Tween 80 and require Tween 80 for expression of antimycobacterial action. Smegmatocin was found to hydrolyze various polyoxyethylene (sorbitan) fatty acyl esters but not sorbitan monooleate and glyceryl esters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrehem K., Zamiri I. Inhibition of Corynebacterium ulcerans toxin production by Tween 80. J Med Microbiol. 1980 Nov;13(4):581–584. doi: 10.1099/00222615-13-4-581. [DOI] [PubMed] [Google Scholar]
- Adámek L., Trnka L., Mion P., Gutová M. Hemmstoffe vom Bakteriocin-Typus bei schnell wachsenden saphrophytischen Stämmen der Mycobakterien. Beitr Klin Erforsch Tuberk Lungenkr. 1968;138(1):51–55. [PubMed] [Google Scholar]
- Akao T., Kusaka T., Kobashi K. Two esterases released from Mycobacterium smegmatis from the hydrolysis of long chain acyl-CoAs and Tween. J Biochem. 1981 Dec;90(6):1661–1669. doi: 10.1093/oxfordjournals.jbchem.a133641. [DOI] [PubMed] [Google Scholar]
- Butcher G. W., King G., Dyke K. G. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J Gen Microbiol. 1976 Jun;94(2):290–296. doi: 10.1099/00221287-94-2-290. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Daneo-Moore L. Effects of fatty acids on lysis of Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1122–1126. doi: 10.1128/jb.141.3.1122-1126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J. The effect of organic acids on mammalian tubercle bacilli. J Exp Med. 1950 Oct 1;92(4):319–332. doi: 10.1084/jem.92.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Dubos R. J. The Inhibitory Effect of Lipase on Bacterial Growth in Media Containing Fatty Acid Esters. J Bacteriol. 1948 Jan;55(1):11–23. doi: 10.1128/jb.55.1.11-23.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway D. L., Dyke K. G. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979 Nov;115(1):233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Lamport P., Dormandy T. L. Autoxidation as a cause of antibacterial activity in unsaturated fatty acids. J Med Microbiol. 1974 Aug;7(3):387–389. doi: 10.1099/00222615-7-3-387. [DOI] [PubMed] [Google Scholar]
- HAWKINS J. E., STEENKEN W. Lipase activity of Mycobacteria. Am Rev Respir Dis. 1963 Apr;87:585–588. doi: 10.1164/arrd.1963.87.4.585. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of Tween 80 on the morphology and physiology of Lactobacillus salivarius strain IV CL-37 grown in a chemostat under glucose limitation. J Gen Microbiol. 1980 Jul;119(1):195–201. doi: 10.1099/00221287-119-1-195. [DOI] [PubMed] [Google Scholar]
- Larsson K., Norén B., Odham G. Antimicrobial effect of simple lipids and the effect of pH and positive ions. Antimicrob Agents Chemother. 1975 Dec;8(6):733–736. doi: 10.1128/aac.8.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K., Norén B., Odham G. Antimicrobial effect of simple lipids with different branches at the methyl end group. Antimicrob Agents Chemother. 1975 Dec;8(6):742–750. doi: 10.1128/aac.8.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI K. Bactericidal action of oleic acid for tubercle bacilli. II. Morphological response. J Bacteriol. 1957 Mar;73(3):345–352. doi: 10.1128/jb.73.3.345-352.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa I., Shiga S., Kageyama M. An esterase on the outer membrane of Pseudomonas aeruginosa for the hydrolysis of long chain acyl esters. J Biochem. 1979 Sep;86(3):643–656. doi: 10.1093/oxfordjournals.jbchem.a132568. [DOI] [PubMed] [Google Scholar]
- Saito H., Watanabe T., Tomioka H. Purification, properties, and cytotoxic effect of a bacteriocin from Mycobacterium smegmatis. Antimicrob Agents Chemother. 1979 Apr;15(4):504–509. doi: 10.1128/aac.15.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Salomon D., Simmons J. L., Sreevalsan T., Freese E. Inhibitory effects of lipophilic acids and related compounds on bacteria and mammalian cells. Antimicrob Agents Chemother. 1975 Mar;7(3):349–363. doi: 10.1128/aac.7.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Solotorovsky M. Interaction of Tween 80 detergent with mycobacteria in synthetic medium. I. Effect of Tween 80 on the growth and turbidimetric response of Mycobacterium avium cultures. Am Rev Respir Dis. 1971 Nov;104(5):717–727. doi: 10.1164/arrd.1971.104.5.717. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Shimamoto M., Mizuguchi Y. Physicochemical and biological properties of mycobacteriocin M12 produced by Mycobacterium smegmatis ATCC 25855. J Gen Microbiol. 1978 Dec;109(2):215–223. doi: 10.1099/00221287-109-2-215. [DOI] [PubMed] [Google Scholar]
- Tokiwa H. [Studies on mycobacteriocin. I. Characterization and classification of mycobacteriocin produced by rapidly growing mycobacteria (author's transl)]. Fukuoka Igaku Zasshi. 1974 Jul 25;65(7):473–480. [PubMed] [Google Scholar]
- WAYNE L. G., DOUBEK J. R., RUSSELL R. L. CLASSIFICATION AND IDENTIFICATION OF MYCOBACTERIA. I. TESTS EMPLOYING TWEEN 80 AS SUBSTRATE. Am Rev Respir Dis. 1964 Oct;90:588–597. doi: 10.1164/arrd.1964.90.4.588. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Langridge W. H., 3rd, Walker R. W. Relationships between oleic acid uptake and lipid metabolism in Mycobacterium smegmatis. Am Rev Respir Dis. 1972 Sep;106(3):450–457. doi: 10.1164/arrd.1972.106.3.450. [DOI] [PubMed] [Google Scholar]



