Abstract
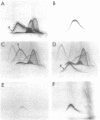
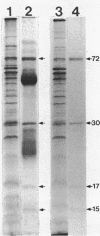
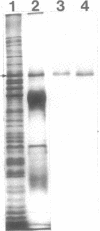
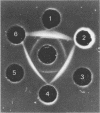
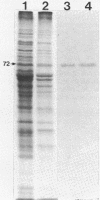
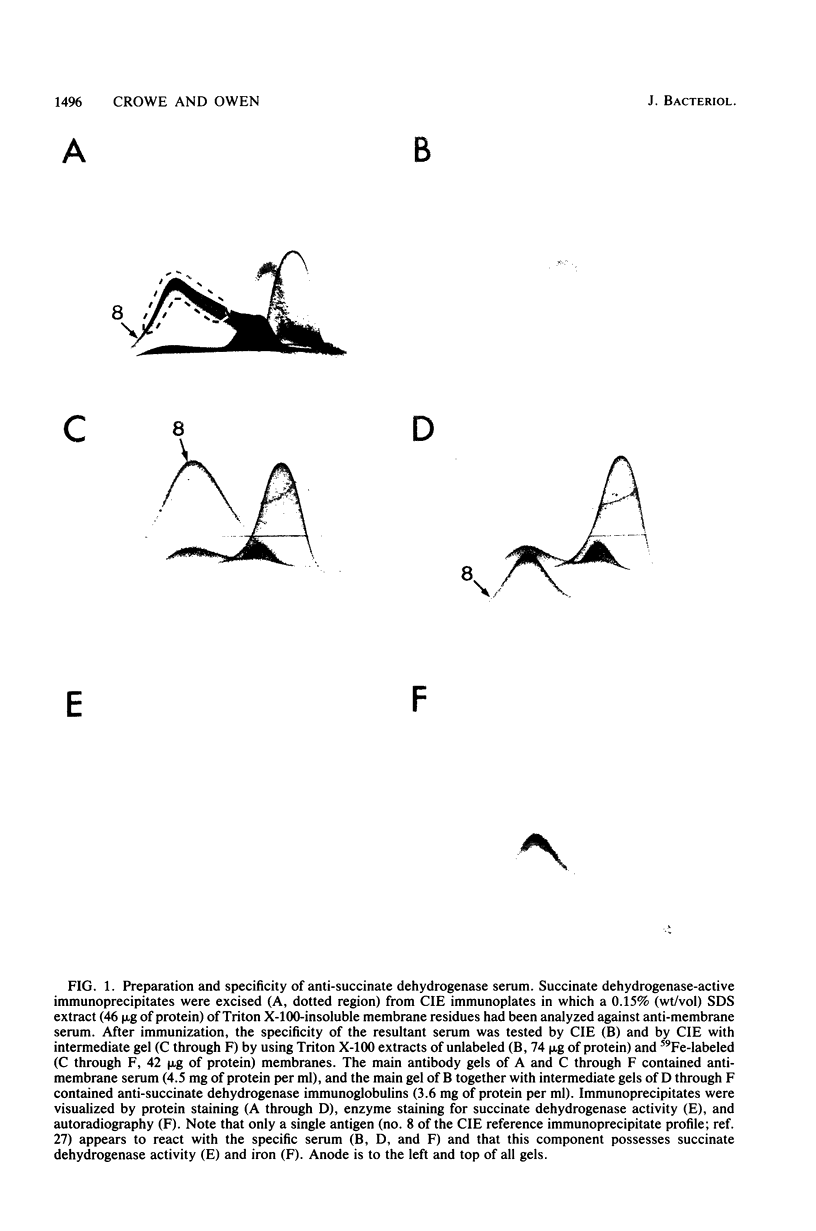
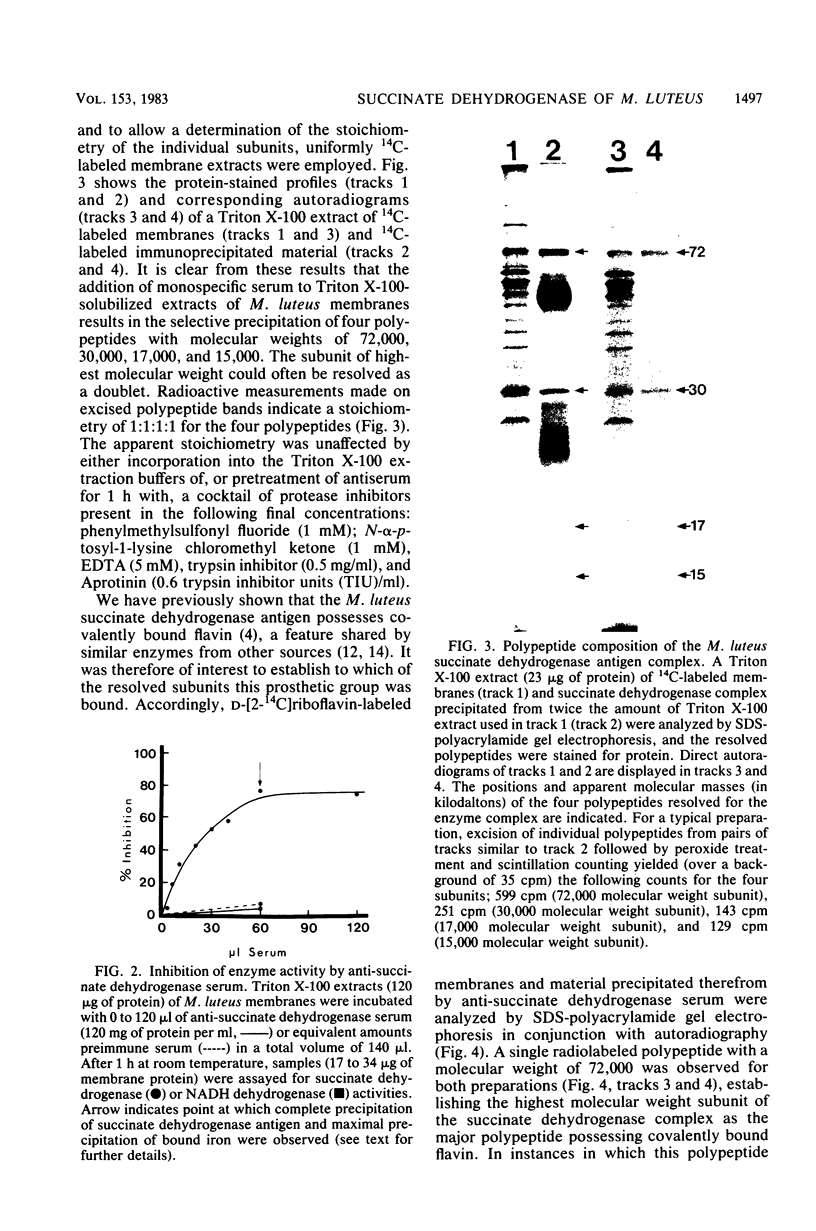
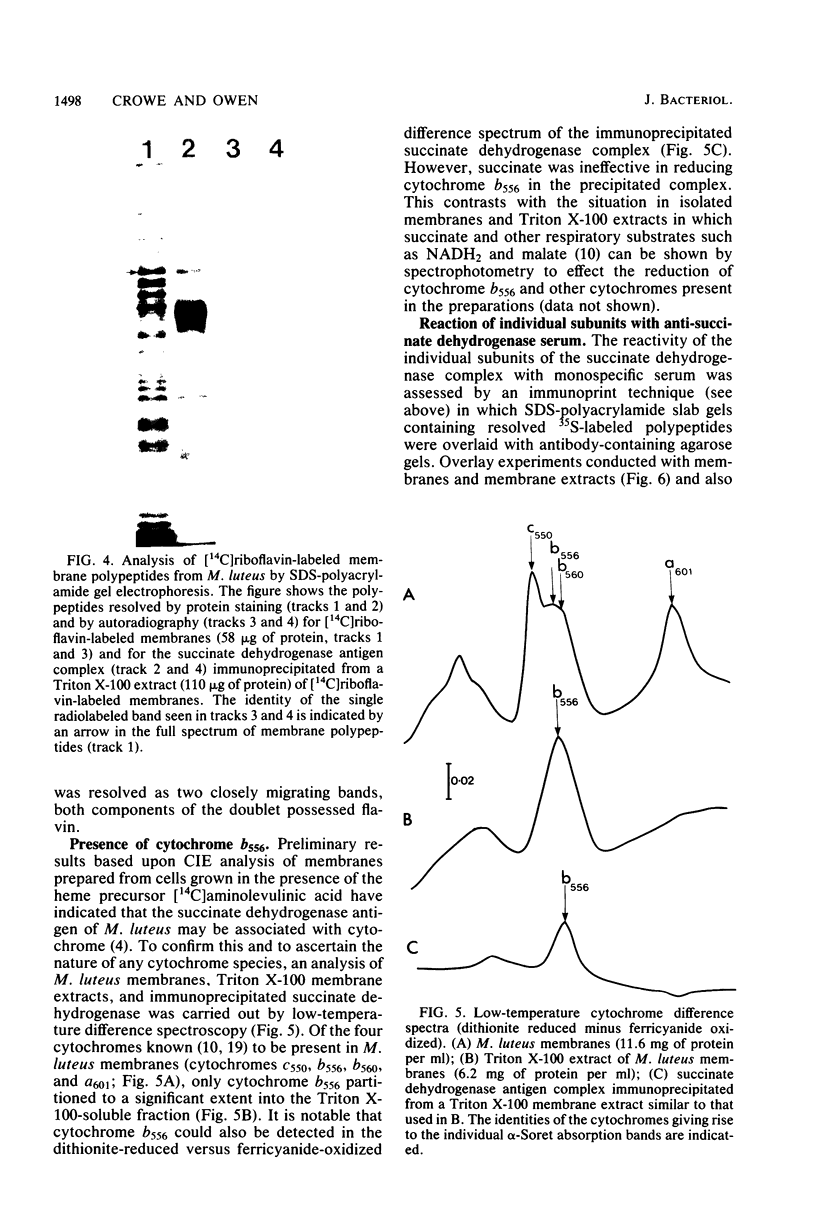
Succinate dehydrogenase (EC 1.3.99.1) of Micrococcus luteus was selectively precipitated from Triton X-100-solubilized membranes by using specific antiserum. The precipitated enzyme contained equimolar amounts of four polypeptides with apparent molecular weights of 72,000, 30,000, 17,000, and 15,000. The 72,000 polypeptide possessed a covalently bound flavin prosthetic group and appeared to be strongly antigenic as judged by immunoprinting experiments. Low-temperature absorption spectroscopy revealed the presence of cytochrome b556 in the antigen complex. By analogy with succinate dehydrogenase purified from other sources, the 72,000 and 30,000 polypeptides were considered to represent subunits of the succinate dehydrogenase enzyme, whereas one (or both) of the low-molecular-weight polypeptides was attributed to the apoprotein of the b-type cytochrome. A succinate dehydrogenase antigen cross-reacting with the M. luteus enzyme complex could be demonstrated in membranes of Micrococcus roseus, Micrococcus flavus, and Sarcina lutea, but not in the membranes isolated from a wide variety of other gram-positive and gram-negative bacteria.
Full text
PDF


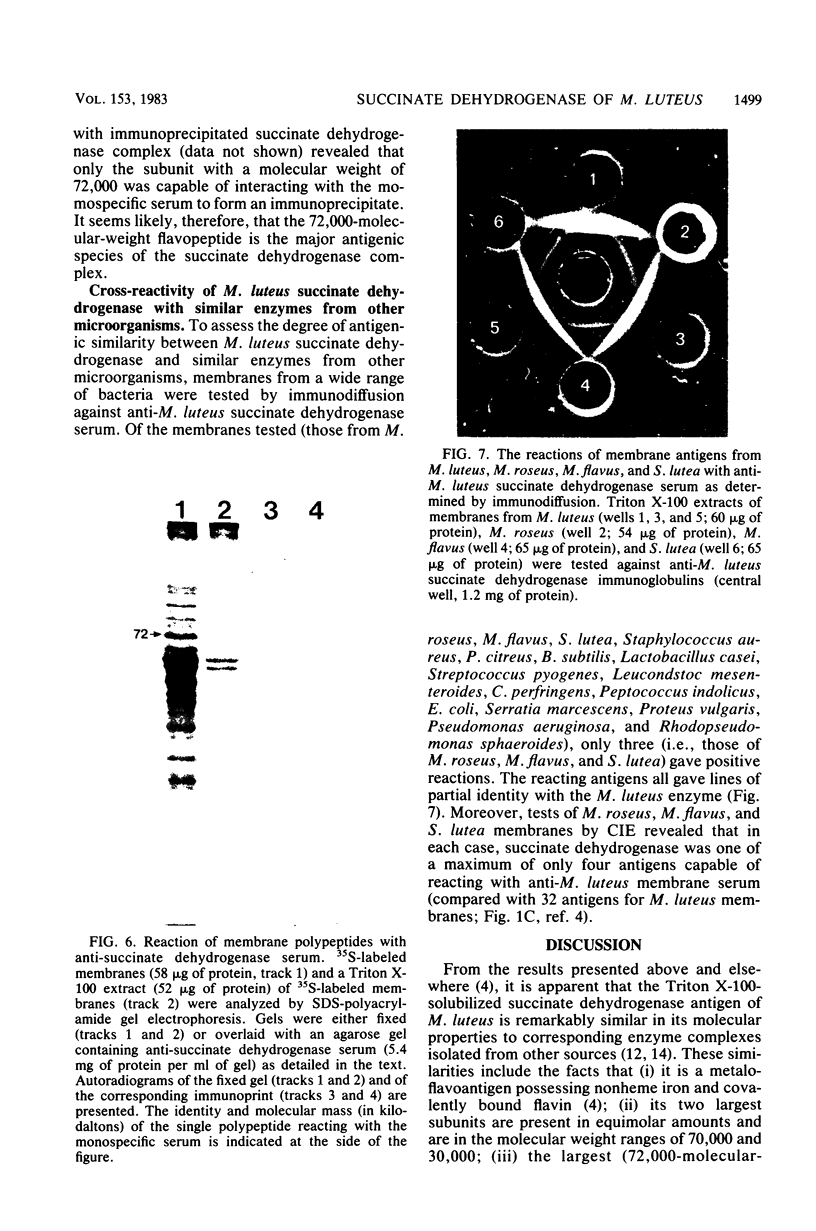


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud P., Wilson G. B., Koistinen J., Fudenberg H. H. Immunofixation after electrofocusing: improved method for specific detection of serum proteins with determination of isoelectric points. I. Immunofixation print technique for detection of alpha-1-protease inhibitor. J Immunol Methods. 1977;16(3):221–231. doi: 10.1016/0022-1759(77)90200-9. [DOI] [PubMed] [Google Scholar]
- Crowe B. A., Owen P. Immunochemical analysis of respiratory-chain components of micrococcus luteus (lysodeikticus). J Bacteriol. 1983 Jan;153(1):498–505. doi: 10.1128/jb.153.1.498-505.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Revis G. J., Jarrett K. Preparatory electroimmunodiffusion for making precipitins to selected native antigens. Immunol Commun. 1972;1(4):325–336. doi: 10.3109/08820137209022946. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Gel'man N. S., Tikhonova G. V., Simakova I. M., Lukoyanova M. A., Taptykova S. D., Mikelsaar H. M. Fragmentation by detergents of the respiratory chain of Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):321–331. doi: 10.1016/0005-2728(70)90188-x. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Galante Y. M. Isolation of cytochrome b560 from complex II (succinateùbiquinone oxidoreductase) and its reconstitution with succinate dehydrogenase. J Biol Chem. 1980 Jun 25;255(12):5530–5537. [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder R., Salton M. R. Affinity chromatography of succinate dehydrogenase from the membranes of Micrococcus lysodeikticus. Prep Biochem. 1975;5(4):349–357. doi: 10.1080/00327487508061582. [DOI] [PubMed] [Google Scholar]
- Lukoianova M. A., Taptykova S. D. Tsitokhromy Micrococcus lysodeikticus. Biokhimiia. 1968 Jul-Aug;33(4):888–894. [PubMed] [Google Scholar]
- MITCHELL P. Metabolism, transport, and morphogenesis: which drives which? J Gen Microbiol. 1962 Sep;29:25–37. doi: 10.1099/00221287-29-1-25. [DOI] [PubMed] [Google Scholar]
- Owen P., Doherty H. Immunochemical analysis of triton X-100-insoluble residues from Micrococcus lysodeikticus membranes. J Bacteriol. 1979 Dec;140(3):881–887. doi: 10.1128/jb.140.3.881-887.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Freer J. H. Factors influencing the activity of succinate dehydrogenase in membrane preparations from Micrococcus lysodeikticus. Biochem J. 1970 Nov;120(2):237–243. doi: 10.1042/bj1200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Isolation and characterization of a mannan from mesosomal membrane vesicles of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Oct 6;406(2):214–234. doi: 10.1016/0005-2736(75)90006-1. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Membrane asymmetry and expression of cell surface antigens of Micrococcus lysodeikticus established by crossed immunoelectrophoresis. J Bacteriol. 1977 Dec;132(3):974–978. doi: 10.1128/jb.132.3.974-985.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- Switalski L. M., Schwam O., Smyth C. J., Wadström T. Peptocoagulase: clotting factor produced by bovine strains of Peptococcus indolicus. J Clin Microbiol. 1978 Apr;7(4):361–367. doi: 10.1128/jcm.7.4.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgenbaeva D. A., Kharat'ian E. F., Zhukova I. G., Lukoianova M. A., Ostrovskii D. N. Kompleksy tsitokhroma b556, soliubilizirovannye iz membran Micrococcus lysodeikticus tritonom X-100. Biokhimiia. 1979 Apr;44(4):729–737. [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Kolb H. J. Isolation of mitochondrial succinate: ubiquinone reductase, cytochrome c reductase and cytochrome c oxidase from Neurospora crassa using nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):139–149. doi: 10.1111/j.1432-1033.1979.tb13240.x. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P., Arnon D. I. Isolation and characterization of bound ion-sulfur proteins from bacterial photosynthetic membranes. I. Ferredoxins III and IV from Rhodospirillum rubrum chromatophores. J Biol Chem. 1977 Nov 10;252(21):7453–7460. [PubMed] [Google Scholar]
- Yu C. A., Yu L. Isolation and properties of a mitochondrial protein that converts succinate dehydrogenase into succinate-ubiquinone oxidoreductase. Biochemistry. 1980 Jul 22;19(15):3579–3585. doi: 10.1021/bi00556a025. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L. Ubiquinone-binding proteins. Biochim Biophys Acta. 1981 Dec 4;639(2):99–128. doi: 10.1016/0304-4173(81)90007-0. [DOI] [PubMed] [Google Scholar]