Abstract
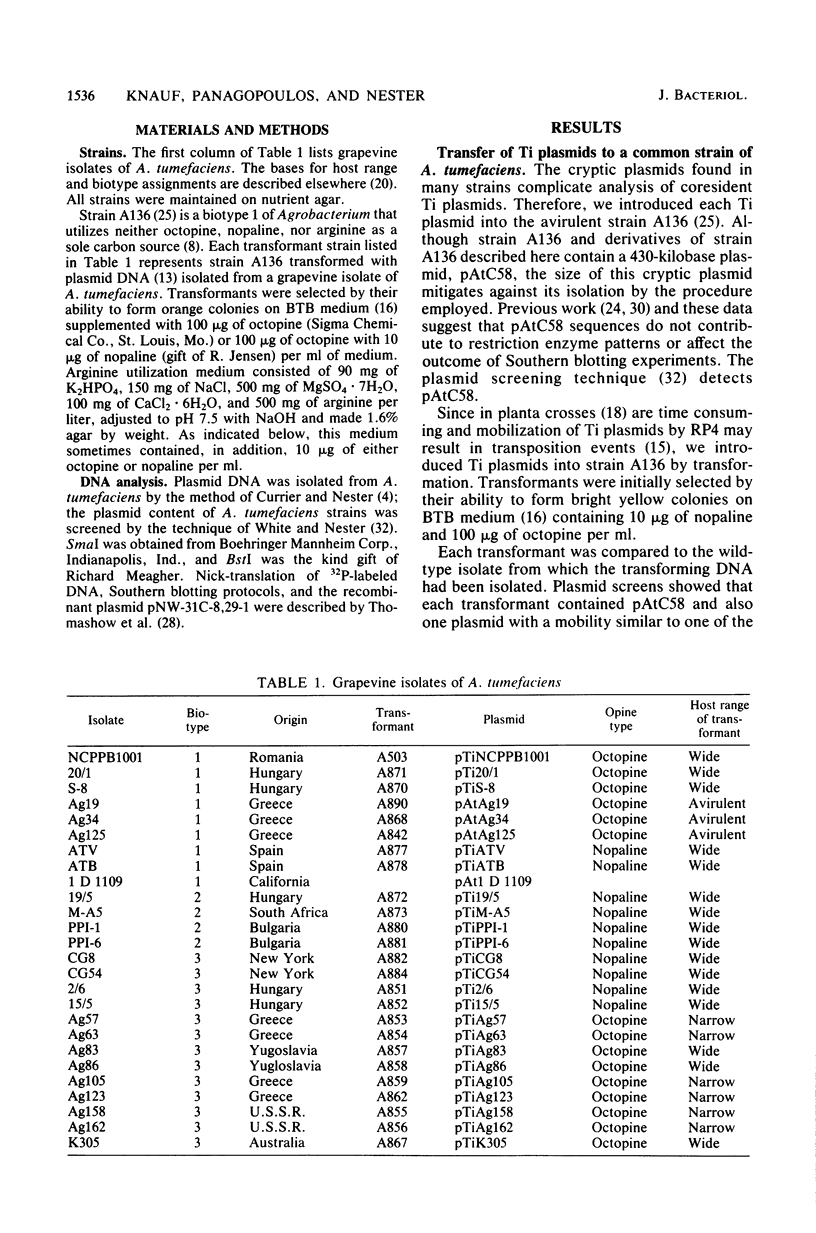
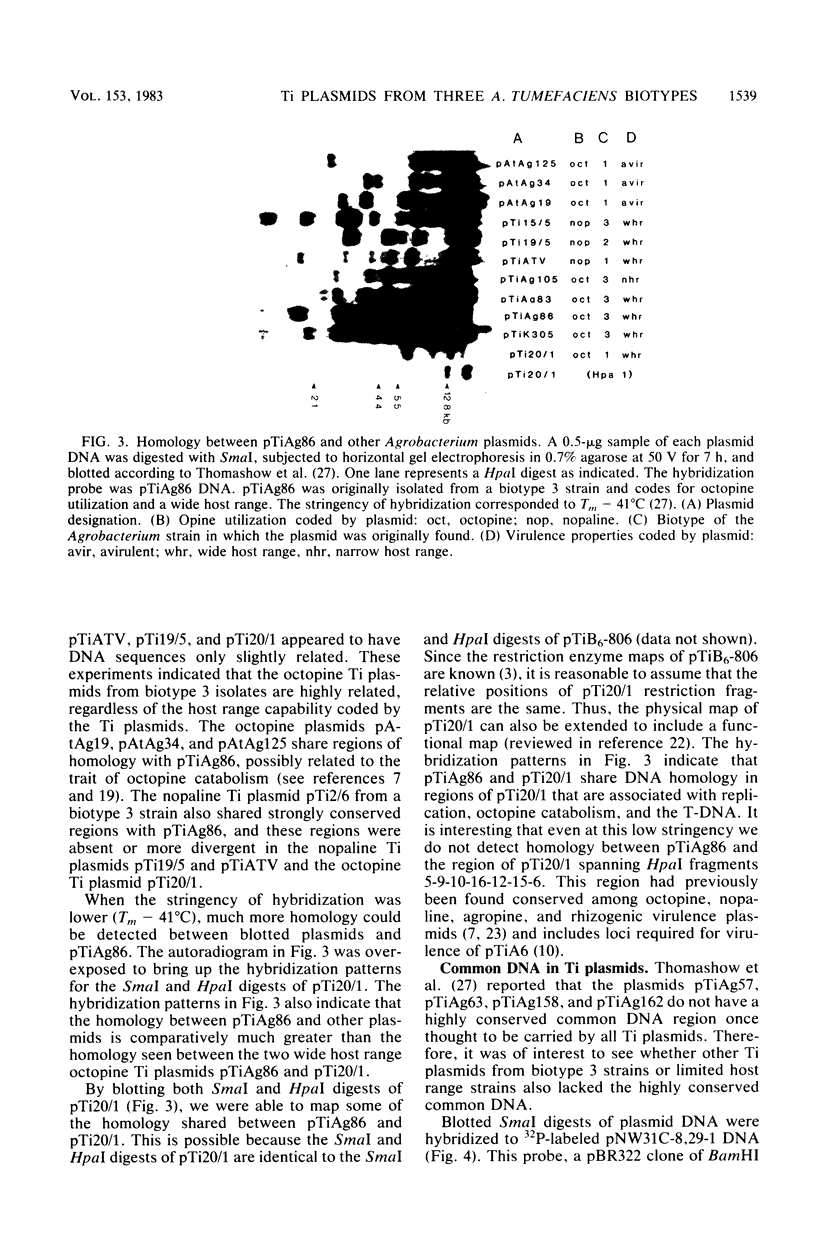
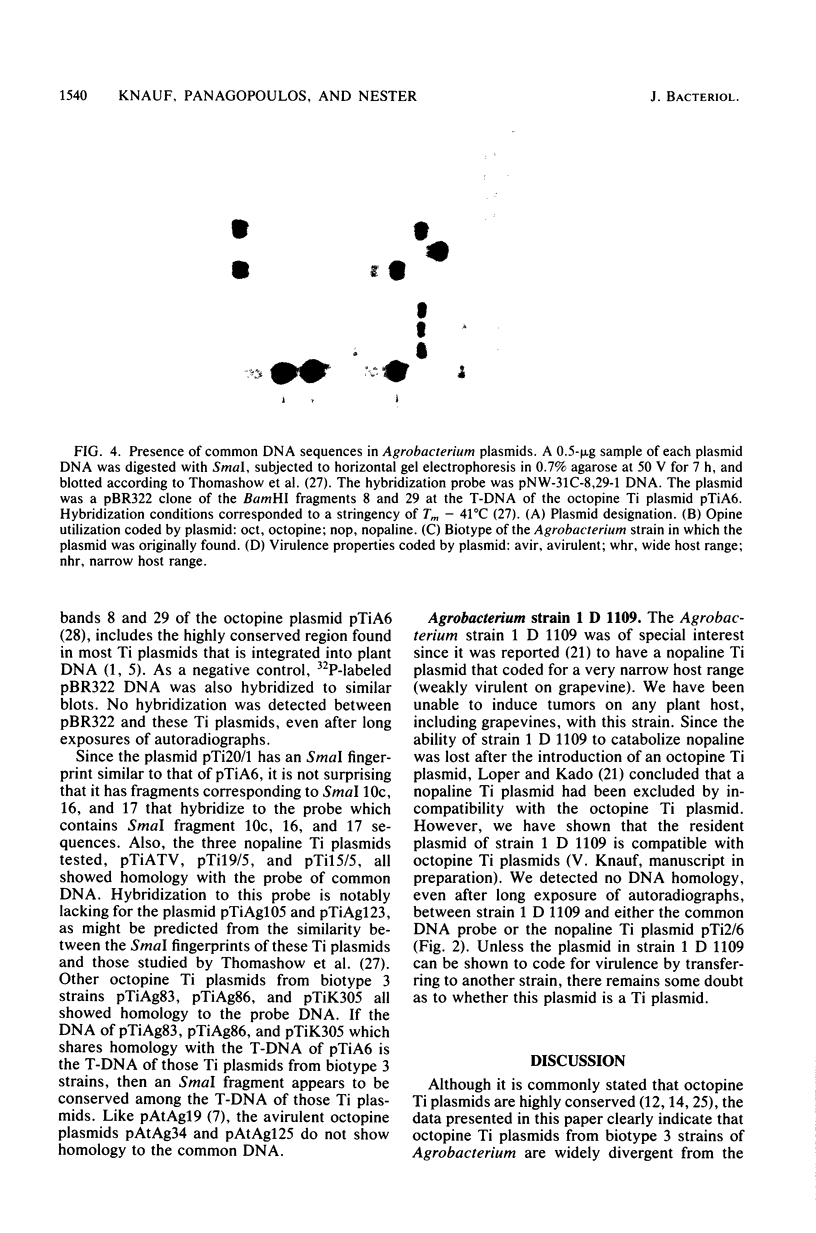
Twenty-six plasmids from grapevine isolates of Agrobacterium tumefaciens were analyzed by SmaI fingerprinting and by hybridization of nick-translated DNA to DNA of another plasmid. These experiments established that octopine Ti plasmids are not highly conserved, although octopine Ti plasmids from biotype 1 A. tumefaciens strains appeared to be very similar. Octopine Ti plasmids from biotype 3 strains are more variable in terms of host range and SmaI fingerprints, but share extensive DNA homology. Fingerprints of nopaline Ti plasmids from strains of a given biotype resemble each other but not fingerprints of Ti plasmids from strains of the other two biotypes. The wide host range octopine Ti plasmid from the biotype 3 strain Ag86 shares more DNA homology with narrow host range Ti plasmids, nopaline Ti plasmids, and octopine catabolism plasmids than with the wide host range octopine Ti plasmid from biotype 1 strain 20/1. pTiAg86 does share homology with the portion of pTi20/1 integrated and expressed in plant tumor cells. Since all wide host range Ti plasmids studied contain these sequences, we suggest that natural selection for a wide host range resulted in the presence of the common sequences in distantly related plasmids. The lack of homology between this "common DNA" and limited host range Ti plasmids shows that the DNA sequences per se are not required for tumorigenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Montoya A. L., Merlo D. J., Drummond M. H., Nutter R., Gordon M. P., Nester E. W. Restriction endonuclease mapping of a plasmid that confers oncogenicity upon Agrobacterium tumefaciens strain B6-806. Plasmid. 1978 Feb;1(2):254–269. doi: 10.1016/0147-619x(78)90043-4. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- De Vos G., De Beuckeleer M., Van Montagu M., Schell J. Restriction endonuclease mapping of the octopine tumor-inducing plasmid pTiAch5 of Agrobacterium tumefaciens. Plasmid. 1981 Sep;6(2):249–253. doi: 10.1016/0147-619x(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Chilton M. D. Tumor-inducing (Ti) plasmids of Agrobacterium share extensive regions of DNA homology. J Bacteriol. 1978 Dec;136(3):1178–1183. doi: 10.1128/jb.136.3.1178-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. G., Kerr A., Tempé J., Petit A. Arginine catabolism: a new function of both octopine and nopaline Ti-plasmids of Agrobacterium. Mol Gen Genet. 1979 Jun 20;173(3):263–269. doi: 10.1007/BF00268636. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Holsters M., de Waele D., Depicker A., Messens E., van Montagu M., Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978 Jul 11;163(2):181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Ooms G., Schilperoort R. A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980 Sep;143(3):1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Schilperoort R. A. Molecular mechanism of Ti plasmid mobilization by R plasmids: isolation of Ti plasmids with transposon-insertions in Agrobacterium tumefaciens. Plasmid. 1980 Jul;4(1):64–75. doi: 10.1016/0147-619x(80)90083-9. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper J. E., Kado C. I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Knauf V. C., Nester E. W. Relationship between the limited and wide host range octopine-type Ti plasmids of Agrobacterium tumefaciens. J Bacteriol. 1981 May;146(2):484–493. doi: 10.1128/jb.146.2.484-493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Postle K., Chilton M. D., Blattner F. R., Powell A., Gordon M. P., Nester E. W. Recombination between higher plant DNA and the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6448–6452. doi: 10.1073/pnas.77.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol. 1980 Mar;141(3):1134–1141. doi: 10.1128/jb.141.3.1134-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Relationship of plasmids responsible for hairy root and crown gall tumorigenicity. J Bacteriol. 1980 Nov;144(2):710–720. doi: 10.1128/jb.144.2.710-720.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]