Abstract
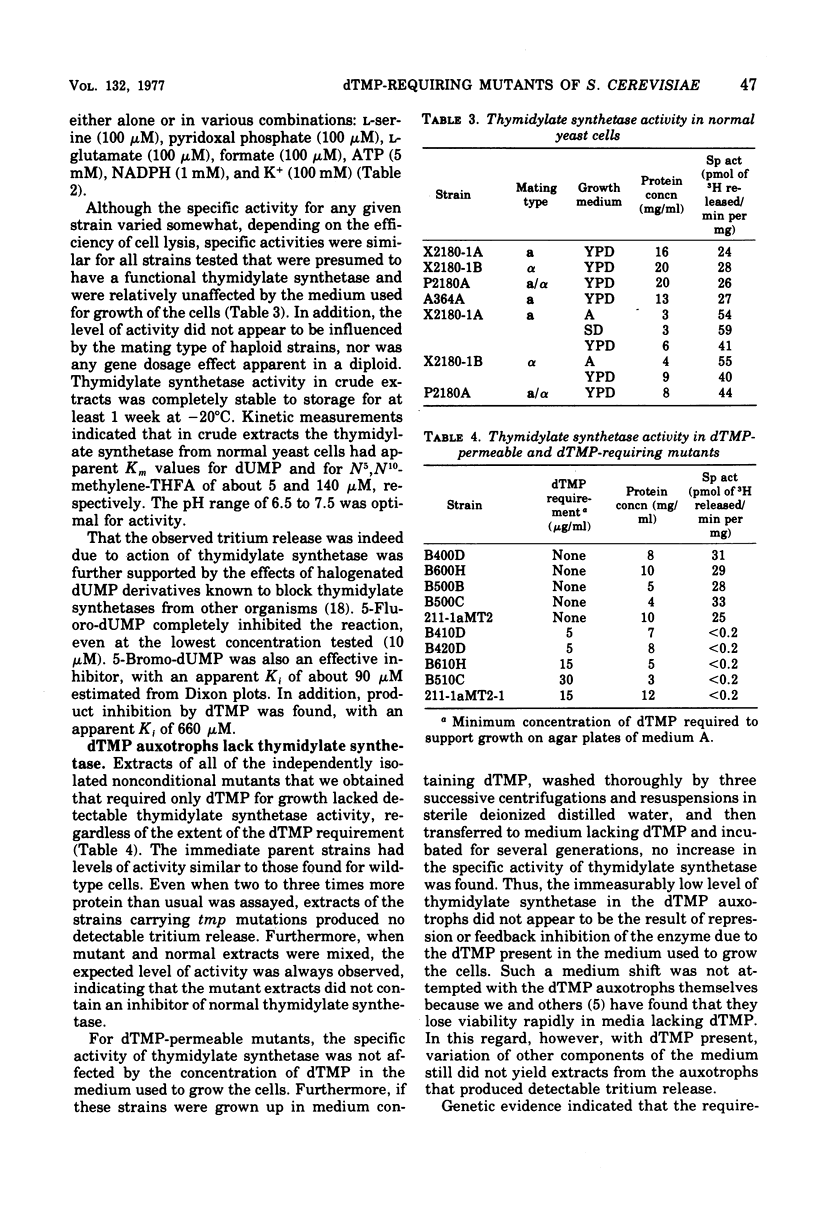
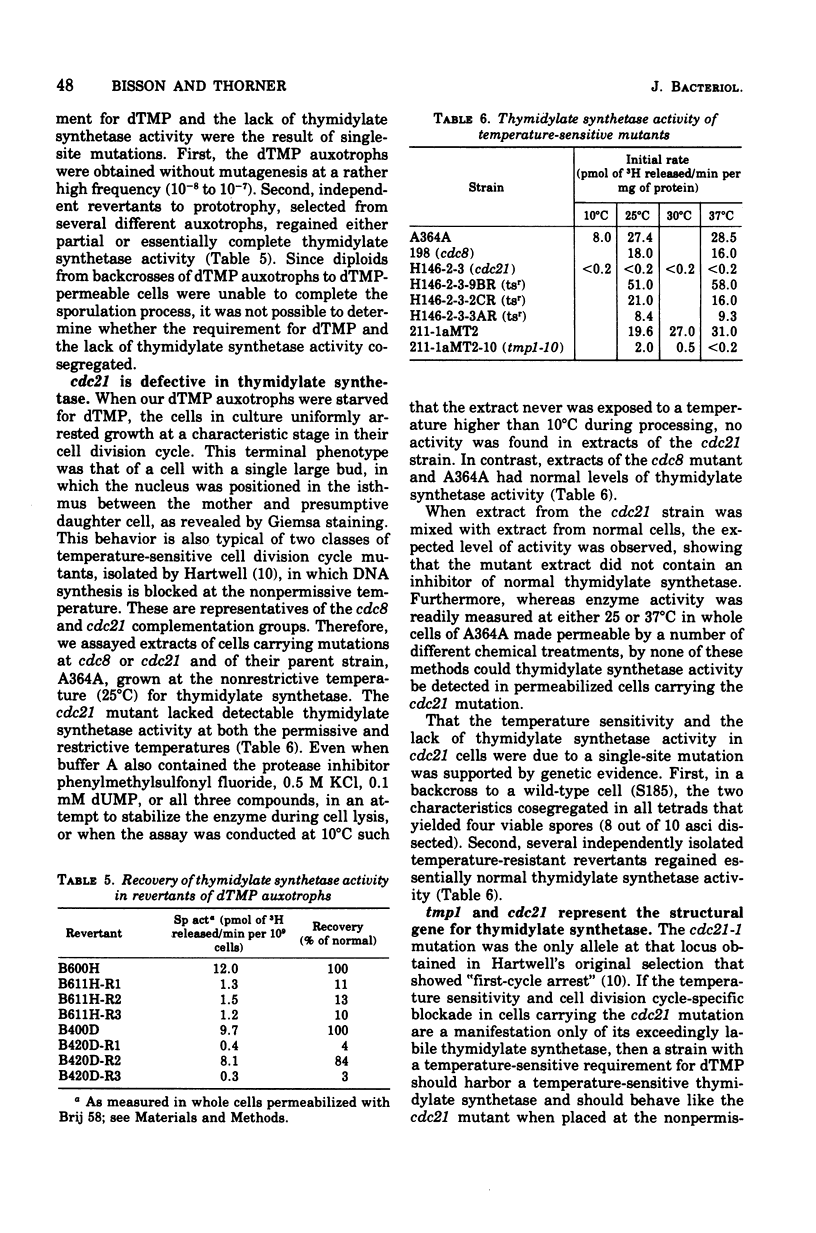
Thymidylate synthetase activity was measured in crude extracts of the yeast Saccharomyces cerevisiae by a sensitive radiochemical assay. Spontaneous non-conditional mutants auxotrophic for thymidine 5′-monophosphate (tmp1) lacked detectable thymidylate synthetase activity in cell-free extracts. In contrast, the parent strains (tup1, -2, or -4), which were permeable to thymidine 5′-monophosphate, contained levels of activity similar to those found in wild-type cells. Specific activity of thymidylate synthetase in crude extracts of normal cells or of cells carrying tup mutations was essentially unaffected by the ploidy or mating type of the cells, by the medium used for growth, by the respiratory capacity of the cells, by concentrations of exogenous thymidine 5′-monophosphate as high as 50 μg/ml, or by subsequent removal of thymidine 5′-monophosphate from the medium. Extracts of a strain bearing the temperature-sensitive cell division cycle mutation cdc21 lacked detectable thymidylate synthetase activity under all conditions tested. Its parent and another mutant (cdc8), which arrests with the same terminal phenotype under restrictive conditions, had normal levels of the enzyme. Cells of a temperature-sensitive thymidine 5′-monophosphate auxotroph arrested with a morphology identical to the cdc21 strain at the nonpermissive temperature and contained demonstrably thermolabile thymidylate synthetase activity. Tetrad analysis and the properties of revertants showed that the thymidylate synthetase defects were a consequence of the same mutation causing, in the auxotrophs, a requirement for thymidine 5′-monophosphate and, in the conditional mutants, temperature sensitivity. Complementation tests indicated that tmp1 and cdc21 are the same locus. These results identify tmp1 as the structural gene for yeast thymidylate synthetase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Method for decryptification of -glucosidase in yeast with dimethyl sulfoxide. Anal Biochem. 1972 Jan;45(1):137–146. doi: 10.1016/0003-2697(72)90014-0. [DOI] [PubMed] [Google Scholar]
- Brendel M., Fäth W. W. Isolation and characterization of mutants of Saccharomyces cerevisiae auxotrophic and conditionally auxotrophic for 5'-dTMP. Z Naturforsch C. 1974 Nov-Dec;29(11-12):733–738. doi: 10.1515/znc-1974-11-1214. [DOI] [PubMed] [Google Scholar]
- Brendel M., Fäth W. W., Laskowski W. Isolation and characterization of mutants of Saccharomyces cerevisiae able to grow after inhibition of dTMP synthesis. Methods Cell Biol. 1975;11:287–294. doi: 10.1016/s0091-679x(08)60329-5. [DOI] [PubMed] [Google Scholar]
- Brendel M., Langjahr U. G. "Thymineless death" in a strain of Saccharomyces cerevisiae auxotrophic for deoxythymidine-5'-monophosphate. Mol Gen Genet. 1974;131(4):351–358. doi: 10.1007/BF00264865. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Scott J. M., Rabinowitz J. C. Folate coenzymes of Clostridium acidi-urici. The isolation of (l)-5,10-methenyltetrahydropteroyltriglutamate, its conversion to (l)-tetrahydropteroyltriglutamate and (l)-10-( 14 C)formyltetrahydropteroyltriglutamate, and the synthesis of (l)-10-formyl-(6,7- 3 H 2 )tetrahydropteroyltriglutamate and (l)-(6,7- 3 H 2 )tetrahydropteroyltriglutamate. J Biol Chem. 1972 Apr 10;247(7):1959–1964. [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Defective DNA synthesis in permeabilized yeast mutants. Nat New Biol. 1971 Dec 8;234(49):171–172. doi: 10.1038/newbio234171a0. [DOI] [PubMed] [Google Scholar]
- Jaspers H. T., Christianse K., van Steveninck J. An improved method for the preparation of yeast enzymes in situ. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1434–1439. doi: 10.1016/s0006-291x(75)80389-5. [DOI] [PubMed] [Google Scholar]
- Kammen H. O. A rapid assay for thymidylate synthetase. Anal Biochem. 1966 Dec;17(3):553–556. doi: 10.1016/0003-2697(66)90192-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Newlon C. S., Fangman W. L. Mitochondrial DNA synthesis in cell cycle mutants of Saccharomyces cerevisiae. Cell. 1975 Aug;5(4):423–428. doi: 10.1016/0092-8674(75)90061-6. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr An enzymatic method for the determination of formic acid. J Biol Chem. 1957 Nov;229(1):321–328. [PubMed] [Google Scholar]
- Roodman S. T., Greenberg G. R. A temperature-sensitive thy mutant blocked in the synthesis of thymidylate synthetase. J Biol Chem. 1971 Apr 25;246(8):2609–2617. [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine 5'-monophosphate into DNA in vivo. Methods Cell Biol. 1975;11:295–302. doi: 10.1016/s0091-679x(08)60330-1. [DOI] [PubMed] [Google Scholar]


