Abstract
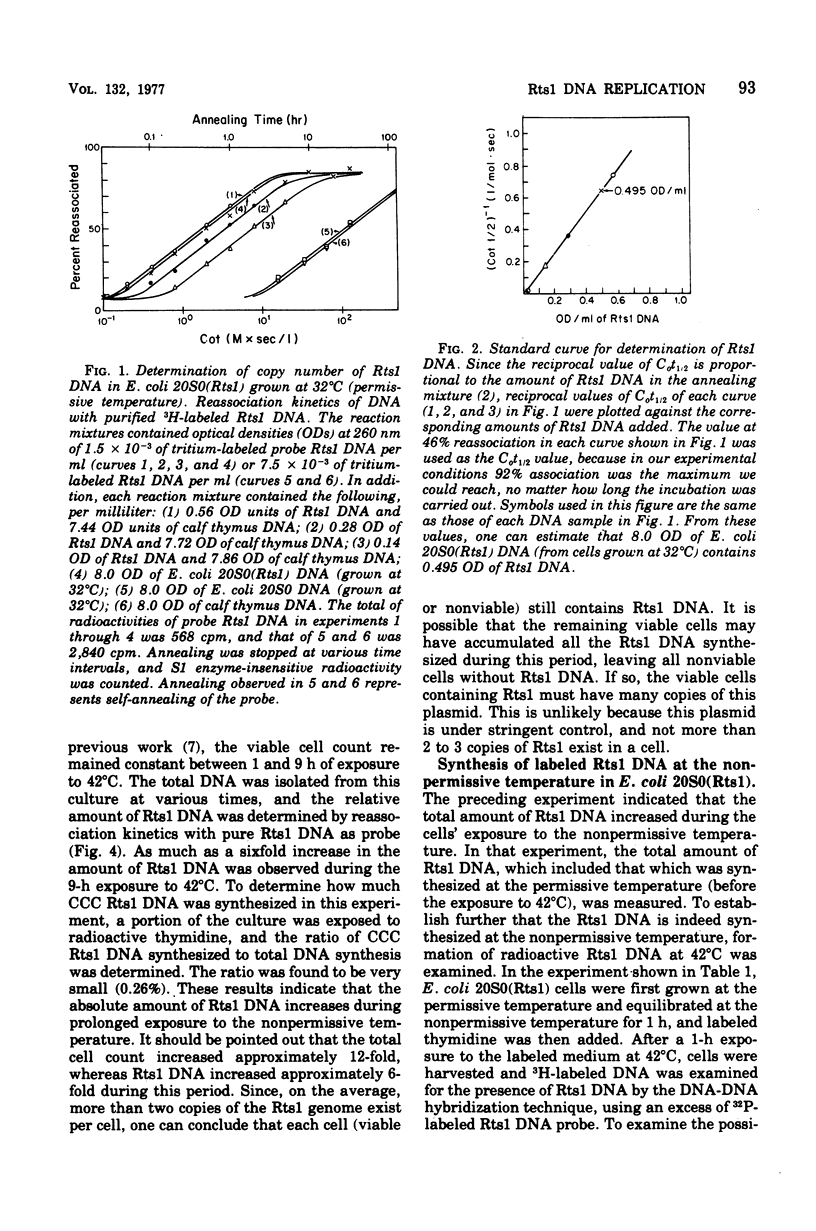
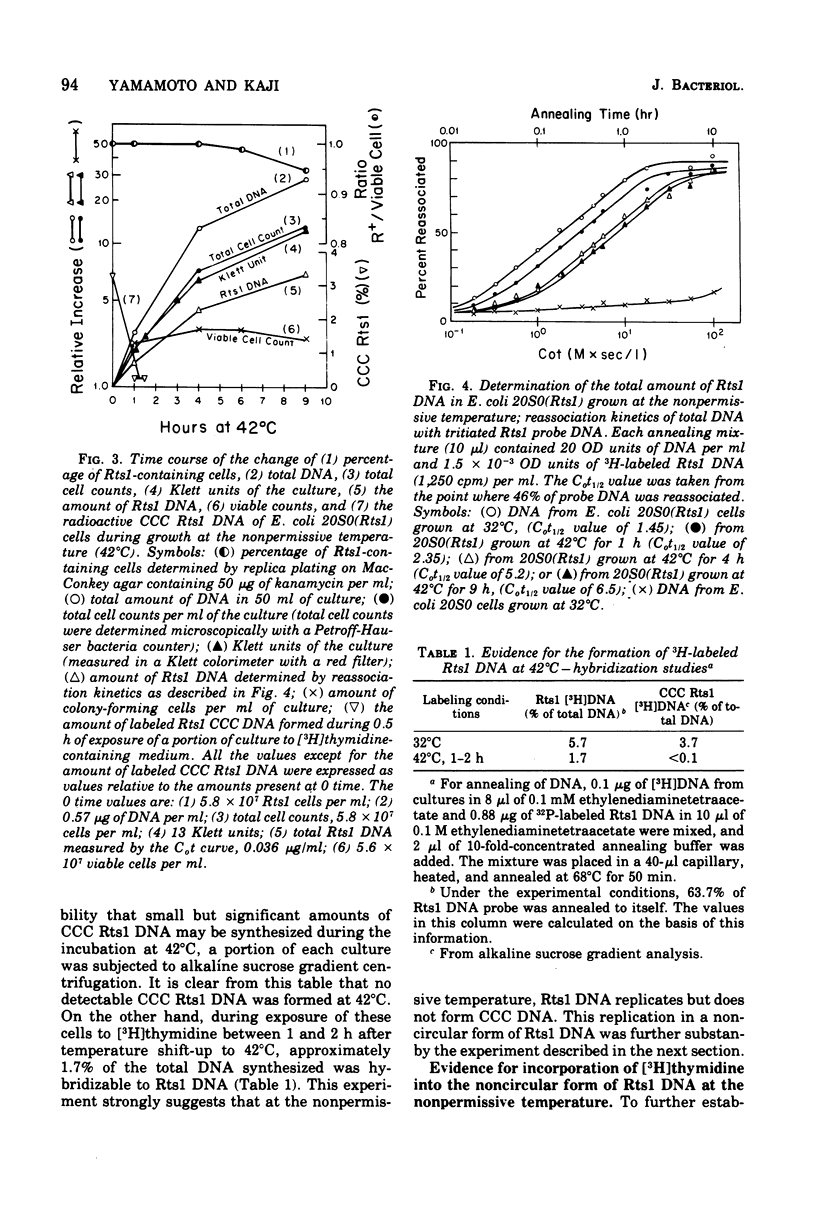
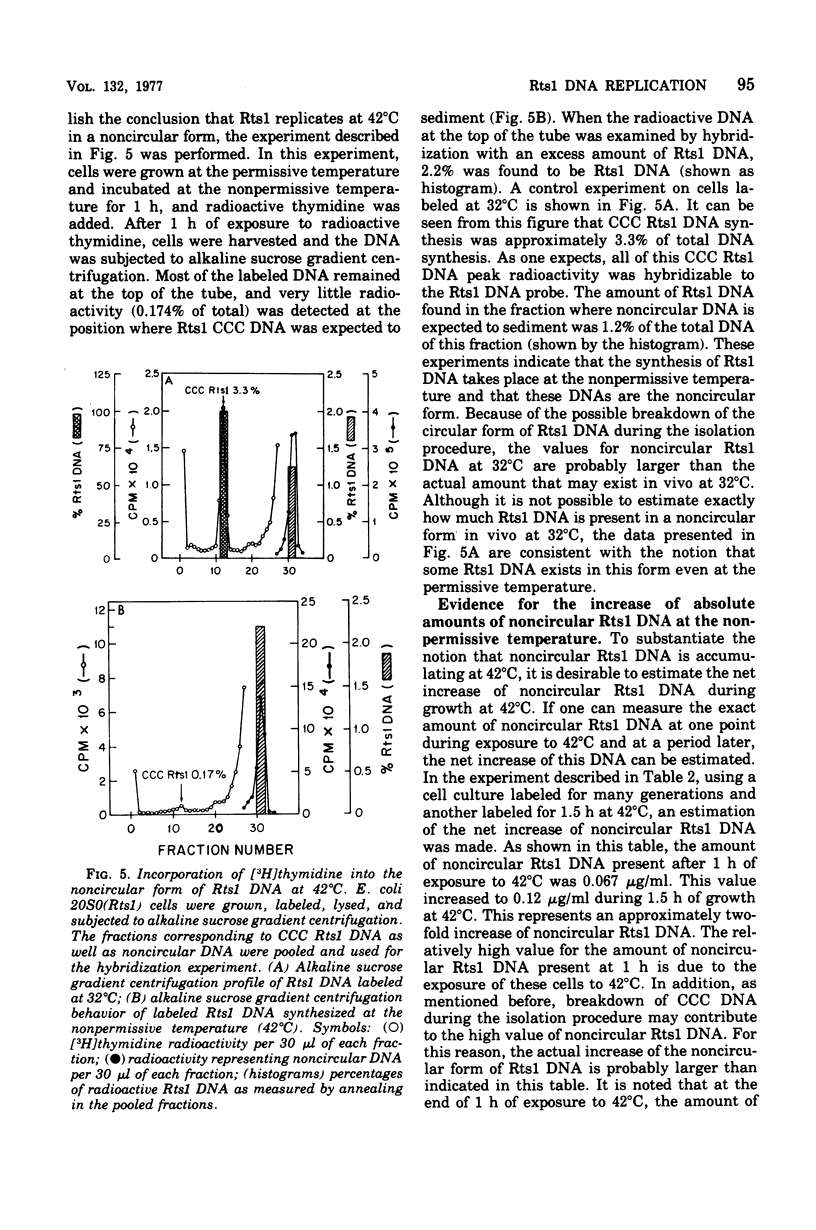
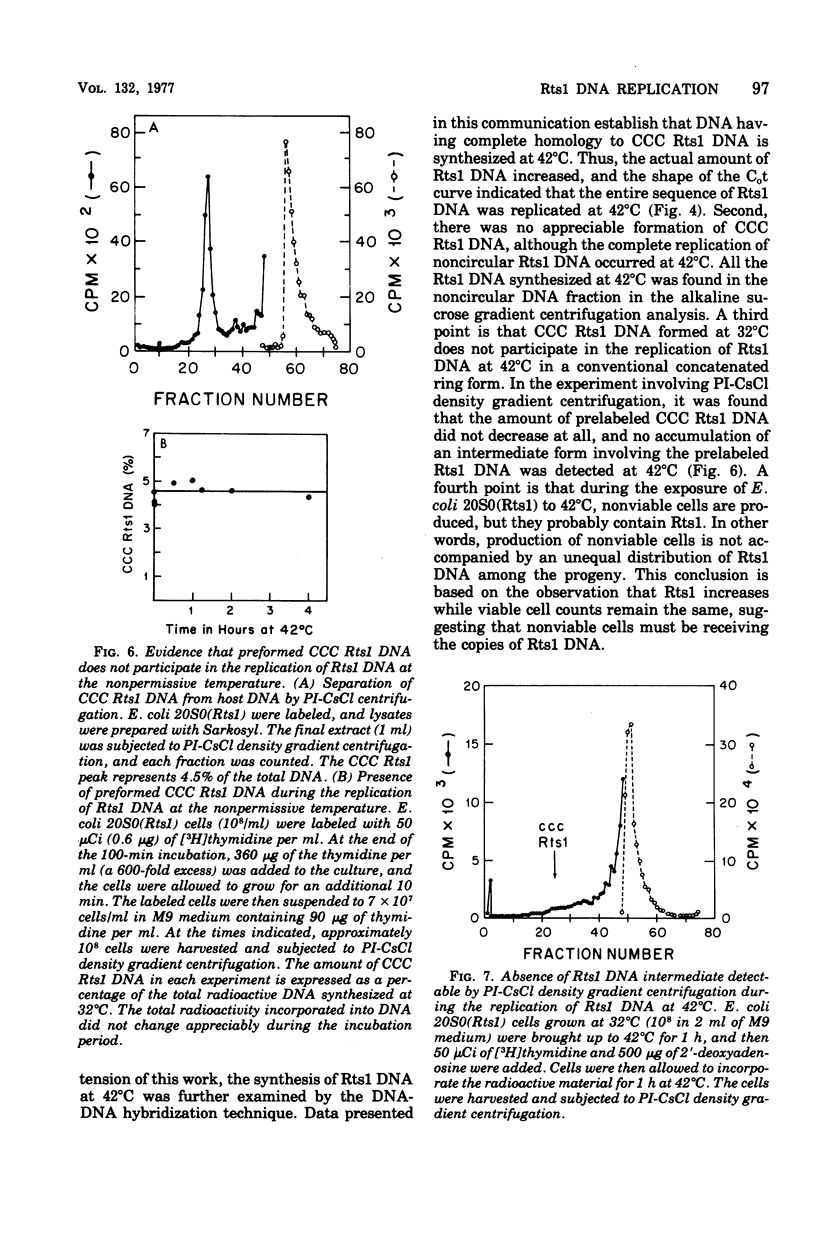
Replication of the thermosensitive drug resistance factor Rts1 was studied at the nonpermissive temperature (42 degrees C). It was concluded from the following observations that replication of this plasmid takes place at 42 degrees C without involving the covalently closed circular (CCC) form of deoxyribonucleic acid (DNA). (i) DNA-DNA- reassociation kinetics studies with purified Rts1 DNA showed that Rts1 DNA increased several-fold during cell growth at 42 degrees C while very little, if any, CCC DNA was synthesized. (ii) When Escherichia coli 20S0(Rts1) was labeled with [3H]thymidine at 42 degrees C, a significant amount of radioactive DNA hybridizable to Rts1 DNA was formed. This DNA was found in a fraction where DNA other than CCC DNA was expected in alkaline sucrose density gradient centrifugation analysis. When E. coli 20S0(Rts1) was labeled at 32 degrees C, the labeled CCC DNA did not disappear during a chase period at 42 degrees C. This indicates that preformed CCC DNA does not participate in replication at the nonpermissive temperature. These results are consistent with the hypothesis that there are two modes of replication of Rts1 DNA, one involving a CCC molecule and the other not involving this form, and that only the latter mode takes place at the nonpermissive temperature.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Mode of replication of the conjugative R-plasmid RSF1040 in Escherichia coli. J Bacteriol. 1976 Apr;126(1):454–466. doi: 10.1128/jb.126.1.454-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Bayer M. E., Kaji A. Host cell growth in the presence of the thermosensitive drug resistance factor, Rts1. J Bacteriol. 1973 Jul;115(1):399–410. doi: 10.1128/jb.115.1.399-410.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Change in the cell envelope of Escherichia coli carrying the thermosensitive drug resistance factor, Rts 1, at the nonpermissive temperature. Antimicrob Agents Chemother. 1975 Oct;8(4):504–509. doi: 10.1128/aac.8.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Molecular nature of the deoxyribonucleic acid of a thermosensitive R factor, Rts1. J Bacteriol. 1974 Dec;120(3):1364–1369. doi: 10.1128/jb.120.3.1364-1369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G. The thermosensitive lesion in the replication of the drug resistance factor, Rts1. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2515–2519. doi: 10.1073/pnas.71.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., CITARELLA R. V. MOLECULAR HOMOLOGY OF F-MEROGENOTE DNA. J Mol Biol. 1965 May;12:138–151. doi: 10.1016/s0022-2836(65)80288-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Helinski D. R. Relaxation complexes of poasmid DNA and protein. III. Association of protein with the 5' terminus of the broken DNA strand in the relaxed complex of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8796–8803. [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Saitoh T. F deoxyribonucleic acid transferred to recipient cells in the presence of rifampin. J Bacteriol. 1975 Mar;121(3):1000–1006. doi: 10.1128/jb.121.3.1000-1006.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Kline B. C. Mechanism and biosynthetic requirements for F plasmid replication in Escherichia coli. Biochemistry. 1974 Jan 1;13(1):139–146. doi: 10.1021/bi00698a022. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Warner R. C., Tewari K. K. The presence of covalently linked ribonucleotides in the closed circular deoxyribonucleic acid from higher plants. J Biol Chem. 1975 Sep 10;250(17):7020–7026. [PubMed] [Google Scholar]
- Koyama A. H., Wada C., Nagata T., Yura T. Indirect selection for plasmid mutants: isolation of ColVBtrp mutants defective in self-maintenance in Escherichia coli. J Bacteriol. 1975 Apr;122(1):73–79. doi: 10.1128/jb.122.1.73-79.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A. H., Yura T. Plasmid mutations affecting self-maintenance and host growth in Escherichia coli. J Bacteriol. 1975 Apr;122(1):80–88. doi: 10.1128/jb.122.1.80-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M. A., Katz L., Helinski D. R. Unidirectional replication of plasmid ColE1 DNA. Nature. 1974 Sep 27;251(5473):337–340. doi: 10.1038/251337a0. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Replication of a specific terminal chromosome segment in Escherichia coli which is required for cell division. J Mol Biol. 1973 Jun 25;78(1):211–228. doi: 10.1016/0022-2836(73)90439-7. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Sheehy R. J., Novick R. P. Studies on plasmid replication. V Replicative intermediates. J Mol Biol. 1975 Apr 5;93(2):237–253. doi: 10.1016/0022-2836(75)90130-8. [DOI] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y., Tomizawa J., Kakefuda T. Location of non-DNA components of closed circular colicin E1 plasmid DNA. Nature. 1975 Feb 20;253(5493):652–654. doi: 10.1038/253652a0. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Kishi H., Nakaya R. Integration of R plasmid Rts1 to the gal region of the Escherichia coli chromosome. J Bacteriol. 1975 Mar;121(3):857–862. doi: 10.1128/jb.121.3.857-862.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Rownd R. Replication of the R factor Rts1 in Proteus mirabilis. J Bacteriol. 1972 Feb;109(2):492–498. doi: 10.1128/jb.109.2.492-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T., Kaji A. Requirement of cyclic adenosine 3',5'-monophosphate for the thermosensitive effects of Rts1 in a cyclic adenosine 3',5'-monophosphate-less mutant of Escherichia coli. J Bacteriol. 1977 Oct;132(1):80–89. doi: 10.1128/jb.132.1.80-89.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T., Kaji A. The role of cyclic AMP in the thermosensitive lesion of the formation of closed covalent circular Rts 1 DNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):546–552. doi: 10.1016/0006-291x(75)90433-7. [DOI] [PubMed] [Google Scholar]
- Yokota T., Kanamaru Y., Mori R., Akiba T. Recombination between a thermosensitive kanamycin resistance factor and a nonthermosensitive multiple-drug resistant factor. J Bacteriol. 1969 Jun;98(3):863–873. doi: 10.1128/jb.98.3.863-873.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto H., Yoshikawa M. Chromosome-plasmid interaction in Escherichia coli K-12 carrying a thermosensitive plasmid, Rts1, in autonomous and in integrated states. J Bacteriol. 1975 Nov;124(2):661–667. doi: 10.1128/jb.124.2.661-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


