Abstract
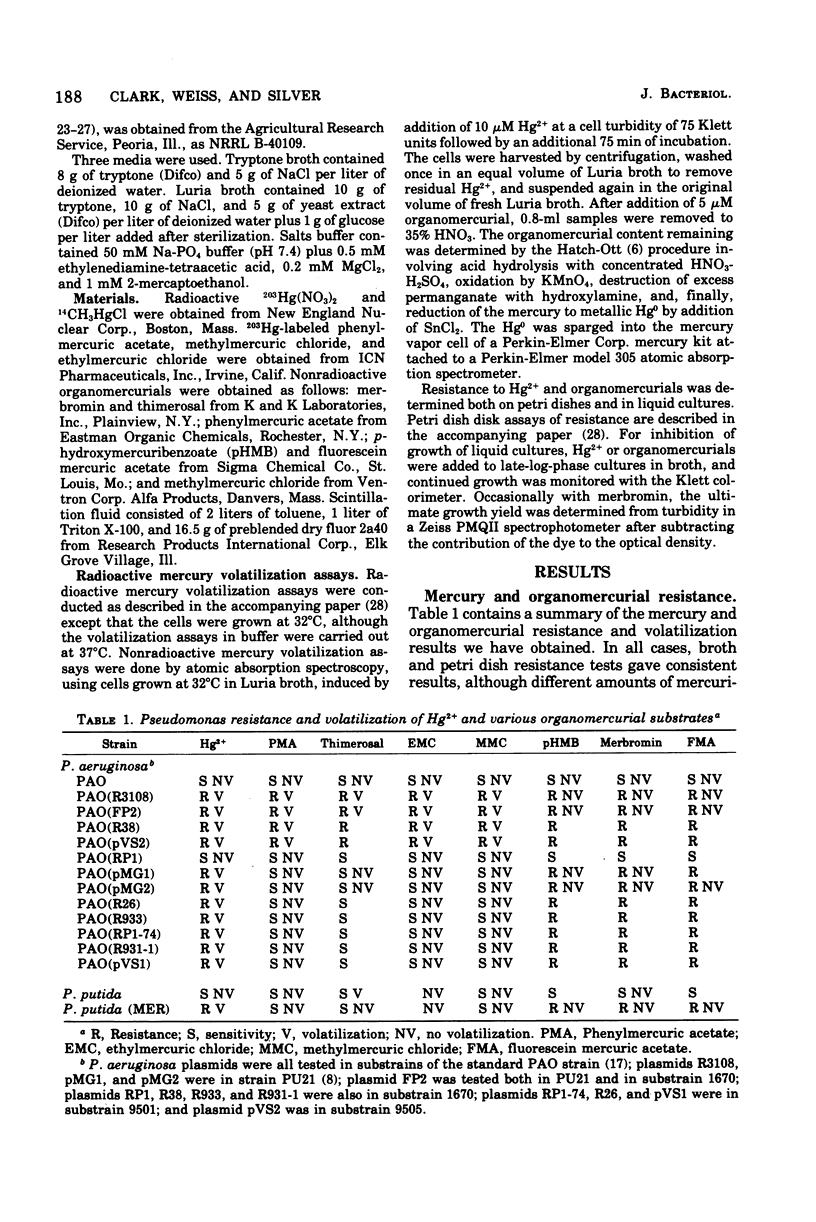
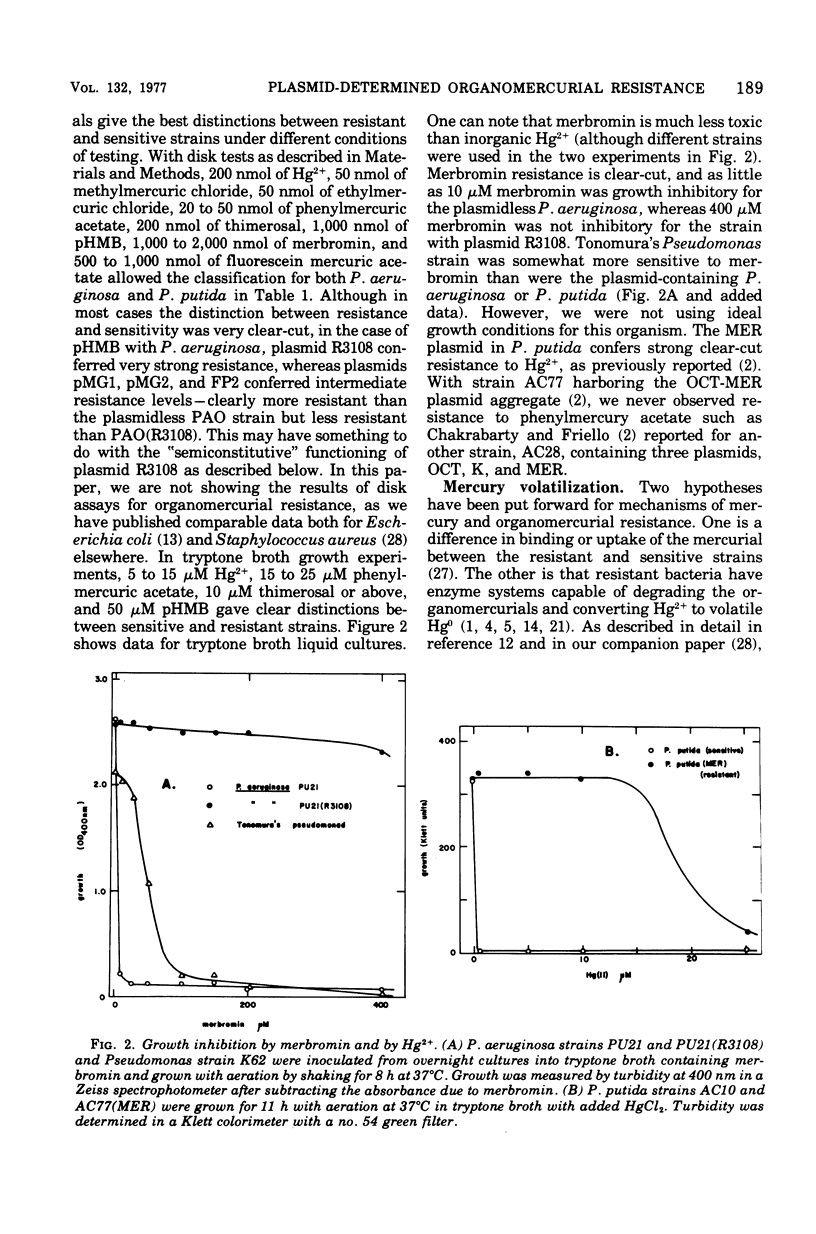
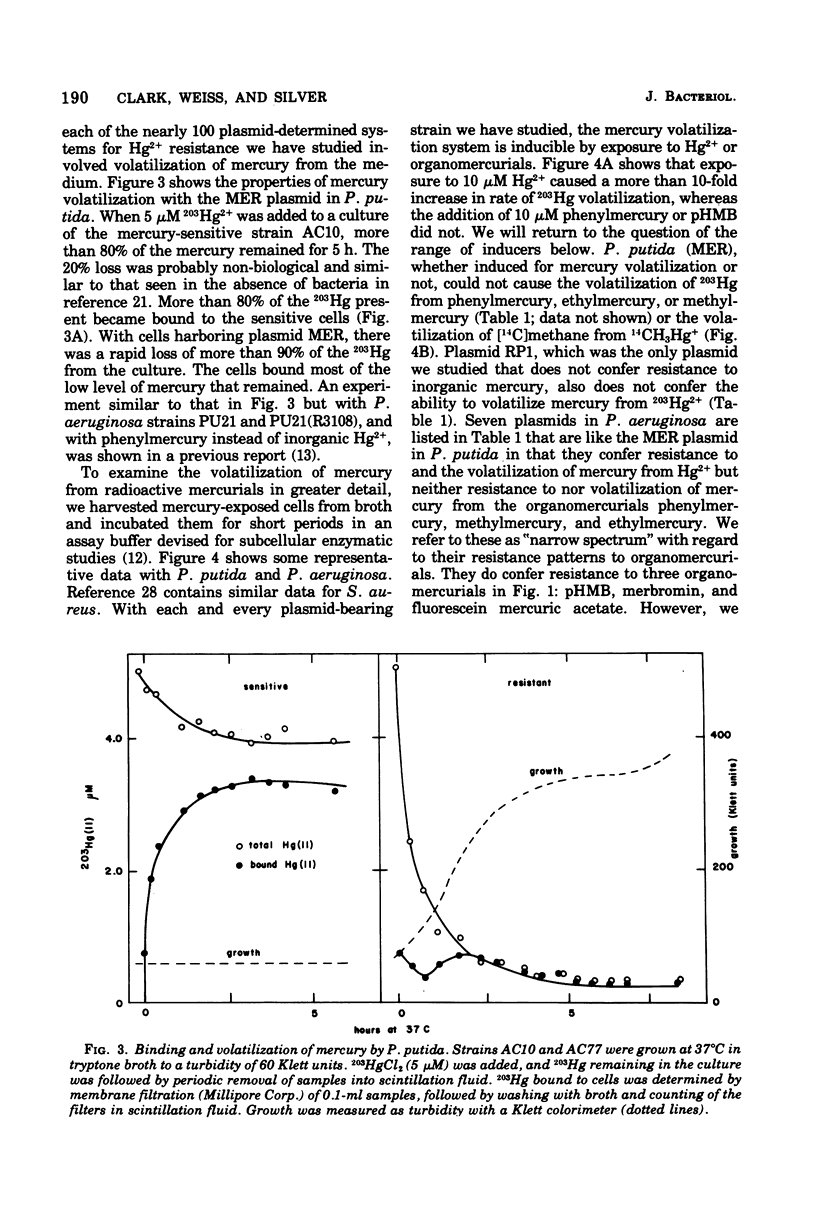
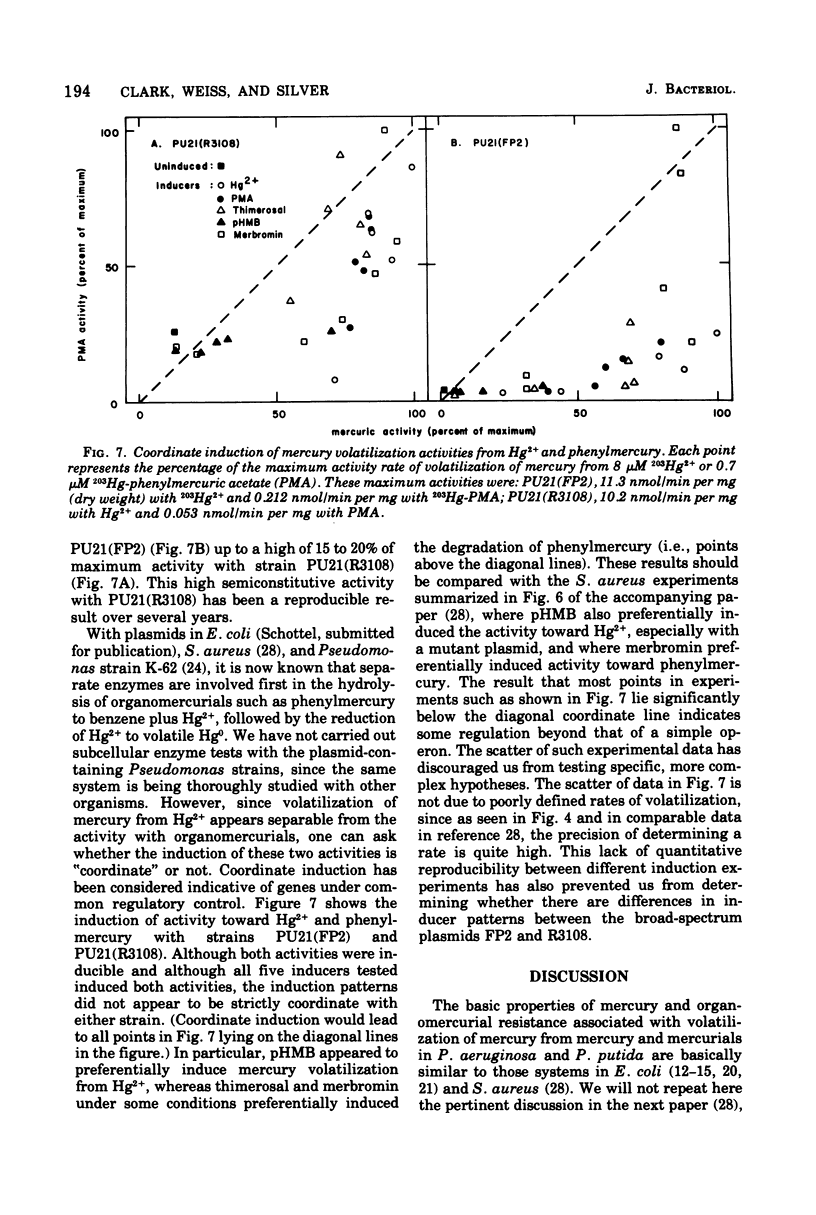
Mercury and organomercurial resistance determined by genes on ten Pseudomonas aeruginosa plasmids and one Pseudomonas putida plasmid have been studied with regard to the range of substrates and the range of inducers. The plasmidless strains were sensitive to growth inhibition by Hg2+ and did not volatilize Hg0 from Hg2+. A strain with plasmid RP1 (which does not confer resistance to Hg2+) similarly did not volatilize mercury. All 10 plasmids determine mercury resistance by way of an inducible enzyme system. Hg2+ was reduced to Hg0, which is insoluble in water and rapidly volatilizes from the growth medium. Plasmids pMG1, pMG2, R26, R933, R93-1, and pVS1 in P. aeruginosa and MER in P. putida conferred resistance to and the ability to volatilize mercury from Hg2+, but strains with these plasmids were sensitive to and could not volatilize mercury from the organomercurials methylmercury, ethylmercury, phenylmercury, and thimerosal. These plasmids, in addition, conferred resistance to the organomercurials merbromin, p-hydroxymercuribenzoate, and fluorescein mercuric acetate. The other plasmids, FP2, R38, R3108, and pVS2, determined resistance to and decomposition of a range of organomercurials, including methylmercury, ethylmercury, phenylmercury, and thimerosal. These plasmids also conferred resistance to the organomercurials merbromin, p-hydroxymercuribenzoate, and fluorescein mercuric acetate by a mechanism not involving degradation. In all cases, organomercurial decomposition and mercury volatilization were induced by exposure to Hg2+ or organomercurials. The plasmids differed in the relative efficacy of inducers. Hg2+ resistance with strains that are organomercurial sensitive appeared to be induced preferentially by Hg2+ and only poorly by organomercurials to which the cells are sensitive. However, the organomercurials p-hydroxymercuribenzoate, merbromin, and fluorescein mercuric acetate were strong gratuitous inducers but not substrates for the Hg2+ volatilization system. With strains resistant to phenylmercury and thimerosal, these organomercurials were both inducers and substrates.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakrabarty A. M., Friello D. A. Dissociation and interaction of individual components of a degradative plasmid aggregate in Pseudomonas. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3410–3414. doi: 10.1073/pnas.71.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Clarkson T. W., Amin-Zaki L., Al-Tikriti S. K. An outbreak of methylmercury poisoning due to consumption of contaminated grain. Fed Proc. 1976 Oct;35(12):2395–2399. [PubMed] [Google Scholar]
- Izaki K., Tashiro Y., Funaba T. Mechanism of mercuric chloride resistance in microorganisms. 3. Purification and properties of a mercuric ion reducing enzyme from Escherichia coli bearing R factor. J Biochem. 1974 Mar;75(3):591–599. doi: 10.1093/oxfordjournals.jbchem.a130427. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. S. Investigation of the mating system of Pseudomonas aeruginosa strain I. VI. Mercury resistance associated with the sex factor (FP). Genet Res. 1970 Oct 2;16(2):179–184. doi: 10.1017/s0016672300002408. [DOI] [PubMed] [Google Scholar]
- Nelson J. D., Blair W., Brinckman F. E., Colwell R. R., Iverson W. P. Biodegradation of phenylmercuric acetate by mercury-resistant bacteria. Appl Microbiol. 1973 Sep;26(3):321–326. doi: 10.1128/am.26.3.321-326.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Spangler W. J., Spigarelli J. L., Rose J. M., Flippin R. S., Miller H. H. Degradation of methylmercury by bacteria isolated from environmental samples. Appl Microbiol. 1973 Apr;25(4):488–493. doi: 10.1128/am.25.4.488-493.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M. The properties of hybrids formed between the P-group plasmid RP1 and various plasmids from Pseudomonas aeruginosa. Mol Gen Genet. 1976 Dec 8;149(2):217–223. doi: 10.1007/BF00332892. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J Bacteriol. 1973 Feb;113(2):1070–1072. doi: 10.1128/jb.113.2.1070-1072.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Sugarman L. I. Cell-free mercury(II)-reducing activity in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1974 Jul;119(1):242–249. doi: 10.1128/jb.119.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Tonomura K. Purification and properties of an enzyme catalyzing the splitting of carbon-mercury linkages from mercury-resistant Pseudomonas K-62 strain. I. Splitting enzyme 1. J Biochem. 1976 Jul;80(1):79–87. doi: 10.1093/oxfordjournals.jbchem.a131261. [DOI] [PubMed] [Google Scholar]
- Tonomura K., Kanzaki F. The reductive decomposition of organic mercurials by cell-free extract of a mercury-resistant pseudomonad. Biochim Biophys Acta. 1969 Jun 17;184(1):227–229. doi: 10.1016/0304-4165(69)90124-x. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


