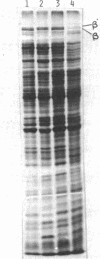
Abstract
Escherichia coli K-12 mutants that are resistant to bacteriophage χ, defective in motility, and unable to grow at high temperature (42°C) were isolated from among those selected for rifampin resistance at low temperature (30°C) after mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine. Genetic analysis of one such mutant indicated the presence of two mutations that probably affect the β subunit of ribonucleic acid (RNA) polymerase: one (rif) causing rifampin resistance and the other (Ts-74) conferring resistance to phage χ (and loss of motility) and temperature sensitivity for growth. Observations with an electron microscope revealed that the number of flagella per mutant cell was significantly reduced, suggesting that the Ts-74 mutation somehow affected flagella formation at the permissive temperature. When a mutant culture was transferred from 30 to 42°C, deoxyribonucleic acid synthesis accelerated normally, but RNA or protein synthesis was enhanced relatively little. The rate of synthesis of β and β′ subunits of RNA polymerase was low even at 30°C and was further reduced at 42°C, in contrast to the parental wild-type strain. Expression of the lactose and other sugar fermentation operons, as well as lysogenization with phage λ, occurred normally at 30°C, suggesting that the mutation does not cause general shut-off of gene expression regulated by cyclic adenosine 3′,5′-monophosphate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., James R., Paradee A. B. Evidence of the involvement of an outer membrane protein in DNA initiation. J Biol Chem. 1976 Jun 10;251(11):3470–3479. [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Genetics and chemistry of bacterial flagella. Bacteriol Rev. 1969 Dec;33(4):454–475. doi: 10.1128/br.33.4.454-475.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Yura T., Yamagishi H. Genetic and physical studies of lambda transducing bacteriophage carrying the beta subunit gene of the Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1247–1256. doi: 10.1128/jb.122.3.1247-1256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A., Yura T. RNA polymerase mutants of Escherichia coli. Streptolydigin resistance and its relation to rifampicin resistance. Mol Gen Genet. 1973 Mar 1;121(2):181–196. doi: 10.1007/BF00277531. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Claeys I. V., Nasi S., Molholt B., Miller J. H. Temperature-sensitive RNA polymerase mutants with altered subunit synthesis and degradation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2375–2379. doi: 10.1073/pnas.72.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Scaife J. Evidence for a lambda transducing phage carrying the genes for the beta and beta' subunits of Escherichia coli RNA polymerase. Mol Gen Genet. 1974;132(3):193–201. doi: 10.1007/BF00269392. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Morishita T., Yura T. Altered nutritional requirements associated with mutations affecting the structures of ribonucleic acid polymerase in Lactobacillus casei. J Bacteriol. 1976 Feb;125(2):416–422. doi: 10.1128/jb.125.2.416-422.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Evidence for a positive regulation of RNA polymerase synthesis in Escherichia coli. J Mol Biol. 1975 Oct 5;97(4):621–642. doi: 10.1016/s0022-2836(75)80063-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Hyperproduction of the sigma subunit of RNA polymerase in a mutant of Escherichia coli. Mol Gen Genet. 1975 Nov 24;141(2):97–111. doi: 10.1007/BF00267677. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. Regulation of RNA polymerase synthesis in Escherichia coli: a mutant unable to synthesize the enzyme at 43 degrees. Proc Natl Acad Sci U S A. 1975 Mar;72(3):911–915. doi: 10.1073/pnas.72.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade S., Adler J. Purification and chemistry of bacteriophage chi. J Virol. 1967 Jun;1(3):591–598. doi: 10.1128/jvi.1.3.591-598.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. H., Boos W. Regulation of the -methylgalactoside transport system and the galatose-binding protein by the cell cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1481–1485. doi: 10.1073/pnas.70.5.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silerman M., Matsumura P., Draper R., Edwards S., Simon M. I. Expression of flagellar genes carried by bacteriophage lambda. Nature. 1976 May 20;261(5557):248–250. doi: 10.1038/261248a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974 Dec;120(3):1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Iino T. Absence of messenger ribonucleic acid specific for flagellin in non-flagellate mutants of Salmonella. J Mol Biol. 1975 Jul 15;95(4):549–556. doi: 10.1016/0022-2836(75)90316-2. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]