Abstract
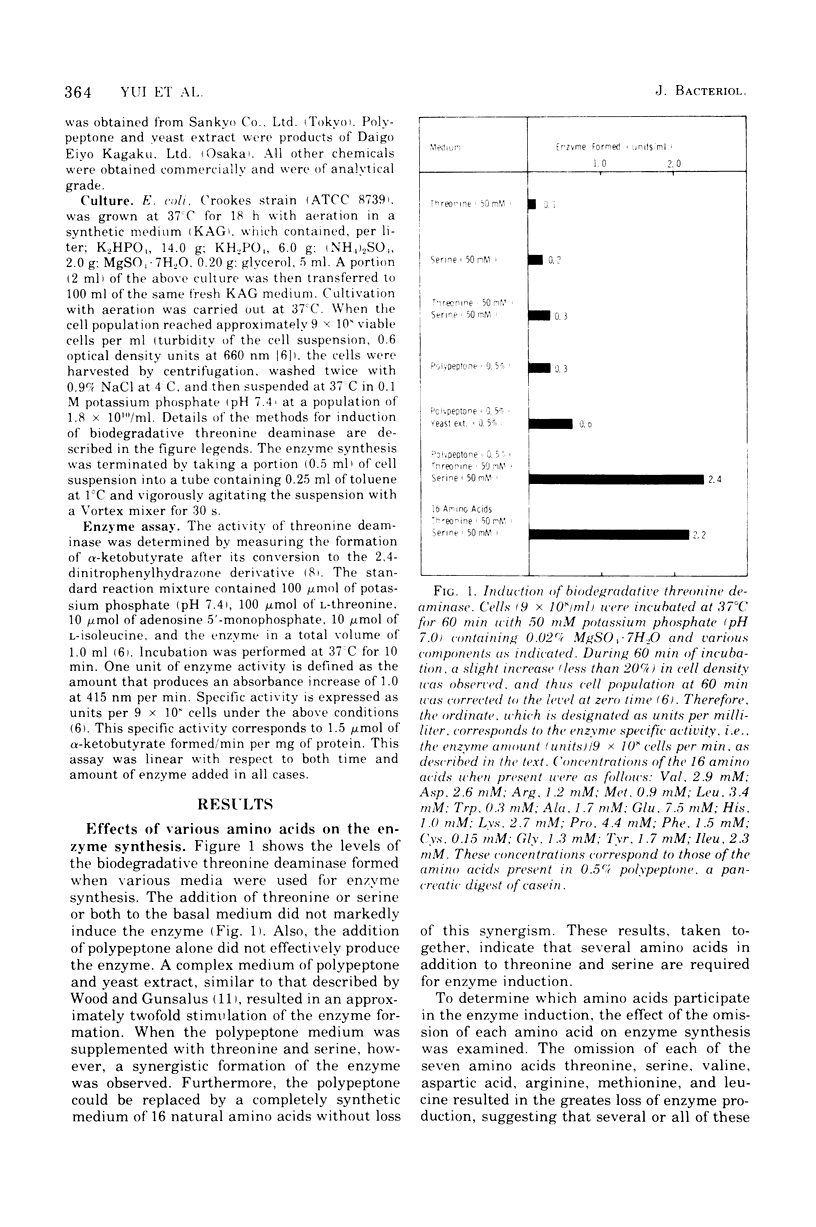
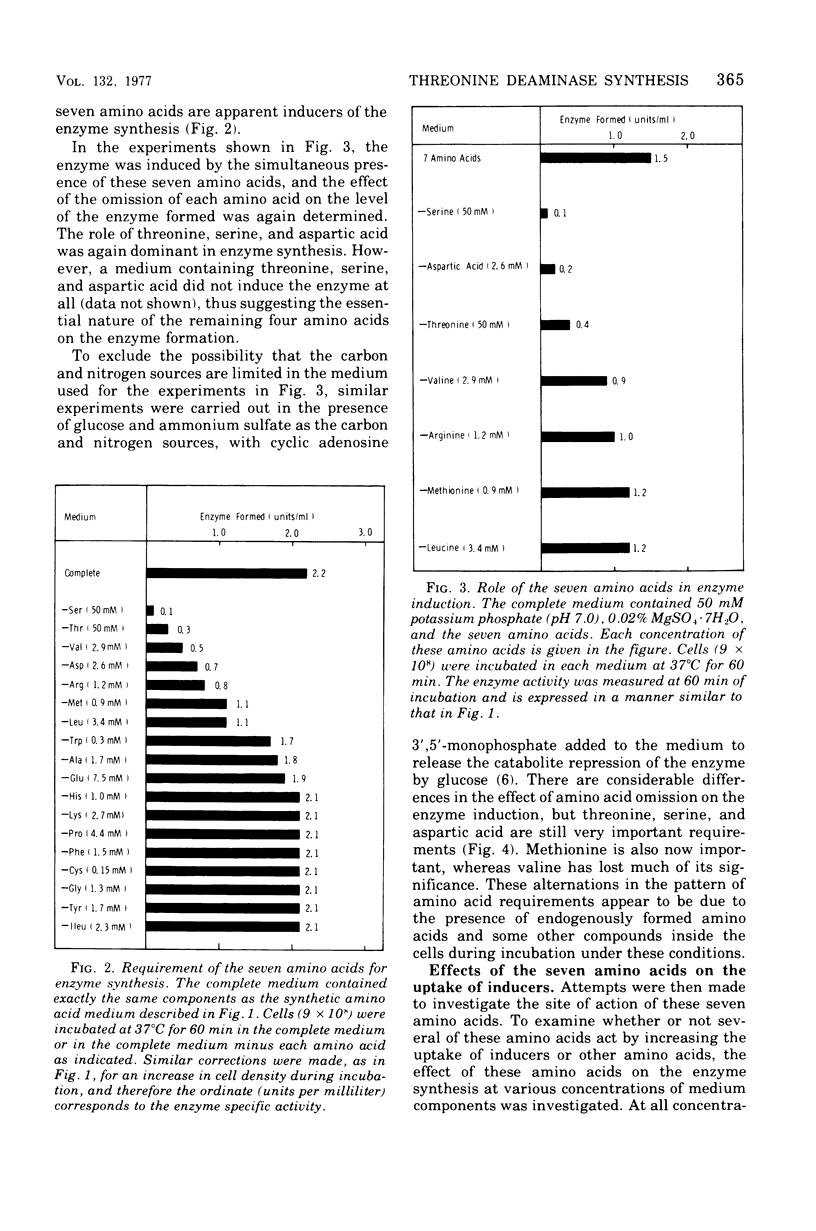
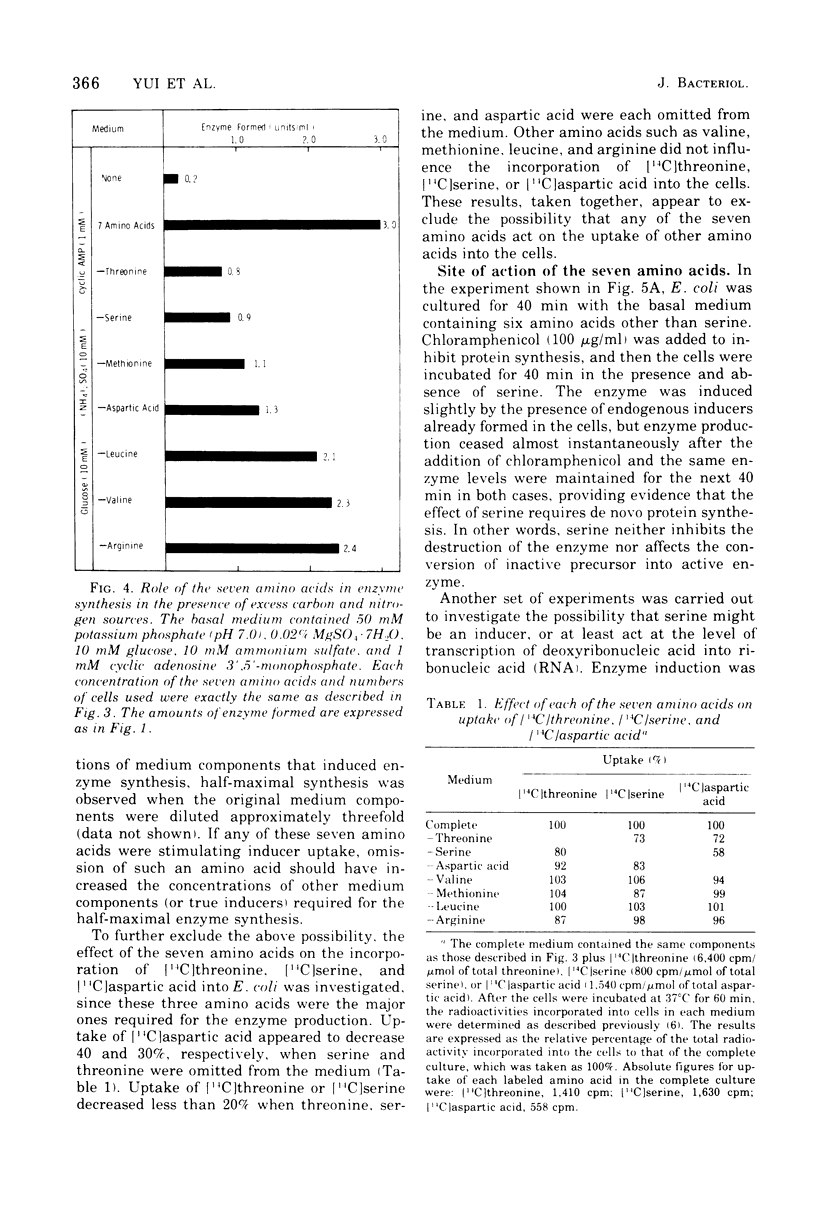
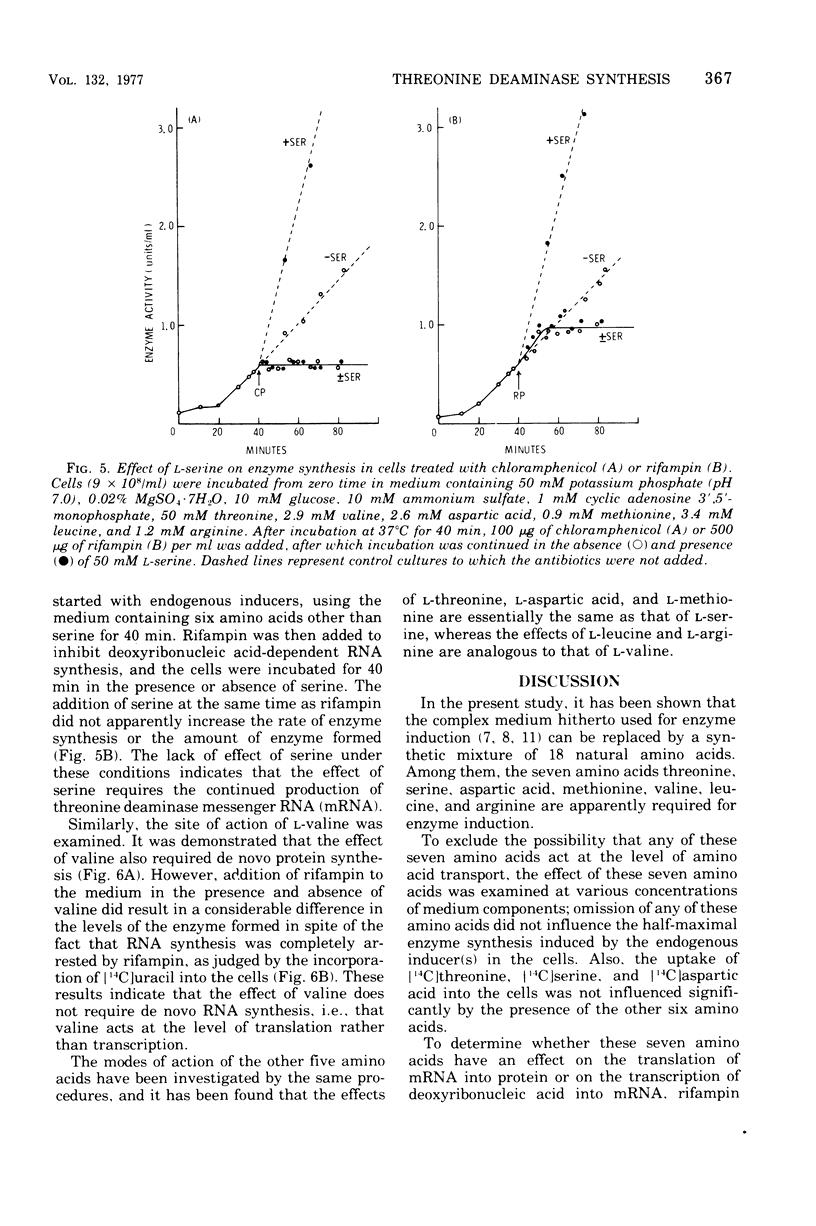
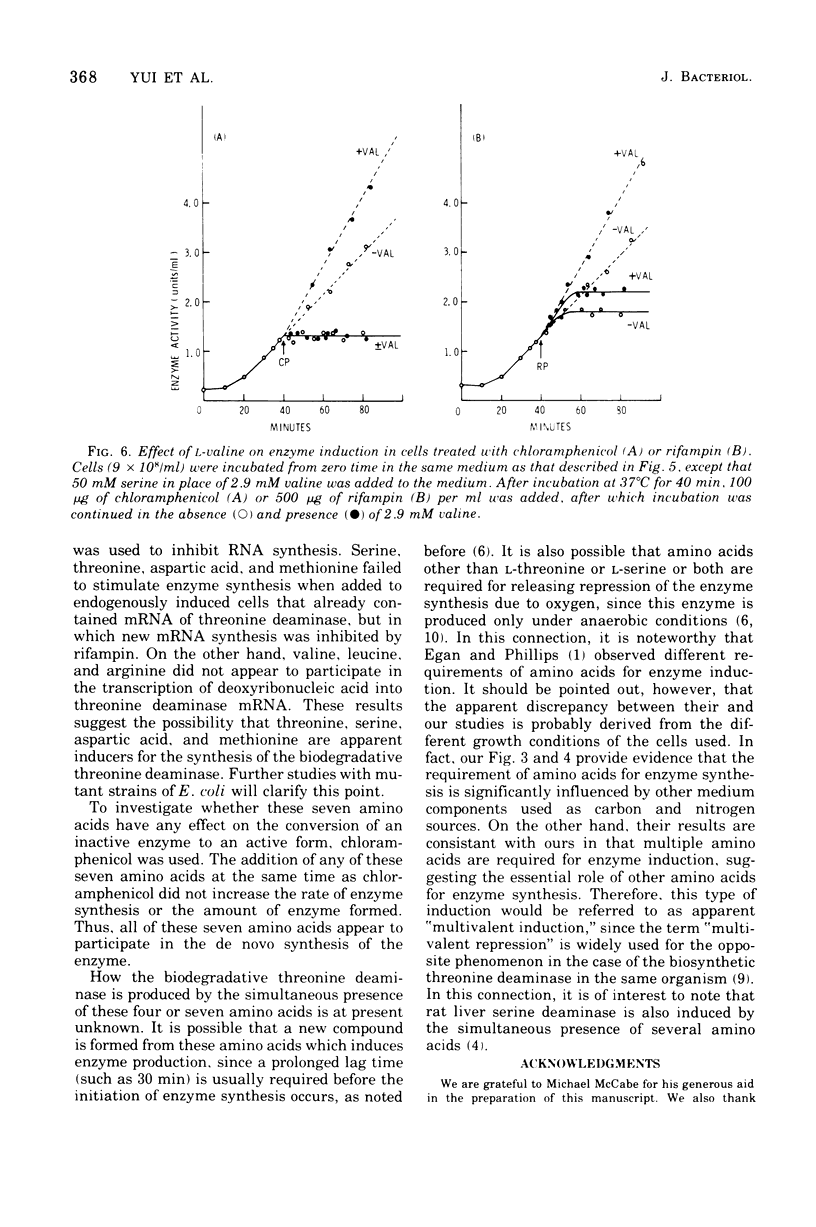
To determine the inducer(s) of the biodegradative threonine deaminase in Escherichia coli, the effects of various amino acids on the synthesis of this enzyme were investigated. The complex medium used hitherto for the enzyme induction can be completely replaced by a synthetic medium composed of 18 natural amino acids. In this synthetic medium, the omission of each of the seven amino acids threonine, serine, aspartic acid, methionine, valine, leucine, and arginine resulted in the greatest loss of enzyme formation. These seven amino acids did not significantly influence the uptake of other amino acids into the cells. Furthermore, they did not stimulate the conversion of inactive enzyme into an active form, since they did not affect the enzyme level in cells in which protein synthesis was inhibited by chloramphenicol. Threonine, serine, aspartic acid, and methionine failed to stimulate enzyme production in cells in which messenger ribonucleic acid synthesis was arrested by rifampin, whereas valine, leucine, and arginine stimulated enzyme synthesis under the same conditions. Therefore, the first four amino acids appear to act as inducers of the biodegradative threonine deaminase in E. coli and the last three amino acids appear to be amplifiers of enzyme production. The term “multivalent induction” has been proposed for this type of induction, i.e., enzyme induction only by the simultaneous presence of several amino acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Egan R. M., Phillips A. T. Requirements for induction of the biodegradative threonine dehydratase in Escherichia coli. J Bacteriol. 1977 Nov;132(2):370–376. doi: 10.1128/jb.132.2.370-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O., Shizuta Y. Binding of pyridoxal phosphate to apoenzymes as studied by optical rotatory dispersion and circular dichroism. Vitam Horm. 1970;28:245–264. doi: 10.1016/s0083-6729(08)60896-1. [DOI] [PubMed] [Google Scholar]
- Kamihara T., Tokushige M. Formation of biodegradative threonine deaminase in Escherichia coli. J Nutr Sci Vitaminol (Tokyo) 1974;20(2):103–112. doi: 10.3177/jnsv.20.103. [DOI] [PubMed] [Google Scholar]
- Mauron J., Mottu F., Spohr G. Reciprocal induction and repression of serine dehydratase and phosphoglycerate dehydrogenase by proteins and dietary-essential amino acids in rat liver. Eur J Biochem. 1973 Jan 15;32(2):331–342. doi: 10.1111/j.1432-1033.1973.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Regulation of gene transcription in Escherichia coli by cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:11–16. [PubMed] [Google Scholar]
- Shizuta Y., Hayaishi O. Regulation of biodegradative threonine deaminase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1970 Oct 25;245(20):5416–5423. [PubMed] [Google Scholar]
- Shizuta Y., Hayaishi O. Regulation of biodegradative threonine deaminase. Curr Top Cell Regul. 1976;11:99–146. doi: 10.1016/b978-0-12-152811-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Shizuta Y., Nakazawa A., Tokushige M., Hayaishi O. Studies on the interaction between regulatory enzymes and effectors. 3. Crystallization and characterization of adenosine 5'-monophosphate-dependent threonine deaminase from Escherichia coli. J Biol Chem. 1969 Apr 10;244(7):1883–1889. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Threonine deaminases. Adv Enzymol Relat Areas Mol Biol. 1973;37:349–395. doi: 10.1002/9780470122822.ch6. [DOI] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]


