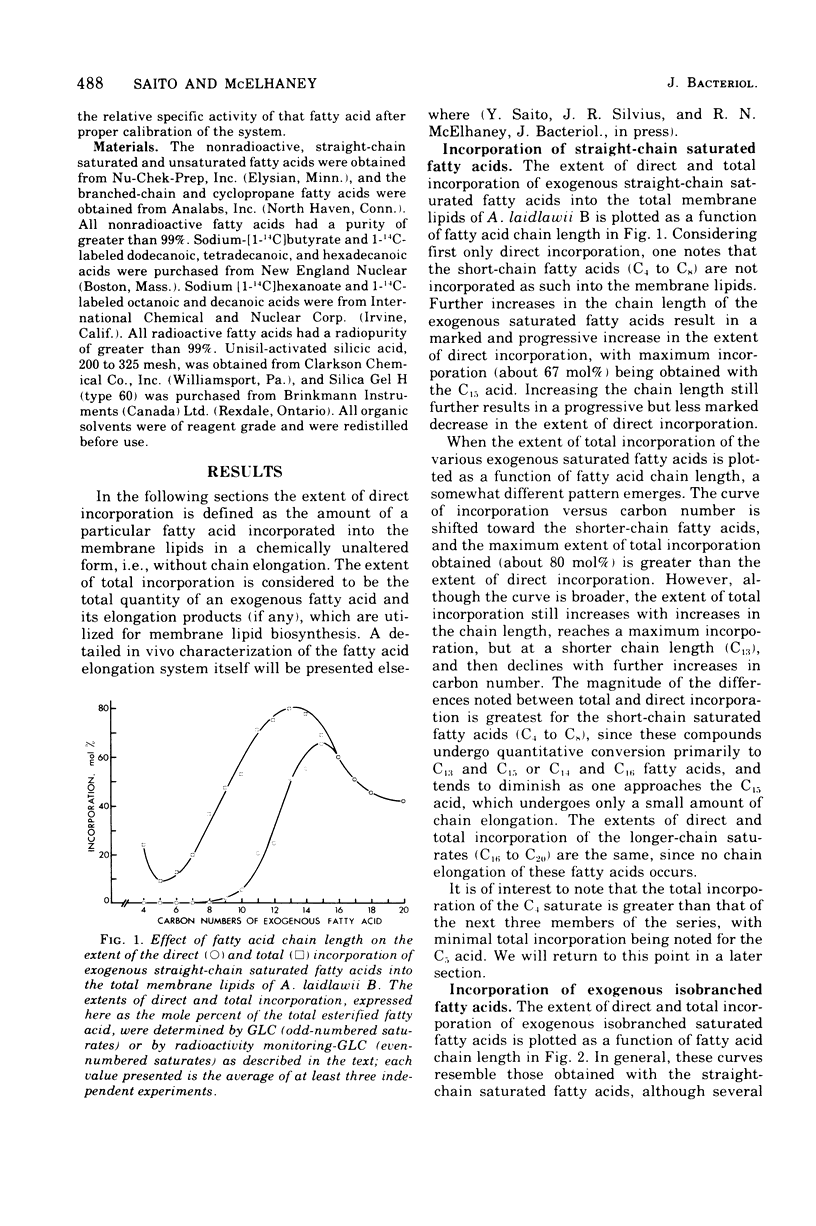
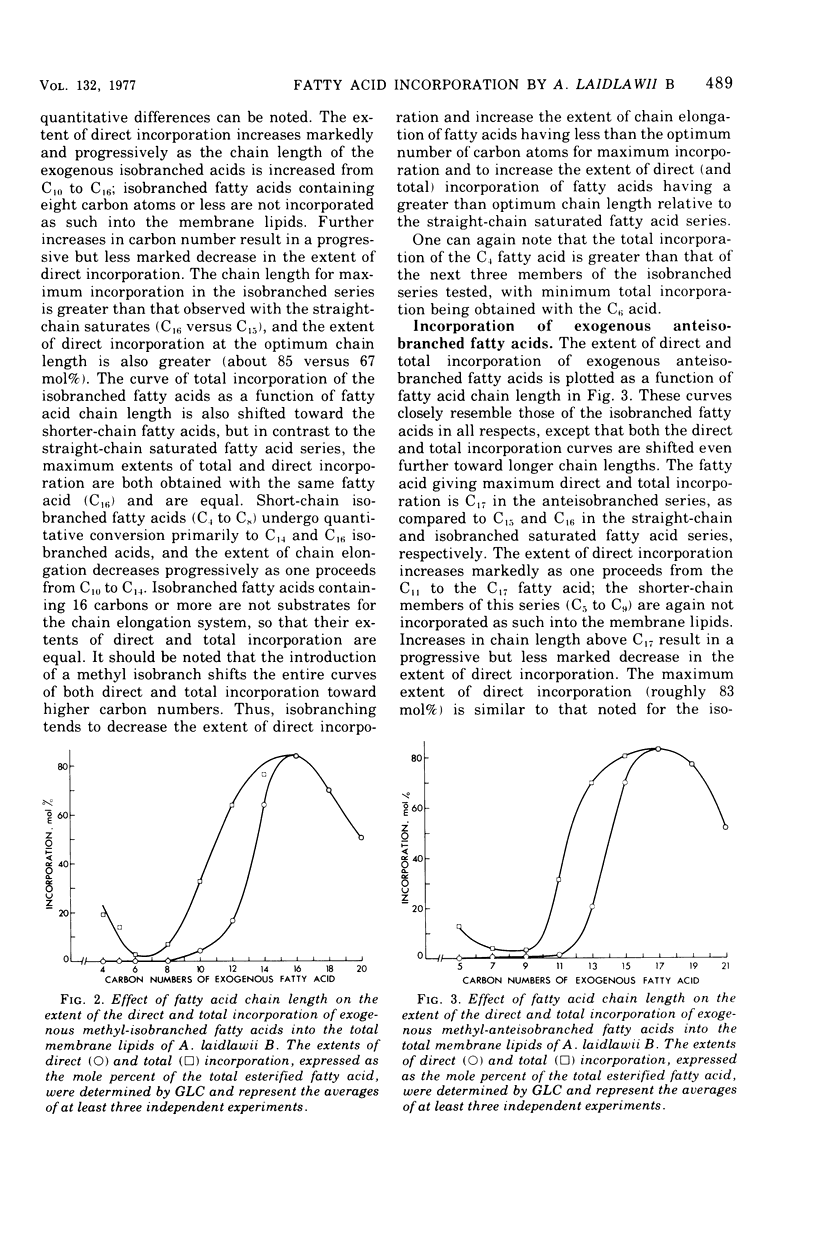
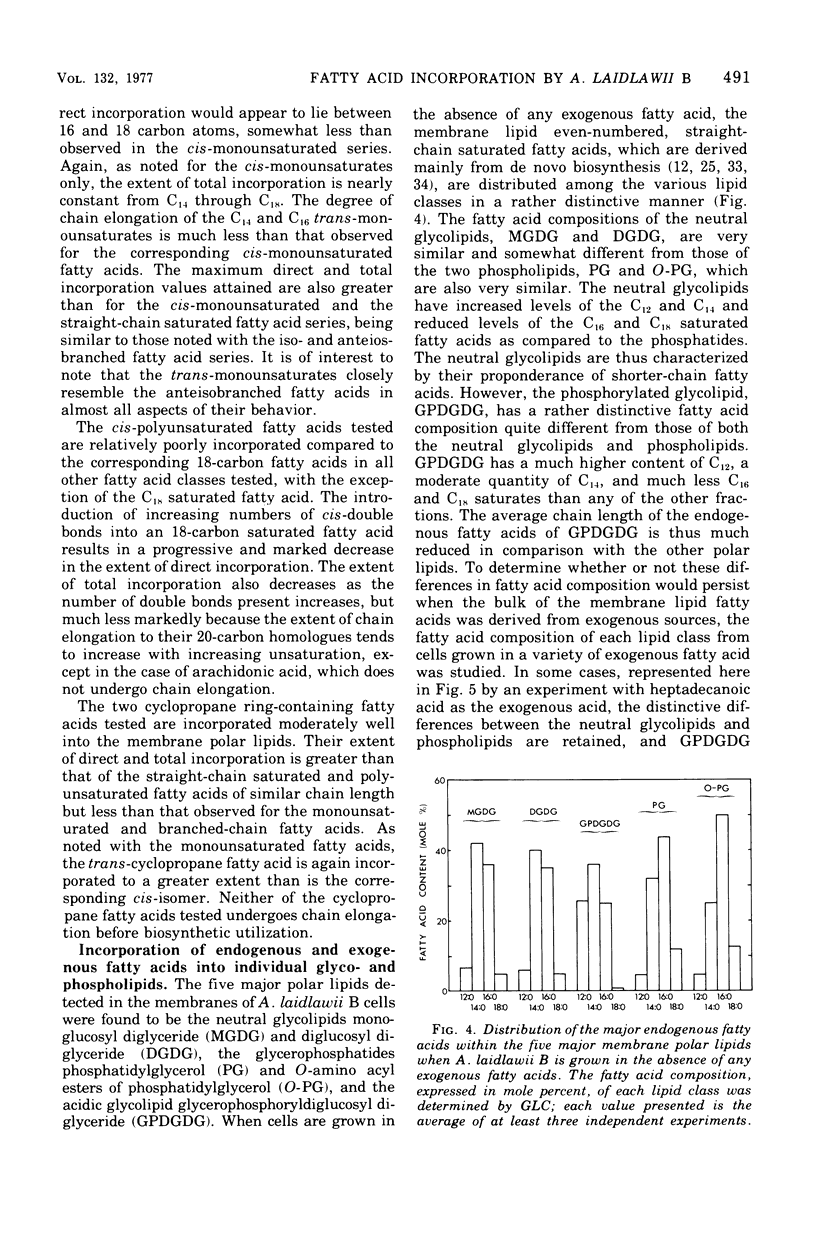
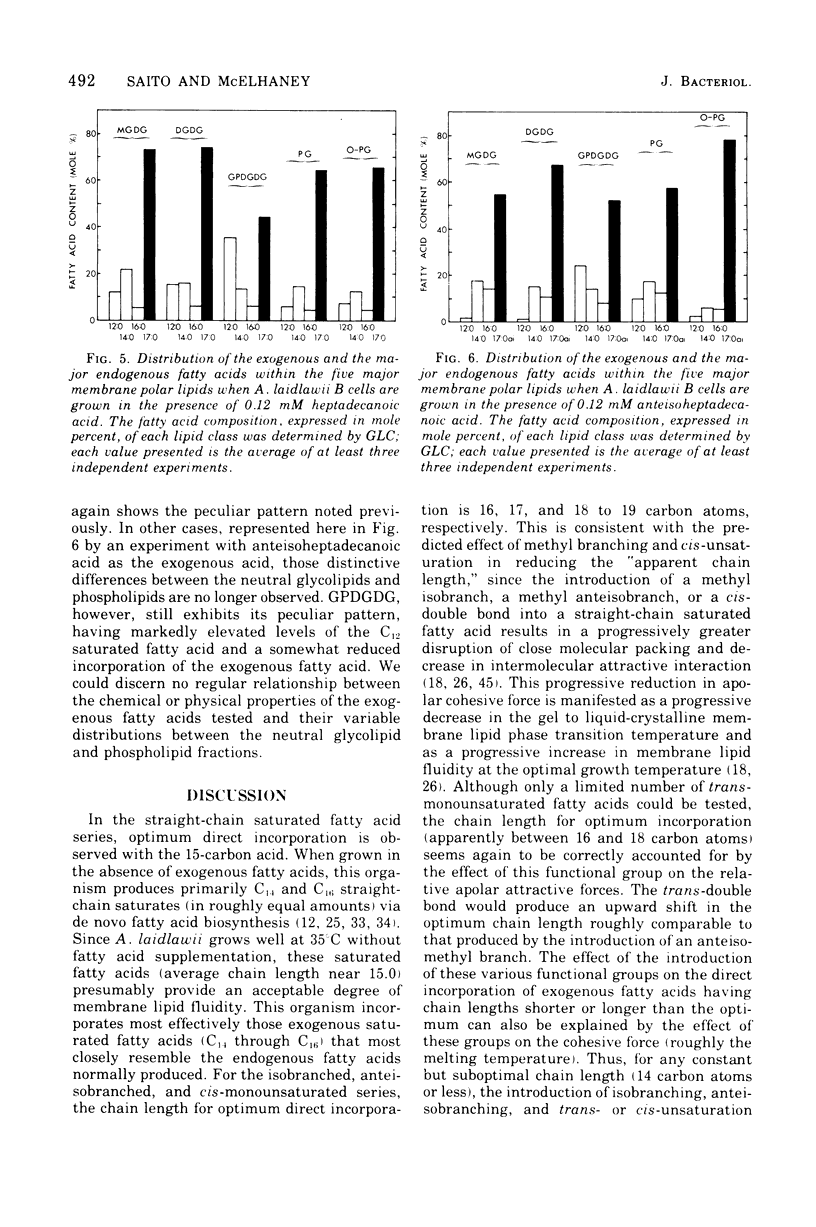
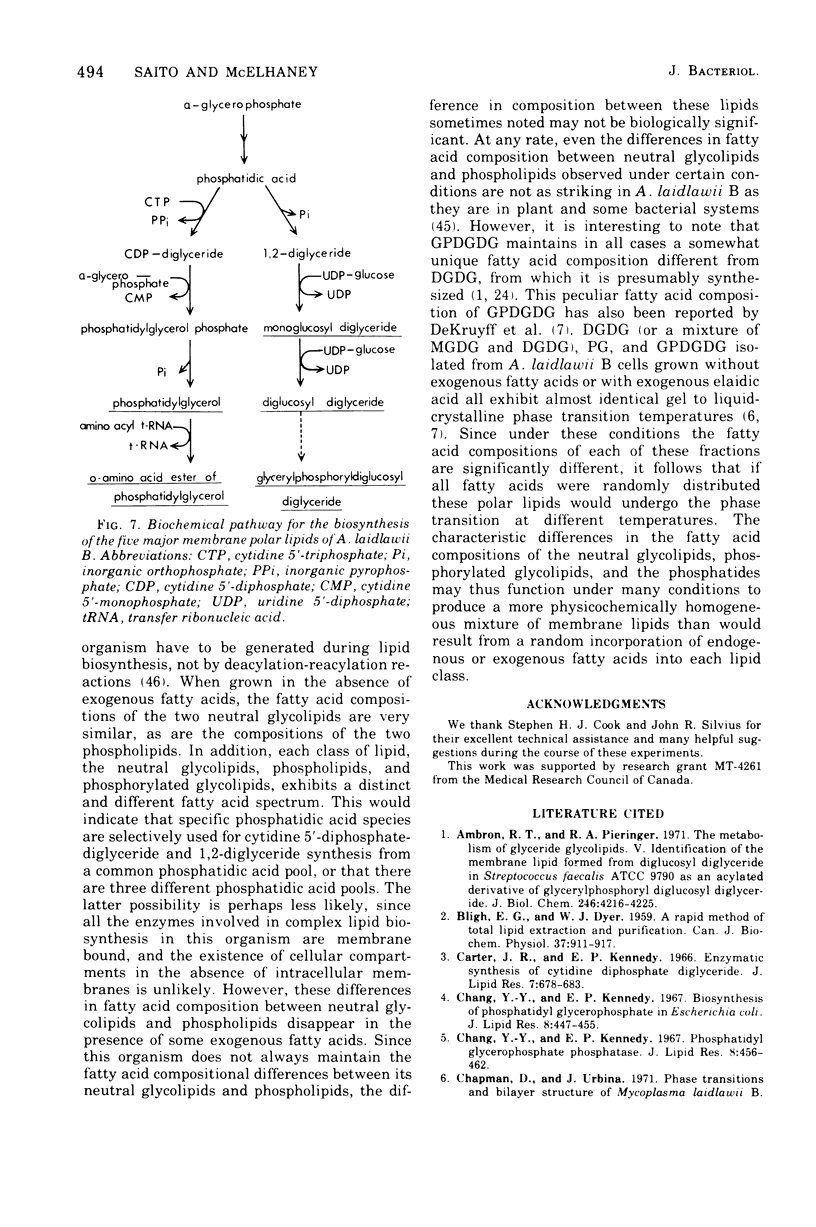
Abstract
The extent of incorporation of a wide variety of exogenous saturated, unsaturated, branched-chain, and cyclopropane fatty acids into the membrane lipids of Acholeplasma laidlawii B was systematically studied. Within each fatty acid class the extent of incorporation generally increased markedly with increasing chain length, reached a maximum, and then declined progressively but less sharply with further increases above that chain length giving maximal direct incorporation. Certain shorter-chain members of each fatty acid class underwent complete or partial conversion to longer-chain homologues before utilization for complex lipid biosynthesis. The degree and extent of chain elongation and direct incorporation and the characteristic dependence of each of these processes on fatty acid chain length and structure correlated well with the physical properties (melting temperatures) of the exogenous fatty acids. The in vivo specificity of the enzyme systems responsible for the incorporation of exogenous fatty acids was such that the fluidity and physical state of the membrane lipids were maintained within a definite, albeit a relatively wide, range. We also observed that the neutral glycolipids typically have similar fatty acid compositions, which are somewhat different from those of the major phosphatides, which also exhibit similar fatty acid spectra. The phosphorylated glycolipid glycerophosphoryldiglucosyl diglyceride, however, always maintained a unique fatty acid composition quite different from that of the diglucosyl diglyceride from which it is presumably derived. These characteristic differences in fatty acid composition appear to function to minimize differences in phase transition temperatures, thus producing a more physicochemically homogeneous mixture of membrane lipids than would result from a nonspecific incorporation of fatty acids.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Pieringer R. A. The metabolism of glyceride glycolipids. V. Identification of the membrane lipid formed from diglucosyl diglyceride in Streptococcus faecalis ATCC 9790 as an acylated derivative of glyceryl phosphoryl diglucosyl glycerol. J Biol Chem. 1971 Jul 10;246(13):4216–4225. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Kennedy E. P. Enzymatic synthesis of cytidine diphosphate diglyceride. J Lipid Res. 1966 Sep;7(5):678–683. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Phosphatidyl glycerophosphate phosphatase. J Lipid Res. 1967 Sep;8(5):456–462. [PubMed] [Google Scholar]
- Chapman D., Urbina J. Phase transitions and bilayer structure of Mycoplasma laidlawii B. FEBS Lett. 1971 Jan 12;12(3):169–172. doi: 10.1016/0014-5793(71)80060-1. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Law J. H., Tsukagoshi N., Wilson G. A density label for membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):598–605. doi: 10.1073/pnas.67.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrikson C. V., Panos C. Fatty acid composition, distribution, and requirements of two nonsterol-requiring mycoplasmas from complex but defatted growth media. Biochemistry. 1969 Feb;8(2):646–651. doi: 10.1021/bi00830a028. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- Koostra W. L., Smith P. F. D- and L-Alanylphosphatidylglycerols from Mycoplasma laidlawii, strain B. Biochemistry. 1969 Dec;8(12):4794–4806. doi: 10.1021/bi00840a022. [DOI] [PubMed] [Google Scholar]
- Macfarlane M. G. Phosphatidylglycerols and lipoamino acids. Adv Lipid Res. 1964;2:91–125. doi: 10.1016/b978-1-4831-9938-2.50009-1. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974 Mar 25;84(1):145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of membrane-lipid phase transitions on membrane structure and on the growth of Acholeplasma laidlawii B. J Supramol Struct. 1974;2(5-6):617–628. doi: 10.1002/jss.400020509. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Metabolic turnover of the polar lipids of Mycoplasma laidlawii strain B. J Bacteriol. 1970 Jan;101(1):72–76. doi: 10.1128/jb.101.1.72-76.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. The relationship between fatty acid structure and the positional distribution of esterified fatty acids in phosphatidyl glycerol from Mycoplasma laidlawii B. Biochim Biophys Acta. 1970 Feb 10;202(1):120–128. doi: 10.1016/0005-2760(70)90223-7. [DOI] [PubMed] [Google Scholar]
- Pieringer R. A. The metabolism of glyceride glycolipids. I. Biosynthesis of monoglucosyl diglyceride and diglucosyl diglyceride by glucosyltransferase pathways in Streptococcus faecalis. J Biol Chem. 1968 Sep 25;243(18):4894–4903. [PubMed] [Google Scholar]
- Pollack J. D., Tourtellotte M. E. Synthesis of saturated long chain fatty acids from sodium acetate-1-C14 by Mycoplasma. J Bacteriol. 1967 Feb;93(2):636–641. doi: 10.1128/jb.93.2.636-641.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Tourtellotte M. E., McElhaney R. N., Pollack J. D. Influence of lipid components of Mycoplasma laidlawii membranes on osmotic fragility of cells. J Bacteriol. 1966 Feb;91(2):609–616. doi: 10.1128/jb.91.2.609-616.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwall A. Fatty-acid composition of Mycoplasma lipids: biomembrane with only 1 fatty acid. Science. 1968 Jun 21;160(3834):1350–1351. doi: 10.1126/science.160.3834.1350. [DOI] [PubMed] [Google Scholar]
- Rodwell A. W., Peterson J. E. The effect of straight-chain saturated, monoenoic and branched-chain fatty acids on growth and fatty acid composition of mycoplasma strain Y. J Gen Microbiol. 1971 Oct;68(2):173–186. doi: 10.1099/00221287-68-2-173. [DOI] [PubMed] [Google Scholar]
- Rodwell A. W. The incorporation of elaidate, oleate and straight-chain saturated fatty acids by mycoplasma strain Y. J Gen Microbiol. 1971 Oct;68(2):167–172. doi: 10.1099/00221287-68-2-167. [DOI] [PubMed] [Google Scholar]
- Romijn J. C., van Golde L. M., McElhaney R. N., van Deenen L. L. Some studies on the fatty acid composition of total lipids and phosphatidylglycerol from Acholeplasma laidlawii B and their relation to the premeability of intact cells of this organism. Biochim Biophys Acta. 1972 Sep 7;280(1):22–32. doi: 10.1016/0005-2760(72)90209-3. [DOI] [PubMed] [Google Scholar]
- Saito Y., Silvius J. R., McElhaney N. Membrane lipid biosynthesis in Acholeplasma laidlawii B: de novo biosynthesis of saturated fatty acids by growing cells. J Bacteriol. 1977 Nov;132(2):497–504. doi: 10.1128/jb.132.2.497-504.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Silvius J. R., McElhaney R. N. Membrane lipid biosynthesis in Acholeplasma laidlawii B. Relationship between fatty acid structure and the positional distribution of esterified fatty acids in phospho- and glycolipids from growing cells. Arch Biochem Biophys. 1977 Aug;182(2):443–454. doi: 10.1016/0003-9861(77)90525-2. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Overath P. Lipids containing trans-unsaturated fatty acids change the temperature characteristic of thiomethylgalactoside accumulation in Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):209–214. doi: 10.1016/0022-2836(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Koostra W. L. The lipid composition of Mycoplasma laidlawii strain B. Biochem J. 1968 Apr;107(3):329–333. doi: 10.1042/bj1070329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Verheij H. M. The structure of a glycerylphosphoryldiglucosyl diglyceride from the lipids of Acholeplasma laidlawii strain B. Biochem J. 1972 Aug;129(1):167–173. doi: 10.1042/bj1290167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Cronan J. E., Jr, Beacham I. R., Harder M. E. Proceedings: Genetic engineering of membrane lipid. Fed Proc. 1974 Jun;33(6):1725–1732. [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., Saito Y., McElhaney R. N. Membrane lipid biosynthesis in Acholeplasma laidlawii B. Investigations into the in vivo regulation of the quantity and hydrocarbon chain lengths of de novo biosynthesized fatty aicds in response to exogenously supplied fatty acids. Arch Biochem Biophys. 1977 Aug;182(2):455–464. doi: 10.1016/0003-9861(77)90526-4. [DOI] [PubMed] [Google Scholar]
- Smith P. F. Biosynthesis of glucosyl diglycerides by Mycoplasma laidlawii strain B. J Bacteriol. 1969 Aug;99(2):480–486. doi: 10.1128/jb.99.2.480-486.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., Slotboom A. J., van Deenen L. L., Rosenthal A. F. The effect of the polar headgroup on the lipid-cholesterol interaction: a monolayer and differential scanning calorimetry study. Biochim Biophys Acta. 1973 Apr 25;307(1):1–19. doi: 10.1016/0005-2736(73)90020-5. [DOI] [PubMed] [Google Scholar]
- van Golde L. M., McElhaney R. N., van Deenen L. L. A membrane-bound lysophospholipase from Mycoplasma laidlawii strain B. Biochim Biophys Acta. 1971 Feb 2;231(1):245–249. doi: 10.1016/0005-2760(71)90275-x. [DOI] [PubMed] [Google Scholar]


