Abstract
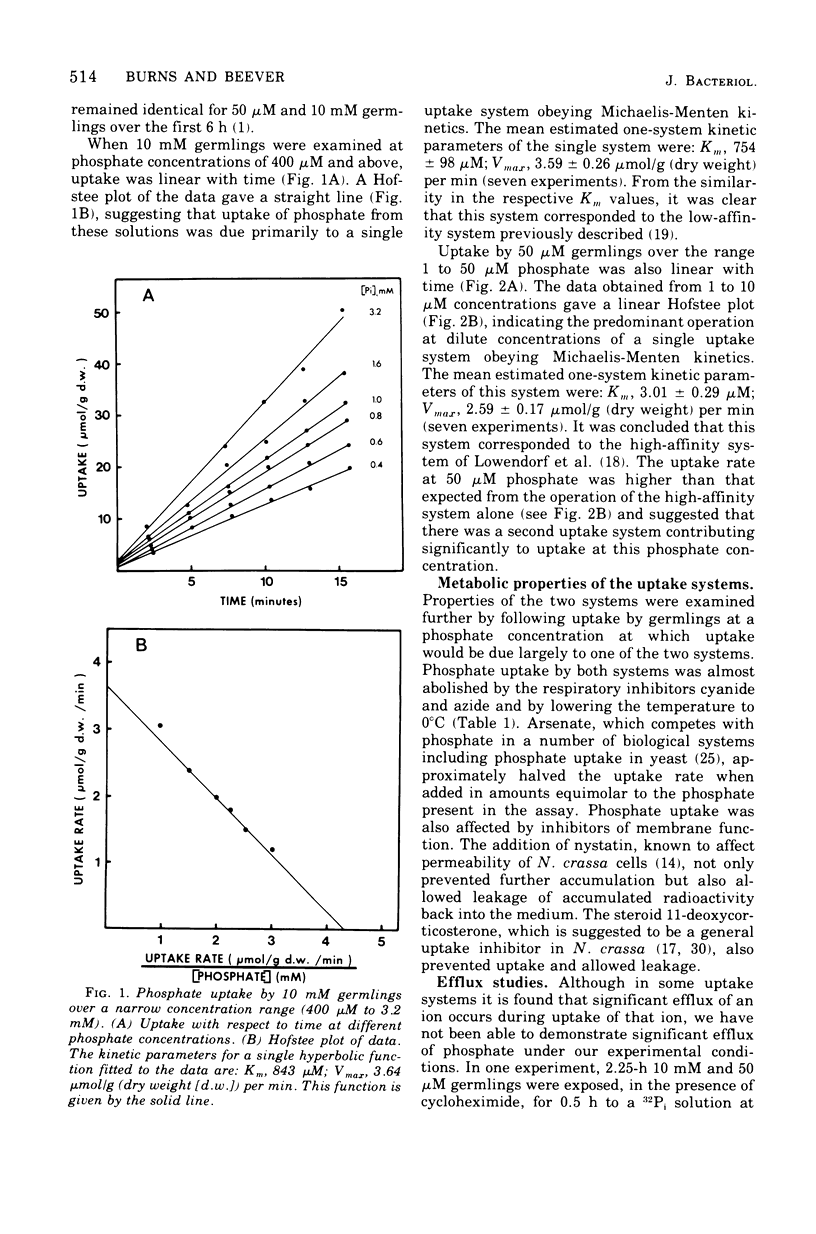
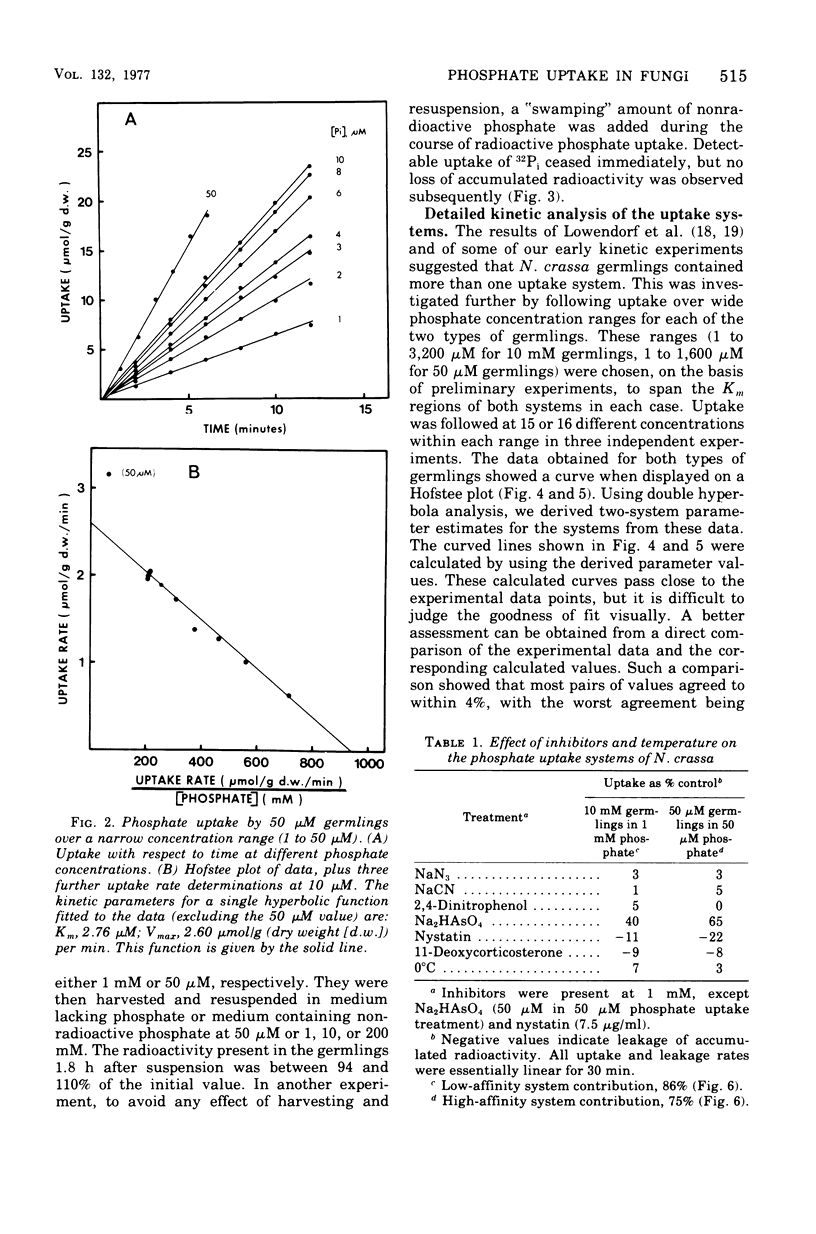
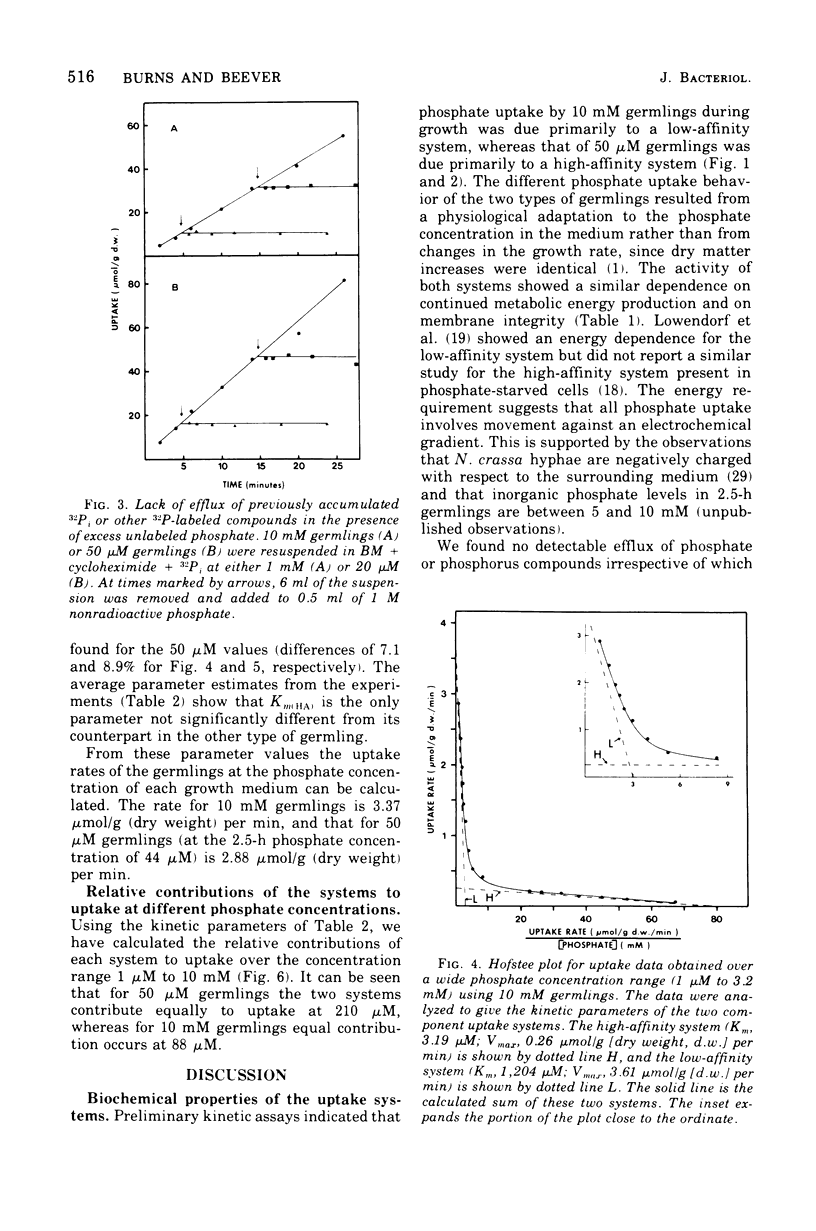
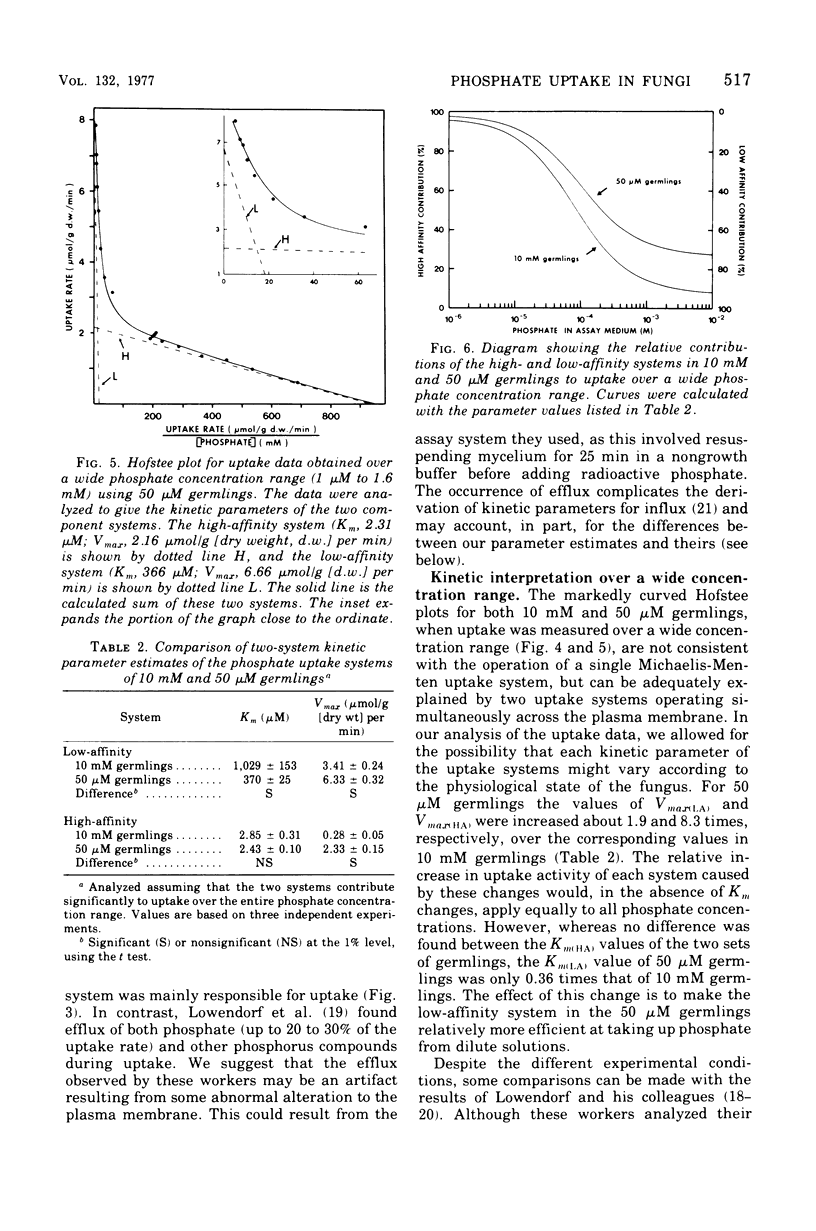
The kinetics of phosphate uptake by exponentially growing Neurospora crassa were studied to determine the nature of the differences in uptake activity associated with growth at different external phosphate concentrations. Conidia, grown in liquid medium containing either 10 mM or 50 micronM phosphate, were harvested, and their phosphate uptake ability was measured. Initial experiments, where uptake was examined over a narrow concentration range near that of the growth medium, indicated the presence of a low-affintiy (high Km) system in germlings from 50 micronM phosphate. Uptake by each system was energy dependent and sensitive to inhibitors of membrane function. No efflux of phosphate or phosphorus-containing compounds could be detected. When examined over a wide concentration range, uptake was consistent with the simultaneous operation of low- and high-affinity systems in both types of germlings. The Vmax estimates for the two systems were higher in germlings from 50 micronM phosphate than for the corresponding systems in germlings from 10 mM phosphate. The Km of the high-affinity system was the same in both types of germlings, whereas the Km of the low-affinity system in germlings from 10 mM phosphate was about three three times that of the system in germlings from 50 micronM phosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beever R. E., Burns D. J. Adaptive changes in phosphate uptake by the fungus Neurospora crassa in response to phosphate supply. J Bacteriol. 1977 Nov;132(2):520–525. doi: 10.1128/jb.132.2.520-525.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Dunker S. S., Morse M. L. Continuous culture of Rhodotorula rubra: kinetics of phosphate-arsenate uptake, inhibition, and phosphate-limited growth. J Bacteriol. 1973 Feb;113(2):599–611. doi: 10.1128/jb.113.2.599-611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN J., ROTHSTEIN A. The active transport of phosphate into the yeast cell. J Gen Physiol. 1957 Jul 20;40(6):915–923. doi: 10.1085/jgp.40.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G. I., D'Ambrosio S. M., Jensen R. A. Versatile properties of a nonsaturable, homogeneous transport system in Bacilus subtilis: genetic, kinetic, and affinity labeling studies. Proc Natl Acad Sci U S A. 1975 Mar;72(3):814–818. doi: 10.1073/pnas.72.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W. PROCEDURE FOR SIMULTANEOUS ASSAY OF TWO BETA-EMITTING ISOTOPES WITH THE LIQUID SCINTILLATION COUNTING TECHNIQUE. Anal Biochem. 1964 Jan;7:110–120. doi: 10.1016/0003-2697(64)90125-3. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- HSU K. S. THE GENETIC BASIS OF ACTIDIONE RESISTANCE IN NEUROSPORA. J Gen Microbiol. 1963 Sep;32:341–347. doi: 10.1099/00221287-32-3-341. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Feldman H. M., Pall M. L. Derepression of tyrosinase synthesis in Neurospora by cycloheximide, actinomycin D, and puromycin. J Biol Chem. 1970 Jun 10;245(11):2784–2788. [PubMed] [Google Scholar]
- KINSKY S. C. The effect of polyene antibiotics on permeability in Neurospora crassa. Biochem Biophys Res Commun. 1961 Apr 7;4:353–357. doi: 10.1016/0006-291x(61)90217-0. [DOI] [PubMed] [Google Scholar]
- LESTER G., STONE D., HECHTER O. The effects of deoxycorticosterone and other steroids on Neurospora crassa. Arch Biochem Biophys. 1958 May;75(1):196–214. doi: 10.1016/0003-9861(58)90410-7. [DOI] [PubMed] [Google Scholar]
- Leggett J. E. Entry of phosphate into yeast cell. Plant Physiol. 1961 May;36(3):277–284. doi: 10.1104/pp.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. F., Gleason M. K., Ahlgren S. K., Metzenberg R. L. Regulation of phosphate metabolism in Neurospora crassa. Characterization of regulatory mutants. Genetics. 1973 Sep;75(1):61–73. doi: 10.1093/genetics/75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowendorf H. S., Bazinet G. F., Jr, Slayman C. W. Phosphate transport in Neurospora. Derepression of a high-affinity transport system during phosphorus starvation. Biochim Biophys Acta. 1975 May 21;389(3):541–549. doi: 10.1016/0005-2736(75)90164-9. [DOI] [PubMed] [Google Scholar]
- Lowendorf H. S., Slayman C. L., Slayman C. W. Phosphate transport in Neurospora. Kinetic characterization of a constitutive, low-affinity transport system. Biochim Biophys Acta. 1974 Dec 24;373(3):369–382. doi: 10.1016/0005-2736(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Lowendorf H. S., Slayman C. W. Genetic regulation of phosphate transport system II in Neurospora. Biochim Biophys Acta. 1975 Nov 17;413(1):95–103. doi: 10.1016/0005-2736(75)90061-9. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A. Interactions of arsenate with the phosphate-transporting system of yeast. J Gen Physiol. 1963 May;46:1075–1085. doi: 10.1085/jgp.46.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYMAN C. L., SLAYMAN C. W. Measurement of membrane potentials in Neurospora. Science. 1962 Jun 8;136(3519):876–877. doi: 10.1126/science.136.3519.876. [DOI] [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. J Biol Chem. 1970 Apr 10;245(7):1694–1698. [PubMed] [Google Scholar]
- Siegenthaler P. A., Belsky M. M., Goldstein S., Menna M. Phosphate uptake in an obligately marine fungus. II. Role of culture conditions, energy sources, and inhibitors. J Bacteriol. 1967 Apr;93(4):1281–1288. doi: 10.1128/jb.93.4.1281-1288.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. W., Slayman C. L. Potassium transport in Neurospora. Evidence for a multisite carrier at high pH. J Gen Physiol. 1970 Jun;55(6):758–786. doi: 10.1085/jgp.55.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]


