Abstract
Environmental and social factors have important effects on aggressive behaviors. We examined the effect of reproductive experience on aggression in a biparental species of mouse, Peromyscus californicus. Estrogens are important in mediating aggressive behavior so we also examined estrogen receptor expression and c-fos for insights into possible mechanisms of regulation. Parental males were significantly more aggressive than virgin males, but no significant differences in estrogen receptor alpha or beta expression were detected. Patterns of c-fos following aggression tests suggested possible parallels with maternal aggression. Parental males had more c-fos positive cells in the medial amygdala, and medial preoptic area relative to virgin males. The medial preoptic area is generally considered to be relatively less important for male-male aggression in rodents, but is known to have increased activity in the context of maternal aggression. We also demonstrated through habituation–dishabituation tests that parental males show exaggerated investigation responses to chemical cues from a male intruder, suggesting that heightened sensory responses may contribute to increased parental aggression. These data suggest that, in biparental species, reproductive experience leads to the onset of paternal aggression that may be analogous to maternal aggression.
Keywords: Aggressive behavior, Rodents, c-fos, Social behavior, Parental behavior, California mouse, Peromyscus californicus, Ventromedial hypothalamus
Introduction
Aggressive behavior is often considered to be a unitary process, but recent studies suggest that different physiological processes regulate aggression in different contexts (Nelson and Trainor, 2007). In humans, a distinction has been drawn between impulsive aggression and instrumental aggression (Vitiello and Stoff, 1997). Impulsive aggression is considered more spontaneous, and mediated by reduced serotonergic function (Blair, 2004; Manuck et al., 2006). In contrast, instrumental aggression is thought to be more premeditated and more closely resemble motivated behaviors (Berkowitz, 1993; Barratt et al., 1999), suggesting a role for dopaminergic function. In female mammals, reproductive context has substantial effects on aggressive behavior. In most mammalian species, females are less aggressive than males, but will become very aggressive after giving birth (Lonstein and Gammie, 2002). This increased parental aggression is directed primarily at male intruders that could be infanticidal. In rodents, this heightened aggression is maintained with tactile stimulation from pups (Svare et al., 1980; Stern and Kolunie, 1993). Patterns of neuronal activity (as inferred by measuring immediate early gene expression) following maternal aggression tests differ from those observed in resident–intruder aggression tests in males (Gammie, 2005), suggesting that there are differences in the neurobiological mechanisms regulating aggression in these contexts. In a few biparental mammalian species, males provide high levels of parental care to their offspring. Whether males of these species display increased aggression as parents has not been examined in detail.
In rats, estradiol facilitates the onset of maternal aggression (Gandelman, 1980; Mayer and Rosenblatt, 1987), but in mice estradiol inhibits the onset of maternal aggression (Ghiraldi et al., 1993). These species differences parallel findings of variable effects of estrogens on male aggression. In some species estrogens increase male aggression, whereas estrogens inhibit male aggression in other species (Trainor et al., 2006b). Estrogens can affect aggression in males because circulating androgens can be converted to estrogens by aromatase present in the brain. One possible mechanism for this intraspecies variability could be differential activation of estrogen receptor subtypes. Mammals have two estrogen receptor subtypes, estrogen receptor α(ERα) and estrogen receptor β (ERβ). In knock-out mouse studies, selective deletion of ERαdecreases male-male aggression (Ogawa et al., 1997; Scordalakes and Rissman, 2003), whereas deletion of ERβ is usually associated with increased male-male aggression (Ogawa et al., 1999; Nomura et al., 2006). Another possible mechanism is that the environment can regulate the molecular actions of estrogen receptors. In Peromyscus polionotus, estrogens act rapidly to increase aggression when males are housed in winter-like short days (Trainor et al., 2007a), apparently via non-genomic processes. When males are housed in summer-like long days, estrogens act over a longer time frame to decrease aggression (apparently via genomic action).
We examined the effects of reproductive experience on aggressive behavior in California mice (P. californicus). Field studies show that individuals of this species form monogamous pairs (Ribble, 1991) and laboratory studies show that males provide a high level of parental care to their pups (Gubernick and Alberts, 1987; Bester-Meredith et al., 1999). We tested virgin and parental males in resident–intruder aggression tests to determine the presence of heightened aggression analogous to maternal aggression in male parents. Because estrogens are known to facilitate the onset of maternal aggression, we examined ERα and ERβ immunoreactivity in virgin males and parents. A previous study reported increased ERα in the MPOA of maternal rats (Champagne et al., 2003), but no study has examined whether ERα or ERβ expression changes with parental experience in a biparental species. We also stained for c-fos, an indirect marker of neuronal activity, following aggression tests. To examine whether parents and virgin males differed in their behavioral responses to intruders (independent of aggressive behavior), we assessed responsiveness to intruder odors with a habituation–dishabituation test.
Methods
Animals
We used California mice (P. californicus) obtained from Dr. Catherine Marler (University of Wisconsin, Madison, WI, USA). Males were individually-housed and provided with filtered tap water and Harlan Teklad 8640 food (Madison, WI) ad libitum. All experimental procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and animals were maintained in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Effect of photoperiod on behavior and physiology
In experiment 1, half of the males were randomly assigned to be paired with a female (parental, n=6) and one half of the males remained sexually inexperienced (virgin, n=7). Virgin males were singly housed through the duration of the study. Field studies show that male P. californicus defend exclusive territories (Ribble and Salvioni, 1990), which indicates that to a certain extent, single housing approximates the social organization of young unpaired males in this species. One week before behavioral testing, males were anesthetized with isoflurane and a blood sample was collected from the retroorbital sinus to measure testosterone. Total testosterone was measured in plasma with a 125I testosterone RIA kit (DSL-4100; Diagnostic Systems Laboratories, Webster, TX). The intra-assay coefficient of variation was 2.8%. Hormone concentrations were not normally distributed, and were log transformed for statistical analyses.
Parental males were allowed to breed with females and were not tested in resident–intruder aggression tests until one litter had been weaned. All parental males had pups between 5 and 8 days old when aggression tests were conducted. Ten minutes before tests were conducted, the female and pups were removed from the parental male's home cage. A sham removal was conducted with virgin males. Resident–intruder tests were conducted 1 h after lights out (1500 EDT) under dim red light. A group-housed, sexually inexperienced male intruder was introduced into each resident's cage for 7 min. Observations were videotaped and scored offline by an observer blind to treatment assignments. One hour after aggression tests, residents were anesthetized with isoflurane and rapidly decapitated. This time course was chosen because previous studies have reported that maximal c-fos expression occurs between 1 and 2 h after stimulation (Kovacs and Sawchenko, 1996; Hoffman and Lyo, 2002). Brains were quickly removed and fixed in 5% acrolein overnight at 4°C. Brains were then transferred to 30% sucrose for 24 h and frozen on dry ice for immunocytochemistry.
In experiment 2, a different set of parental (n=6) and virgin males (n=8) were tested in habituation–dishabituation tests (Pankevich et al., 2004; Trainor et al., 2007c). To collect urine from group-housed sexually inexperienced mice, males were firmly gripped on the scruff of the neck and urine was collected into centrifuge tubes. Urine was frozen at −20°C and then individual samples were thawed and diluted 1 to 10 in distilled water before testing. For the first three trials (trials 1–3), 10 μL of distilled water was pipetted onto the center of a glass slide and then transferred into the cage for a 2 min trial. As in experiment 1, all parental males had raised one litter and were tested 5–7 days after the birth of a second litter. Ten minutes before tests were conducted, the female and pups were removed from the parental male's home cage. A sham removal was conducted with virgin males. During each trial, the amount of time the mouse investigated the drop was recorded. After the third trial with water, three additional trials (trials 4–6) were conducted with 10 μL of diluted urine placed on a slide. For each mouse, a fresh slide was used in every trial and the same diluted urine sample was used for each of the last three trials.
Immunocytochemistry
Immunocytochemistry for ERα and ERβ was conducted as previously described (Trainor et al., 2007b) with brains from 6 parental males and 6 virgin males. Briefly, brains were sectioned at 40 μm on a cryostat and free-floating sections were then incubated in 1% sodium borohydride in 0.1 M phosphate buffered saline (PBS) for 10 min. Sections were then rinsed in 20% normal goat serum and 0.3% hydrogen peroxide in PBS for 20 min. Alternate sections were incubated in either primary ERα antibody (C1355, Upstate Biotechnology, concentration 1:20K), primary ERβ (D7N, Invitrogen, Carlsbad, CA, concentration 1:400), or primary c-fos (rabbit anti c-fos, Chemicon, Temecula, CA, 1:10K) in 1% normal goat serum in 0.5% PBS+triton X (PBS TX) for 40 h at 4°C. Sections were rinsed in PBS, and incubated for 2 h with biotinylated goat-anti-rabbit antibody (Vector Laboratories, Burlingame, CA, 1:500) in PBS+TX. The sections were rinsed in PBS and then incubated for 30 min in avidin-biotin complex (ABC Elite kit, Vector Laboratories). After rinses in PBS, the sections were developed in hydrogen peroxide and diaminobenzidine for 2 min. Sections were mounted on gel-coated slides, dehydrated and coversliped. We used a Nikon E800 microscope to capture representative photomicrographs of each of the following brain areas using a mouse brain atlas (Paxinos and Franklin, 2002): ventral lateral septum (LS, bregma 0.26), posterior BNST (bregma 0.02), medial preoptic area (mPOA, bregma 0.02), ventrolateral ventromedial hypothalamus (VMH, bregma −1.70), paraventricular nucleus (PVN, bregma −1.22), post-eroventral amygdala (pvMEA, bregma, −1.82), and posterodorsal amygdala (pdMEA bregma −1.82). In these areas, the number of ER and c-fos positive immunoreactive (−ir) cells was counted using Image J (NIMH, Bethesda, MD) by an observer unaware of treatment assignments. We counted the number of cells in a box in the MPOA (l×w, 250×400 μm), LS (480×330 μm), BNST (350× 500 μm), PVN, (480×330 μm), pdMEA (450×450 μm), and pvMEA (450× 450 μm) similar to our previous study quantifying estrogen receptor expression in P. polionotus and P. maniculatus (Trainor et al., 2007b). In the VMH, the number of ERα and c-fos positive cells in a circle with a radius of 180 μm was counted. The distributions of estrogen receptors and c-fos were homogenous in these areas. Our box sizes are similar to those used in previous studies that have quantified steroid receptors in hypothalamic and limbic brain areas (Lonstein et al., 2000; Scordalakes et al., 2002; Chung et al., 2006) and immediate early gene expression in the brain (Kollack-Walker and Newman, 1995; Gammie and Nelson, 2001).
Statistics
We used independent t-tests to compare biting behavior, testosterone ERα, ERβ, and c-fos expression. There was heterogeneous variance in attack latency scores, so we used a Mann–Whitney test to compare virgin and parental mice. We also used Spearman correlations to examine correlations between aggressive behaviors and neurobiological data. In experiment 1, the aggressive behavior data and cell counts from the virgin males in this study are also analyzed (as long day males) in the accompanying manuscript examining the effect of photoperiod on aggression and estrogen receptor expression (Trainor et al., 2008-this issue). For data from habituation–dishabituation tests, we used paired t-tests to make comparisons within each experimental group and independent t-tests to make comparisons between experimental groups. Mean differences were considered statistically significant when p ≤ 0.05.
Results
Parental males bit intruders more frequently than virgin males (Fig. 1, t10 =3.33, p < 0.01) and had significantly shorter attacklatencies (Fig. 1, Mann–Whitney U=45, p<0.02). There were no significant differences in ERα expression in the LS, BNST, PVN, VMH, or pdMEA (Fig. 2, all ps>0.1). In the MPOA, parental males had fewer ERα positive cells than virgins but this difference was not significant (t10 =1.8, p =0.1). There were no significant differences in the number of ERβ positive cells in the BNST, MPOA, PVN, or pdMEA (Fig. 2, all ps>0.18). There was no significant difference (t12 =1.8, p =0.1) in baseline plasma testosterone between virgin (mean±standard error, 0.15± 0.04 ng/mL) and parental males (0.27±0.05 ng/mL).
Fig. 1.

Aggressive behaviors in male virgin and parental California mice tested in resident–intruder aggression tests. Ten minutes before testing, dams and pups were removed from cages of parental males and a sham removal was conducted with virgin males. *p<0.05, **p<0.01.
Fig. 2.
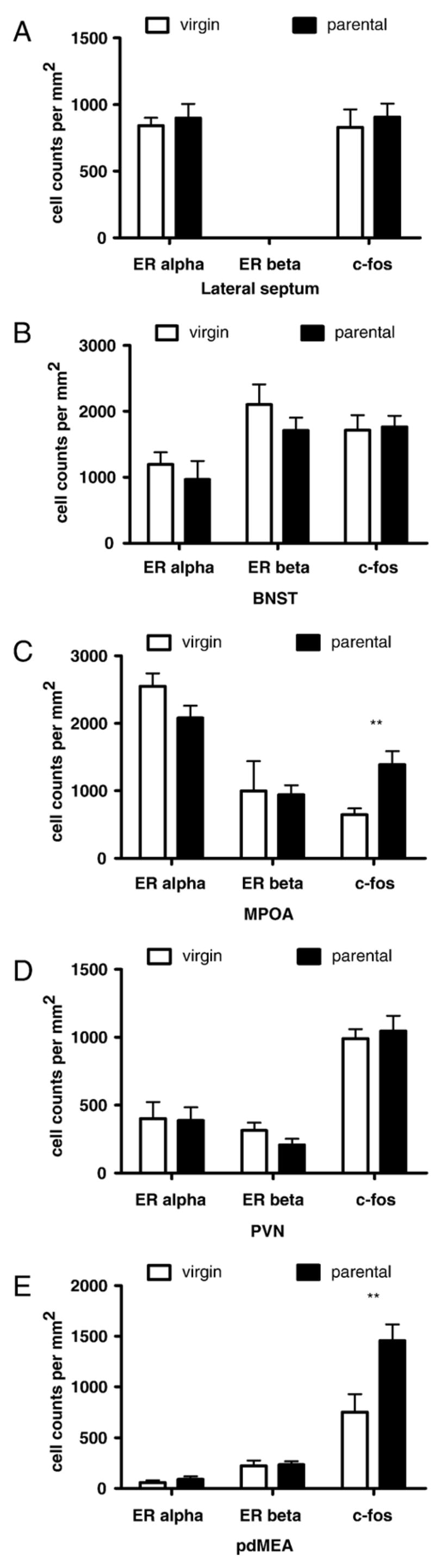
Counts of cells (per mm2) stained positive for estrogen receptor alpha, estrogen receptor beta, or c-fos in virgin and parental mice. (A) Lateral septum (LS), (B) bed nucleus of the stria terminalis (BNST), (C) medial preoptic area (MPOA), (D) paraventricular nucleus (PVN), (E) posterodorsal amygdala (pdMEA) n=6 per group. **p<0.01 effect of treatment group.
Parental males had significantly more c-fos positive cells in the MPOA (Figs. 2C, 3A, B, t10 =3.29, p<0.01), pdMEA (Figs. 2E, 3E, F, t10 =2.92, p =0.01), and pvMEA (virgins 124± 23, parents 221±34, t10 =2.40, p<0.05). There was a non-significant trend for parental males expressing more c-fos positive cells than virgins in the VMH (virgins 101±11, parents 135±13, Fig. 3C, D, t10 =2.23, p =0.06). There were no significant differences in c-fos expression in the LS, BNST, PVN, or AHA.
Fig. 3.
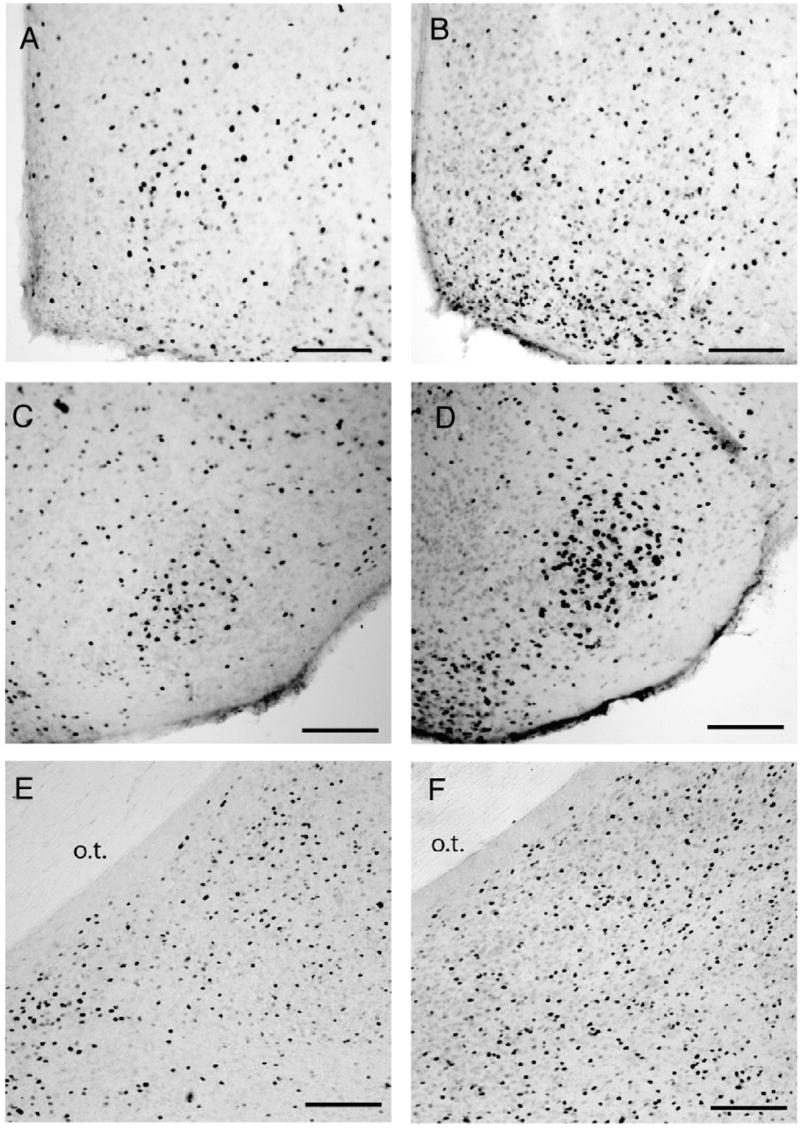
Photomicrographs of c-fos positive cells in male virgin (A, C, E) and parental California mice (B, D, F) in the medial preoptic area (A, B), ventromedial hypothalamus (C, D), and posterodorsal amygdala (E, F). Scale bar=100 μm.
Group differences in c-fos in the pdMEA and MPOA were reflected in correlations with behavior. In the pdMEA, c-fos was positively correlated with number of bites (Fig. 4E, ρ=0.59, p<0.05) and negatively correlated with attack latency (Fig. 4F, ρ=−0.58, p<0.05). Likewise, in the MPOA c-fos was positively correlated with number of bites (Fig. 4A, ρ=0.61, p<0.05) and negatively correlated with attack latency (Fig. 4B, ρ=−0.81, p<0.01). Significant correlations between biting (Fig. 4C, ρ= 0.71, p<0.01) and attack latency (Fig. 4D, ρ=−0.6, p<0.01) were also observed in the VMH. Aggressive behaviors were not significantly correlated with c-fos, ERα, or ERβ in any other brain area.
Fig. 4.
Non-parametric correlations between c-fos positive cells per mm2 and aggressive behaviors in resident–intruder aggression tests. Correlations between bites in resident–intruder tests and c-fos in the (A) MPOA, (C) VMH, and (E) and pdMEA. Correlations between attack latency in resident intruder tests and c-fos in the (B) MPOA, (D) VMH, and (F) pdMEA.
In habituation–dishabituation tests (Fig. 5), both virgin (trial 3–trial 1, paired t7 =3.7, p < 0.01) and parental (paired t5 =3.1, p < 0.05) males showed habituation responses to presentations of water drops, and both virgin (trial 4–trial 3, paired t7 =5.3, p < 0.001) and parental (paired t5 =4.2, p < 0.01) males showed a significant increase in investigation time to the first presentation of urine from an intruder. However, male parents spent twice as much time as virgin males investigating the intruder urine in the fourth trial (trial 4, independent t12 =2.4, p<0.05) and continued to show a stronger response in the fifth trial (trial 5, independent t12 =2.4, p < 0.05). Virgin and parental males did not differ in trial 6.
Fig. 5.
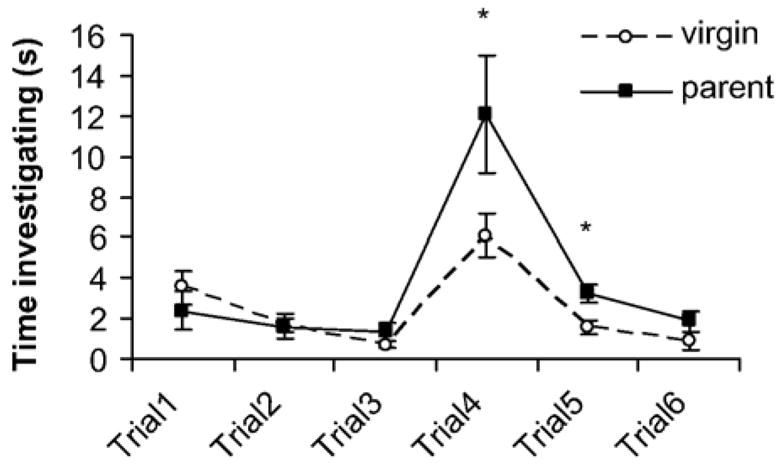
Results from habituation–dishabituation tests. In the first three trials, each mouse was presented with a 10 μL drop of water on a glass slide for 2 min. Both virgin (n=8) and parental males (n=6) habituated to this stimulus by trial 3 (with reduced investigation of the water droplet). In trials 4–6, each mouse was presented with a 10 μL drop of diluted urine from an unfamiliar group-housed virgin male. Both virgin and parental males responded with increased investigation in trial 4. Parental males investigated the diluted urine significantly more than virgin males in trials 4 and 5, indicating a stronger behavioral response to the chemical stimulus of an intruder. *p<0.05 parental vs. virgin males.
Discussion
Many studies have demonstrated that female aggression toward males (maternal aggression) is increased following the birth of offspring. In biparental California mice, we demonstrated that parental males substantially increased aggressive behavior compared to virgin males. Increased aggression was not associated with changes in ERα or ERβ expression in the brain, suggesting that changes in the nuclear expression of these receptors in the hypothalamus and limbic system do not mediate this dramatic behavioral change. Analyses of c-fos expression in parental males suggest that there may be important parallels with previously described neural pathways of maternal aggression. Parental males had increased c-fos expression in the MPOA, VMH, pdMEA, and pvMEA. These brain areas all express c-fos in the context of maternal aggression (Gammie, 2005). The MEA and VMH are known to be important sensory integration nuclei and one possible explanation for our results is that increased c-fos expression in parental males might reflect stronger sensory responses to intruder stimuli. Indeed, parental males investigated chemical stimuli of intruders significantly longer than virgin males. An alternative explanation is that increased c-fos expression in parental males is a consequence of increased aggressive behavior. Further study will be needed to determine the extents to which responses to intruder stimuli and aggressive behavior contribute to the expression of c-fos in parental males. However, these results clearly suggest that mechanisms of maternal aggression may be functional in males of biparental species.
In the current study, male parents had increased c-fos expression in the MPOA. Most studies of male–male aggression have suggested that the role of the MPOA is reduced compared to other nuclei such as the anterior hypothalamus (Albert et al., 1992; Newman, 1999; Delville et al., 2000; Trainor et al., 2006a), and sexually inexperienced female California mice do not express increased c-fos in the MPOA following aggression tests (Davis and Marler, 2004). However, very few studies have examined sexually experienced or parental males. The MPOA has a critical role in the facilitation of reproductive behaviors including mating and parental care (Bridges, 1996; Lee and Brown, 2002; Numan, 2007). Several studies have demonstrated increased c-fos in the MPOA following maternal aggression tests (Gammie and Nelson, 1999, 2001; Hasen and Gammie, 2005) and in California mice, male aggression is positively correlated with male parental behavior (Trainor and Marler, 2001). Collectively, these data suggest that aggression-induced c-fos in the MPOA of parental rodents may reflect communication of information on reproductive status to other brain areas that mediate aggressive behavior.
Before sensory information is received by the MPOA, it is processed by the medial amygdala (MEA). The MEA receives information from the accessory olfactory bulbs and this information is then sent to a several hypothalamic nuclei including the BNST, anterior hypothalamus, VMH, and MPOA (Simerly and Swanson, 1986; Coolen and Wood, 1998). Lesions of the MEA inhibit male–male aggression (Vochteloo and Koolhaas, 1987), presumably by reducing stimulation of brain areas that promote aggression. Lesions of the MEA facilitate the onset of maternal behavior in virgin female rats that typically avoid or even kill pups (Fleming et al., 1980; Numan et al., 1993). The role of the MEA after the onset of maternal aggression is less clear. Expression of c-fos is increased in the MEA during maternal aggression in mice (Gammie and Nelson, 2001; Popeski and Woodside, 2004; Hasen and Gammie, 2005), and we also observed increased c-fos in the pvMEA and pdMEA in parental males compared to virgins. Thus although the MEA has inhibitory effects on parental behavior, it may also play an important role in the facilitation of increased aggression in parents. In experiment 2, we observed that parental males showed stronger olfactory investigation responses to intruder odors compared to virgin males. It is unlikely that this response is due to differences in responses to novelty, because there were no significant differences between virgin and parental males in trials 1–3, when mice were first introduced to the glass slides containing a water droplet. These data suggest that parental males have a stronger behavioral response to intruder cues that could contribute to increased c-fos expression in brain areas such as pdMEA, although further study is needed to test this hypothesis directly.
Increased expression of c-fos was also observed in the ventrolateral VMH. This observation is consistent with previous studies that reported aggression-induced c-fos primarily in the lateral, but not medial aspects of the VMH (Kollack-Walker and Newman, 1995; Delville et al., 2000; Davis and Marler, 2004). The dorsomedial VMH inhibits the onset of maternal behaviors (Sheehan et al., 2001; Mann and Babb, 2004), but the role of the ventrolateral VMH is less clear. It has been hypothesized that neuronal activity in the VMH and MEA could transiently inhibit parental behaviors and simultaneously facilitate aggressive behaviors (Gammie, 2005). Our data suggest that a similar neural mechanism may be present in male parental California mice.
We examined the expression of ERα and ERβ because estrogens are known to regulate aggression in Peromyscus and selective deletion of the two receptors in Mus affects aggression (Ogawa et al., 2006). There were no differences in ERα or ERβ between virgin and parental males, suggesting increased aggression in parental males is not mediated by changes in receptor expression. This does not, however, preclude changes in how estrogens regulate aggression. California mice housed in short days (8L:16D) are more aggressive than males housed in long days (16L:8D) despite a lack of differences in ERα or ERβ expression in the brain (Trainor et al., 2008-this issue). Estrogens decrease aggression in long days (Trainor et al., 2004) and increase aggression in short days in male California mice (Trainor et al. 2008-this issue). Similar results have been reported in beach mice (P. polionotus), and this photoperiod-estrogen interaction appears to be mediated by different pathways that are activated by estrogen receptors (Trainor et al., 2007a). Further study is needed to determine if estrogens differentially regulate aggression in parental and virgin males.
One confounding factor in this study is that virgin males were individually housed whereas parental males were pair-housed until 10 min before testing. Typically individual housing in rodents results in increased aggression instead of the lower aggression we observed in virgin males. Furthermore, aggressive behavior observed in individually housed California mice is comparable to parents if males are singly housed in short-day photoperiods (8L:16D, Trainor et al., 2008-this issue). This suggests that individual housing per se does not inhibit aggression, although further study will be needed to conclusively settle this question. Field studies suggest that unpaired males have little social contact with other males (Ribble and Salvioni, 1990), but cohabitation with a female likely has important effects on affective states influenced by the hypothalamic-pituitary-adrenal axis. For example, individually-housed male California mice have increased plasma corticosterone concentrations compared to males housed with an ovariectomized female behind a wire barrier (Glasper and DeVries, 2004). An additional consequence of social contact with females is the potential for pair bonding. One day after mating and pair bonding with a female, male prairie voles exhibit increased aggression toward male intruders (Getz et al., 1981), a process that is facilitated by changes in vasopressin (Winslow et al., 1993) and dopamine (Aragona et al., 2006). Future studies are necessary to dissect the exact contribution of mating and pups on aggressive behavior in California mice. We also did not observe a difference between virgin and parental males in baseline testosterone. A previous study in P. californicus also reported no significant difference in testosterone between virgin and parental males (Trainor et al., 2003). Although a decrease in male testosterone is associated with the birth of offspring in many biparental mammals (Brown et al., 1995; Reburn and Wynne-Edward, 1999; Storey et al., 2000), this is almost always compared to a relative increase in testosterone immediately before parturition.
Our data indicate that aggression in male parents shares some interesting similarities with previous reports on maternal aggression. In particular, increased c-fos expression in the MPOA of male parents suggests this brain area conveys important information on reproductive state to other brain areas controlling aggression. We also demonstrated that male parents show stronger and more extensive behavioral responses to olfactory cues from intruders, suggesting that the increased aggressive responses may be mediated in part by stronger sensory responses to odors of male intruders. These data suggest that in biparental species the reproductive status of males has distinct physiological effects on aggressive behavior.
Acknowledgments
The authors thank Patience Gallagher for technical assistance. This work was supported by an SBS Undergraduate Research Award to M.S.F., NIH MH076313 to B.C.T., and NIH MH57535 to R.J.N.
References
- Albert DJ, Jonik RH, Walsh ML. Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci Biobehav Rev. 1992;16:177–192. doi: 10.1016/s0149-7634(05)80179-4. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Aggression: It's Causes, Consequences, and Control. McGraw Hill; New York: 1993. [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The neurobiology of aggression. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. Oxford University; Oxford: 2004. pp. 1076–1085. [Google Scholar]
- Bridges RS. Biochemical basis of parental behavior in the rat. Adv Stud Behav. 1996;25:215–242. [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. Expression and estrogen sensitivity in the medial preoptic area. Endocrinol. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor alpha and not estrogen receptor beta. Brain Res. 2006;1082:50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdalaoid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. C-fos changes following an aggressive encounter in female California mice: A synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Vaccarino F, Leubke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is mediated by nitric oxide. J Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Postpartum fighting in the rat: nipple development and the presence of young. Behav Neural Biol. 1980;28:350–360. doi: 10.1016/s0163-1047(80)92357-2. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating systems of the prairie vole, Microtus ochrogaster—field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Ghiraldi LL, Plonsky M, Svare BB. Postpartum aggression in mice: the role of ovarian hormones. Horm Behav. 1993;27:251–268. doi: 10.1006/hbeh.1993.1019. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Socia structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2004;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987;101:169–177. [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84:681–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all “fos-ed out”? J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patters of Fos immunolabeling in the male Syrian hamster brain. Neurosci. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indicies of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female California mice (Peromyscus californicus) Behav Neurosci. 2002;116:968–975. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Greco B, De Vries GJ, Stern JM, Blaustein JD. Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology. 2000;72:91–101. doi: 10.1159/000054576. [DOI] [PubMed] [Google Scholar]
- Mann PE, Babb JA. Disinhibition of maternal behavior following neurotoxic lesions of the hypothalamus in primigravid rats. Brain Res. 2004;1025:51–58. doi: 10.1016/j.brainres.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Lotrich FE. Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of Aggression. Oxford Univ. Press; New York: 2006. pp. 65–113. [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal factors influence the onset of maternal aggression in laboratory rats. Horm Behav. 1987;21:253–267. doi: 10.1016/0018-506x(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev, Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Newman S. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Andersson S, Korach K, Gustafsson J, Pfaff D, Ogawa S. Estrogen receptor-beta gene disruption potentiates estrogen-inducible aggression but not sexual behaviour in male mice. Eur J Neurosci. 2006;23:1860–1868. doi: 10.1111/j.1460-9568.2006.04703.x. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson J, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (bERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Nomura M, Choleris E, Pfaff D. The role of estrogen receptors in the regulation of aggressive behaviors. In: Nelson RJ, editor. Biology of Aggression. Oxford University Press; New York: 2006. pp. 231–249. [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2002. [Google Scholar]
- Popeski N, Woodside B. Central nitric oxide synthase inhibition disrupts maternal behavior in the rat. Behav Neurosci. 2004;118:1305–1316. doi: 10.1037/0735-7044.118.6.1305. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edward KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- Ribble DO, Salvioni M. Social organization and nest coocupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neurosci. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the rat medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk but not by nipple removal. Physiol Behav. 1993;54:861–868. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Svare B, Mann M, Samuels O. Mice: suckling stimulation but not lactation important for maternal aggression. Behav Neural Biol. 1980;29:453–462. doi: 10.1016/s0163-1047(80)92654-0. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Marler CA. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm Behav. 2004;45:115–121. doi: 10.1016/j.yhbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor α in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006a;50:338–345. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: how interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006b;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and non-genomic pathways. Proc Natl Acad Sci U S A. 2007a;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Rowland MR, Nelson RJ. Photoperiod affects estrogen receptor alpha, estrogen receptor beta, and aggressive behavior. Eur J Neurosci. 2007b;26:207–218. doi: 10.1111/j.1460-9568.2007.05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Workman JL, Jessen R, Nelson RJ. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci. 2007c;121:362–369. doi: 10.1037/0735-7044.121.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.09.016. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psych. 1997;36:307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Vochteloo JD, Koolhaas JM. Medial amygdala lesions in male rats reduce aggressive behavior: interference with experience. Physiol Behav. 1987;41:99–102. doi: 10.1016/0031-9384(87)90137-5. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]



