Abstract
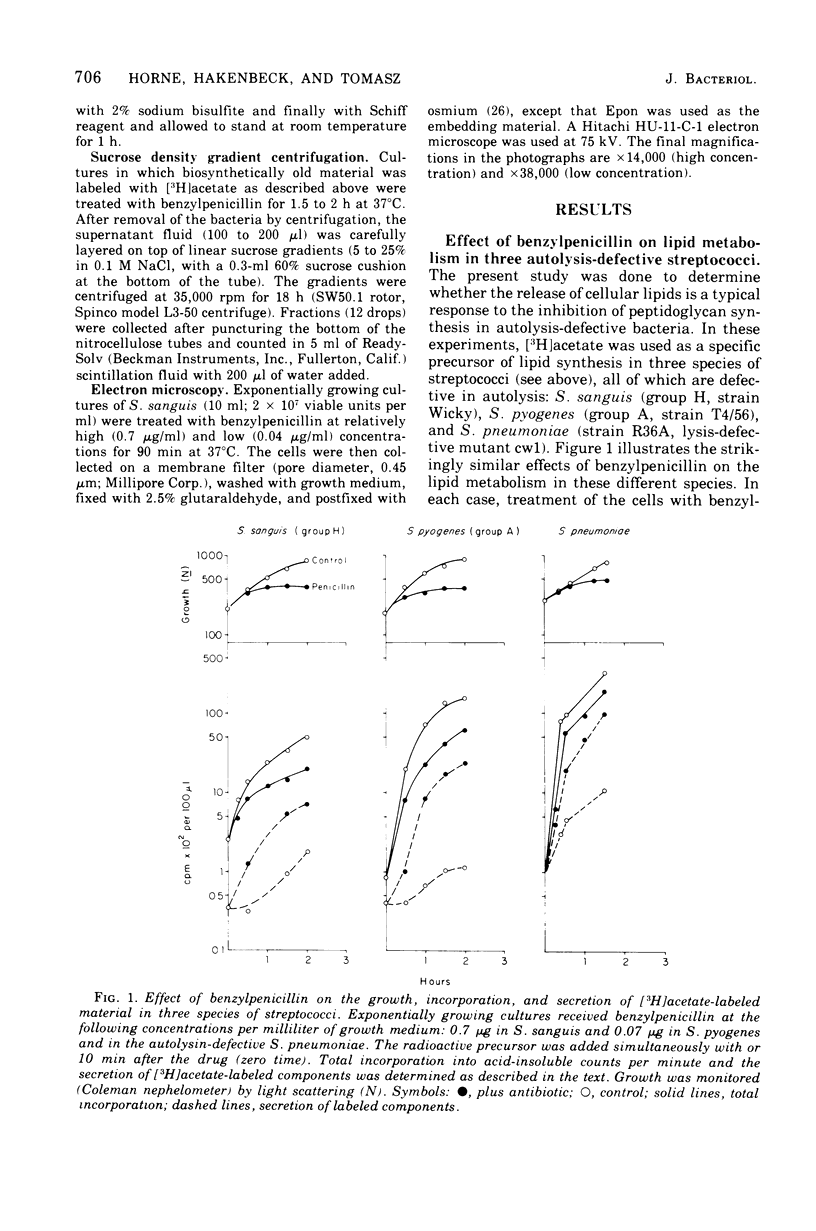
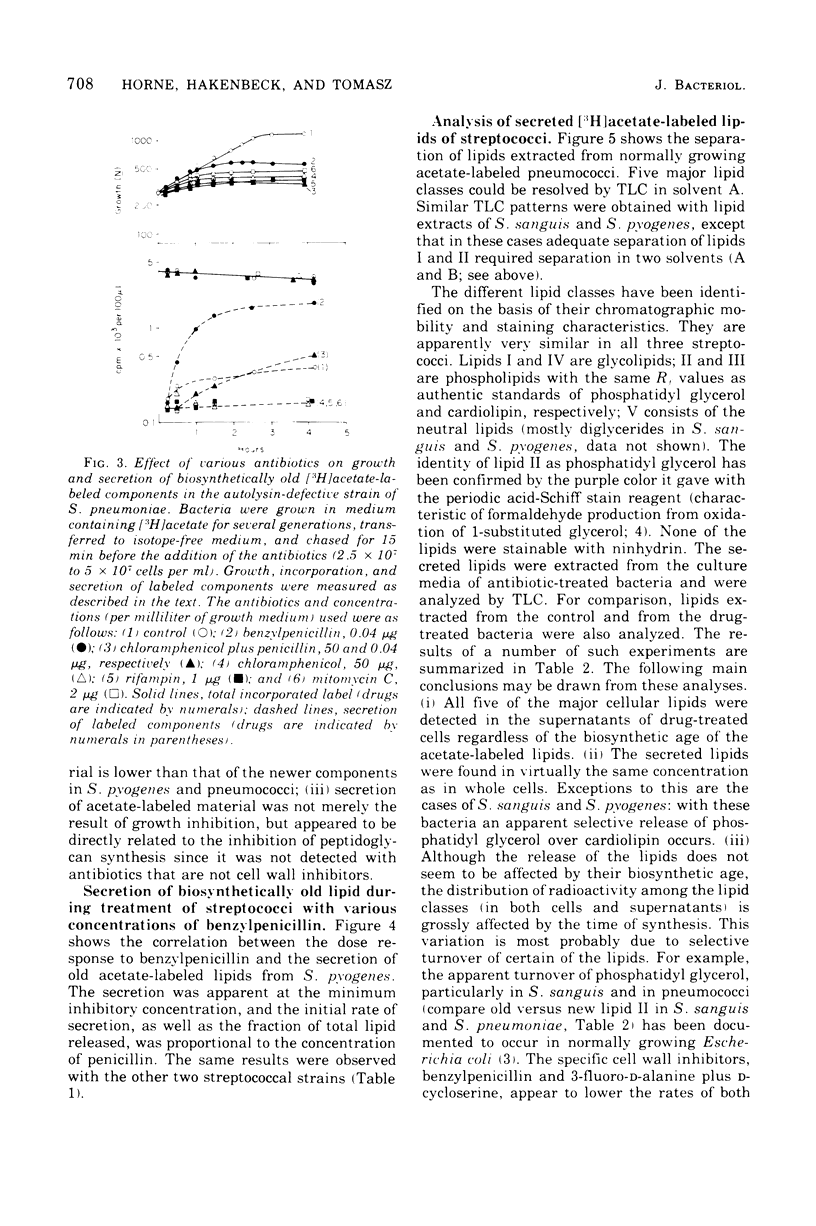
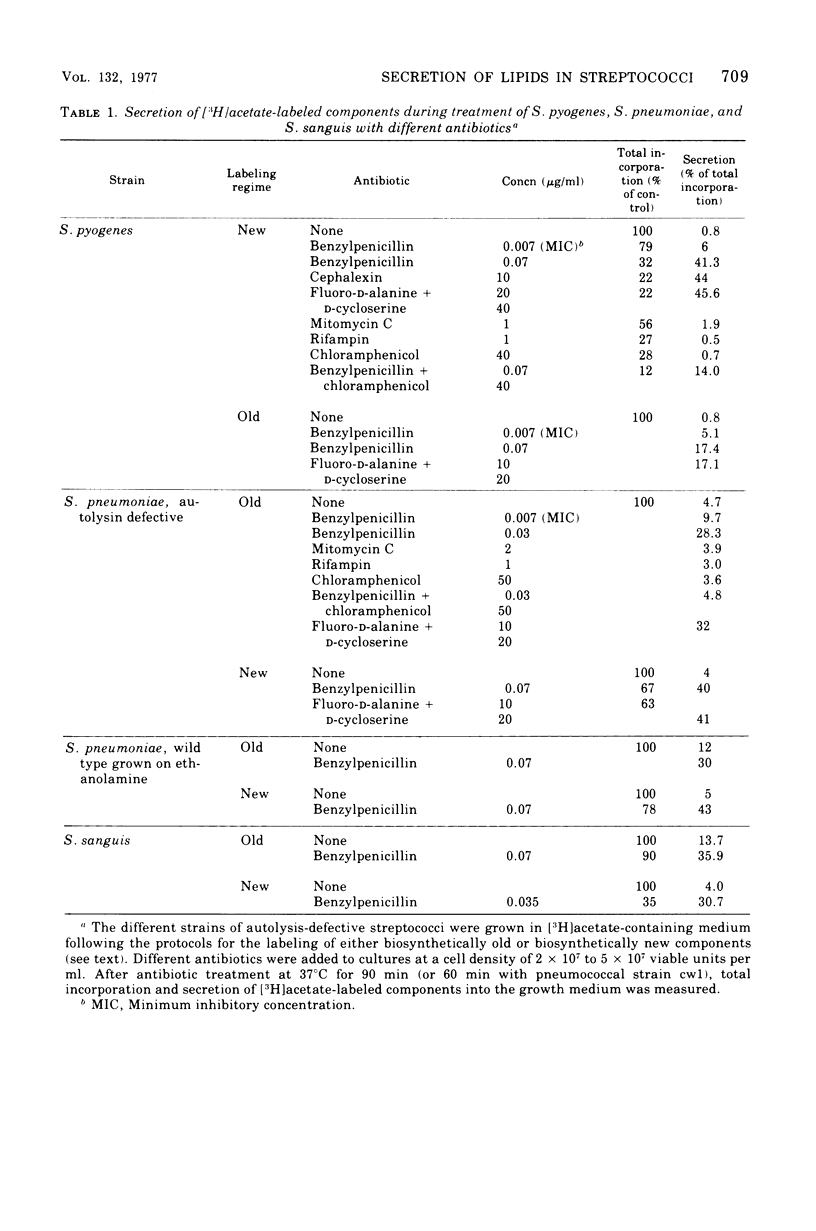
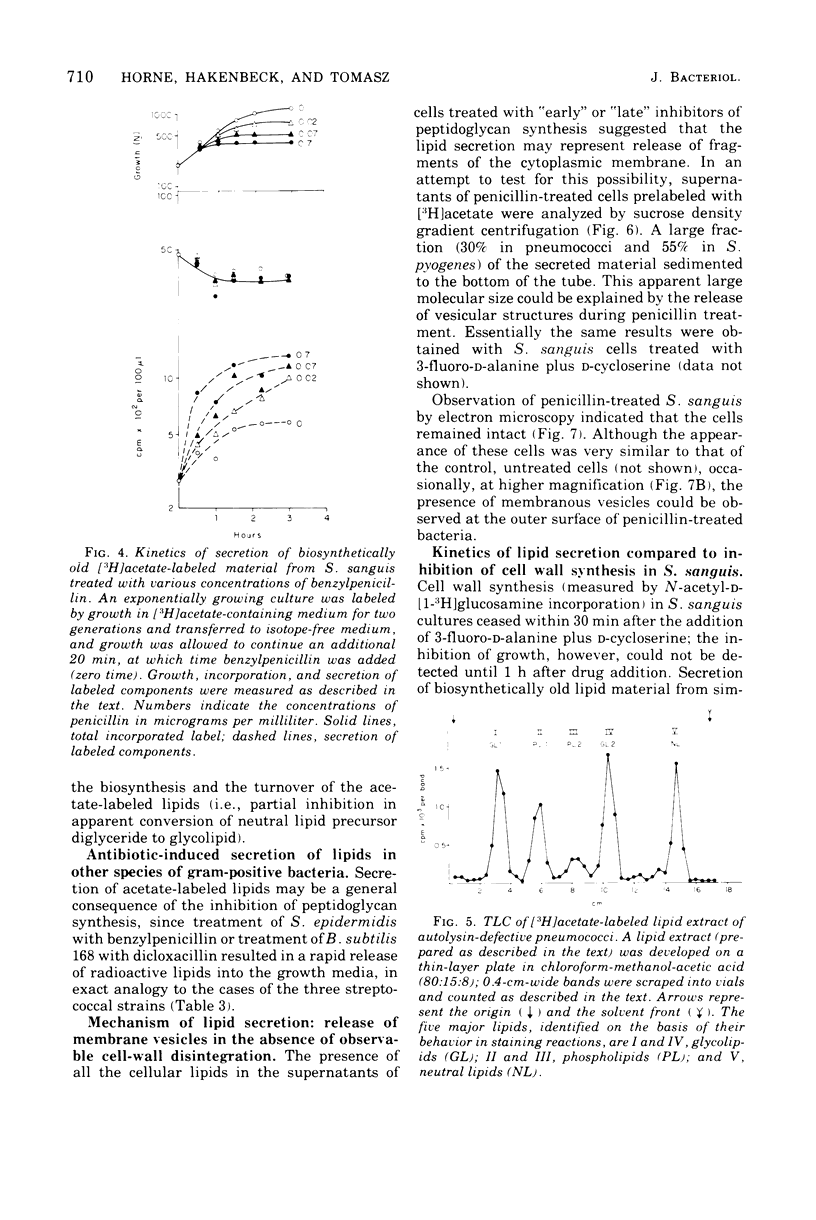
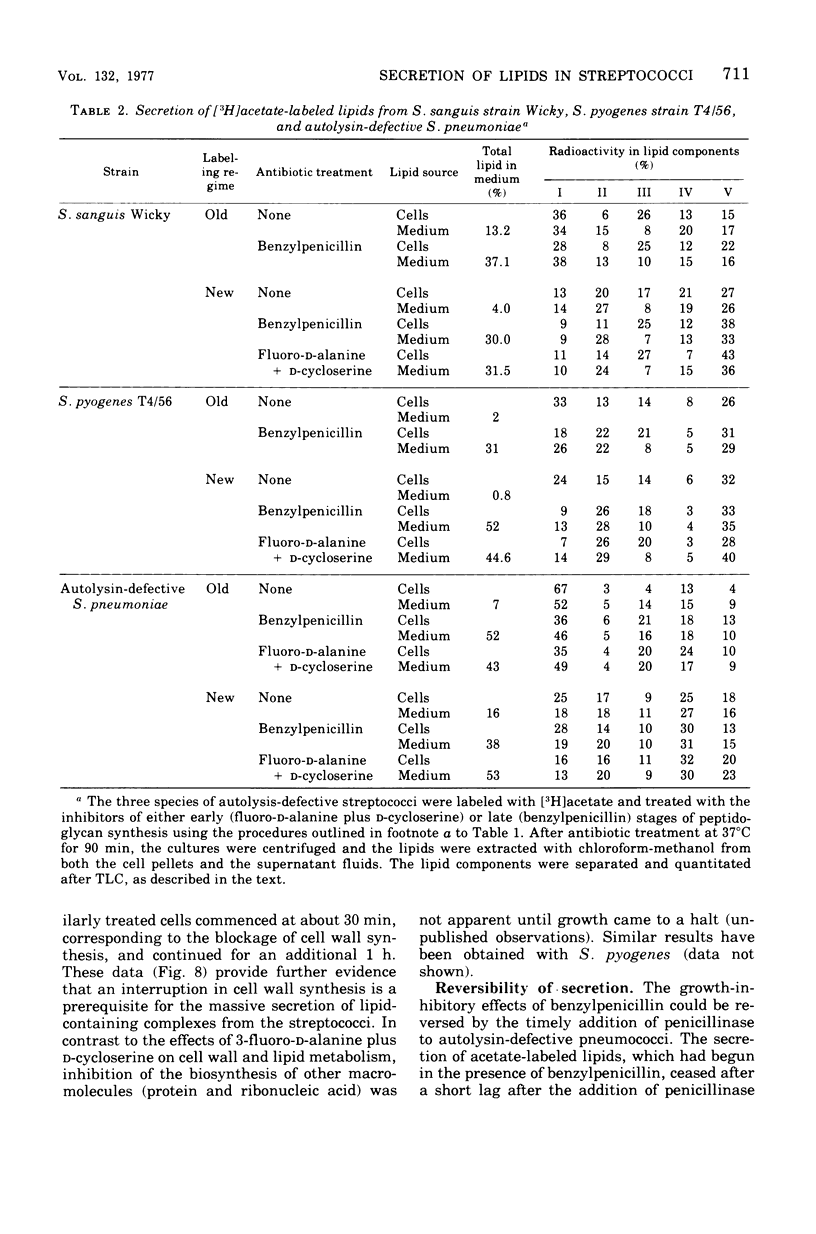
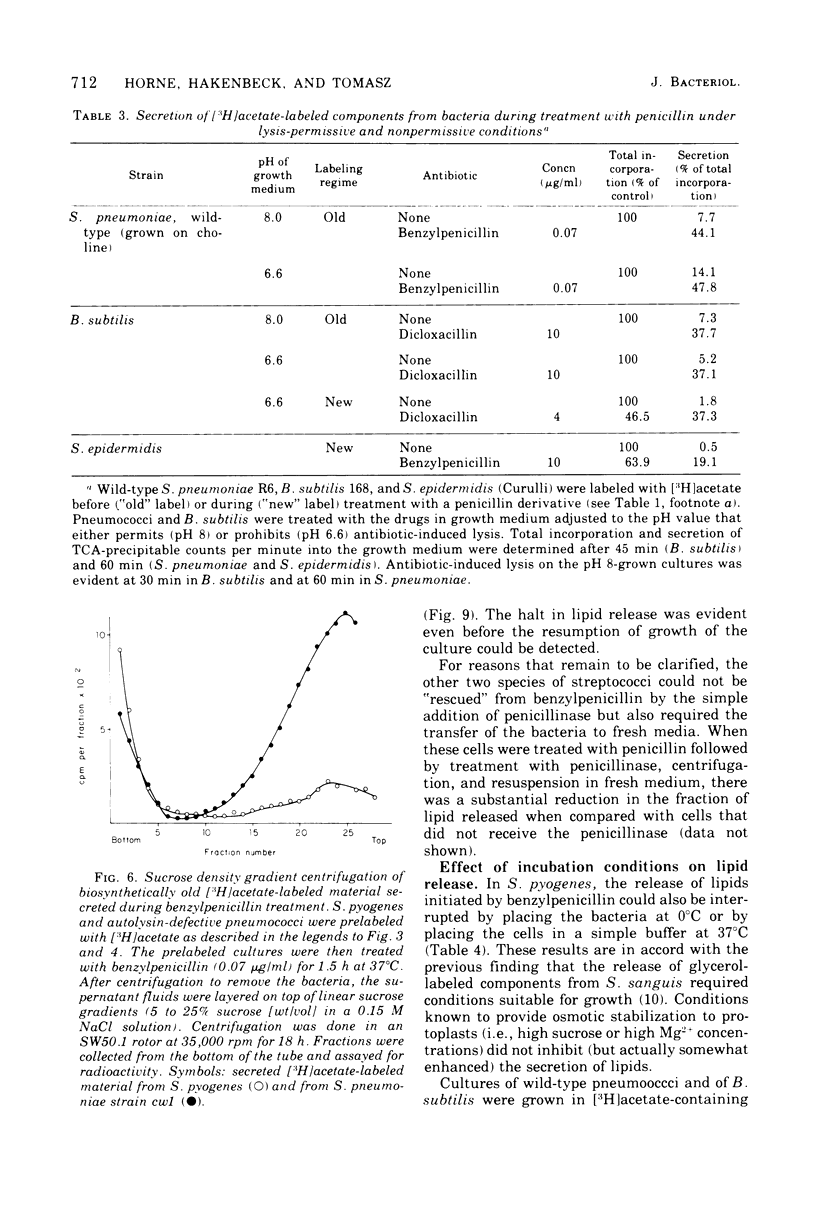
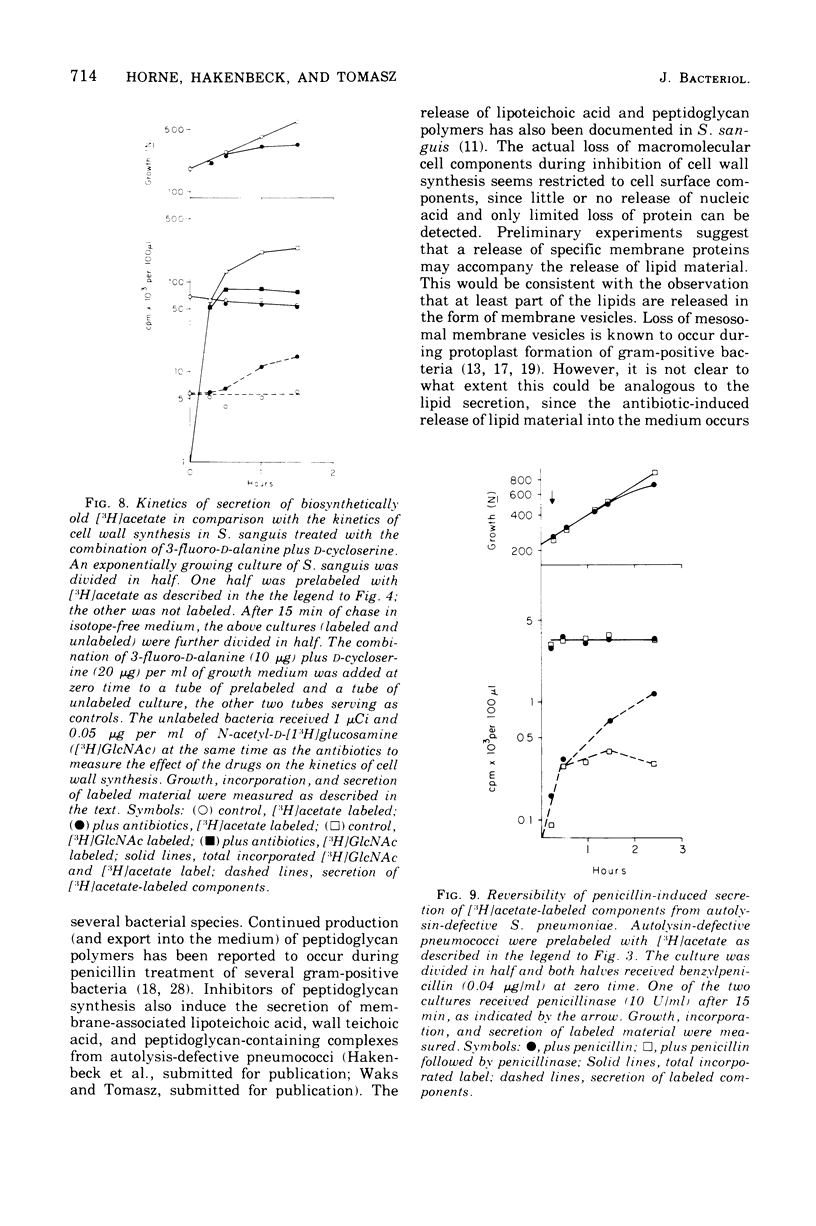
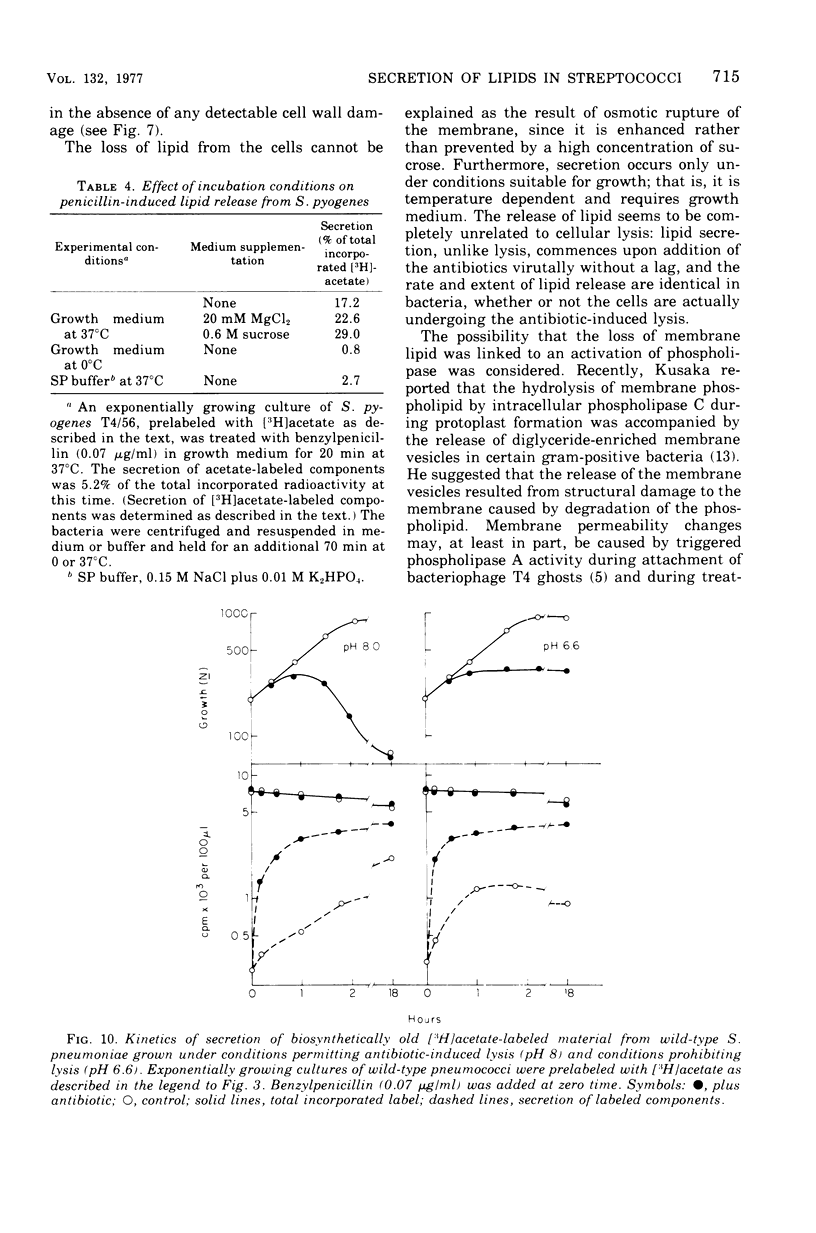
Inhibition of peptidoglycan synthesis causes an immediate and massive secretion of both newly synthesized and "old" lipids from several species of bacteria, including streptococci, Staphylococcus epidermidis, and Bacillus subtilis. Lipid secretion occurs in the absence of detectable bacterial lysis. This novel phenomenon was examined in more detail in three strains of streptococci: S. sanguis (group H), S. pyogenes (group A), And S. pneumoniae. The secretion of lipids is specifically induced by inhibitors of peptidoglycan synthesis; it is not caused by inhibitors of protein, ribonucleic acid, or deoxyribonucleic acid synthesis. The occurrence appears to be reversible since penicillin-induced secretion comes to a halt upon the timely addition of penicillinase, correlating with resumption of culture growth. All cellular lipids are secreted in essentially the same proportions as those found in the drug treated bacteria. It is suggested that continued peptidoglycan synthesis may be essential for the integration and retention of lipid material in the plasma membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast L. Y., Henderson T. O. Effect of inhibition of protein synthesis on lipid metabolism in Lactobacillus plantarum. J Bacteriol. 1975 Sep;123(3):962–971. doi: 10.1128/jb.123.3.962-971.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNDISH D. E., SHAW N., BADDILEY J. THE GLYCOLIPIDS FROM A ROUGH STRAIN OF PNEUMOCOCCUS TYPE I. Biochem Biophys Res Commun. 1965 Feb 3;18:308–311. doi: 10.1016/0006-291x(65)90704-7. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Schaechter M. Effect of shift-down and growth inhibition on phospholipid metabolism of Escherichia coli. J Bacteriol. 1971 Jul;107(1):251–258. doi: 10.1128/jb.107.1.251-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. W., Schaechter M. The mode of segregation of the bacterial cell membrane. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2312–2316. doi: 10.1073/pnas.69.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D. S., Perry D. Effect of competence induction on macromolecular synthesis in a group H streptococcus. J Bacteriol. 1974 Jun;118(3):830–836. doi: 10.1128/jb.118.3.830-836.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN B., KUNDIG D., DISTLER J., ROSEMAN S. ENZYMATIC SYNTHESIS AND STRUCTURE OF TWO GLYCOLIPIDS FROM TYPE XIV PNEUMOCOCCUS. Biochem Biophys Res Commun. 1965 Feb 3;18:312–318. doi: 10.1016/0006-291x(65)90705-9. [DOI] [PubMed] [Google Scholar]
- Kusaka I. Degradation of phospholipid and release of diglyceride-rich membrane vesicles during protoplast formation in certain gram-positive bacteria. J Bacteriol. 1975 Mar;121(3):1173–1179. doi: 10.1128/jb.121.3.1173-1179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Dales S. Membrane synthesis in Bacillus subtilis. 3. The morphological localization of the sites of membrane synthesis. J Cell Biol. 1972 Oct;55(1):32–41. doi: 10.1083/jcb.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Patch C. T., Landman O. E. Comparison of the biochemistry and rates of synthesis of mesosomal and peripheral membranes in Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):345–357. doi: 10.1128/jb.107.1.345-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieringer R. A. The metabolism of glyceride glycolipids. I. Biosynthesis of monoglucosyl diglyceride and diglucosyl diglyceride by glucosyltransferase pathways in Streptococcus faecalis. J Biol Chem. 1968 Sep 25;243(18):4894–4903. [PubMed] [Google Scholar]
- Shaw N. Bacterial glycolipids. Bacteriol Rev. 1970 Dec;34(4):365–377. doi: 10.1128/br.34.4.365-377.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- Weiss J., Franson C., Schmeidler K., Elsbach P. Reversible envelope effects during and after killing of Escherichia coli w by a highly-purified rabbit polymorpho-nuclear leukocyte fraction. Biochim Biophys Acta. 1976 Jun 4;436(1):154–169. doi: 10.1016/0005-2736(76)90227-3. [DOI] [PubMed] [Google Scholar]



