Abstract
Lyme borreliosis is a complex infection, where some individuals develop so-called ‘chronic borreliosis’. The pathogenetic mechanisms are unknown, but the type of immune response is probably important for healing. A strong T helper cell type 1 (Th1)-like response has been suggested as crucial for eradication of Borrelia and for avoiding development of chronic disease. Many studies aimed at altering the Th1/Th2 balance in Lyme arthritis employed mice deficient in cytokine genes, but the outcome has not been clear-cut, due possibly to the high redundancy of cytokines. This study aimed at studying the importance of the Th1/Th2 balance in murine Borrelia arthritis by using the Th2-deviating effect of subtoxic doses of inorganic mercury. Ninety-eight C3H/HeN mice were divided into four groups: Borrelia-infected (Bb), Borrelia-infected exposed to HgCl2 (BbHg), controls exposed to HgCl2 alone and normal controls. Mice were killed on days 3, 16, 44 and 65 post-Borrelia inoculation. Arthritis severity was evaluated by histology, spirochaetal load determined by Borrelia culture, IgG2a- and IgE-levels analysed by enzyme-linked immunosorbemt assay (ELISA) and cytokine-secreting cells detected by enzyme-linked immunospot (ELISPOT). BbHg mice showed less severe histological arthritis, but delayed eradication of spirochaetes compared to Bb mice, associated with increased levels of IgE (Th2-induced) and decreased levels of IgG2a (Th1-induced), consistent with a Th2-deviation. Both the numbers of Th1 and Th2 cytokine-secreting cells were reduced in BbHg mice, possibly explained by the fact that numbers of cytokine-secreting cells do not correlate with cytokine concentration. In conclusion, this study supports the hypothesis that a Th1-like response is required for optimal eradication of Borrelia.
Keywords: arthritis, Borrelia burgdorferi, cytokines/interleukins, immunoglobulins
Introduction
Lyme borreliosis is a complex infection caused by the bacterium Borrelia burgdorferi and related species. The clinical characteristics of the disease differ depending on the subspecies and the geographical location [1,2]. A common manifestation in Europe is neuroborreliosis, whereas arthritis is a more general outcome in the United States [1,2]. The course of the illness differs among individuals, ranging from an asymptomatic infection [3–5] to so-called ‘chronic borreliosis’, i.e. symptoms persisting for more than 6 months despite adequate antibiotic treatment [6–10].
The mechanisms leading to chronic borreliosis are not known and remain a subject of controversy [11]. Current hypotheses include (i) long-term persistence of the spirochaete (despite treatment with antibiotics) [12–14], (ii) an aberrant immune response leading to chronic inflammation (possibly including post-infectious autoimmunity) [15,16] or (iii) genomic differences among subspecies of B. burgdorferi [17,18]. Irrespective of which of these hypotheses holds true, the establishment of a certain quality of the immune response against Borrelia is of crucial importance for resolution of the disease.
The Borrelia-specific T cell response in patients with Lyme borreliosis is predominantly a T helper cell type 1 (Th1)-like response [19–26], especially in target organs such as joints and the central nervous system [22,25,27–29]. Mice of the BALB/c strain eradicate the spirochaetes and resolve arthritis more rapidly than C3H/HeN mice, a phenomenon associated with a stronger initial Th1-like response in BALB/c mice. The stronger Th1 response in BALB/c mice was down-regulated due to the ensuing Th2-like response, in contrast to the gradual increase in the Th1-like response in C3H/HeN mice [30]. Studies on the importance of Th1/Th2 responses using mice with targeted deletions of Th1- or Th2-associated cytokine genes have shown divergent results [31–35]. This may be due to the high degree of functional redundancy, whereby silencing of a single cytokine gene may be compensated for by other cytokine genes.
Exposure to subtoxic doses of mercury leads to a Th2 deviation in most mouse strains [36–42], which is a robust way of influencing the Th1/Th2 balance [43]. This model has been used previously to assess the importance of the Th1/Th2 balance in murine infections [44]. For example, resistance to Leishmania major in mice is dependent upon an adequate initial interferon (IFN)-γ production, whereas an early interleukin (IL)-4 production results in a non-healing phenotype [45,46], resembling our working hypothesis for Lyme borreliosis [47–49]. Treatment with HgCl2 prior to L. major infection is associated with increased production of IL-4 and IgE, and causes increased foot pad swelling and parasite burden [44].
In the present study we used exposure to mercury as a means of inducing Th2-deviation in Borrelia-susceptible C3H/HeN mice to test the hypothesis that a strong Th1-like response is required early in infection for optimal eradication of Borrelia and to avoid chronic inflammatory Th1-like responses, leading potentially to chronic borreliosis. Our results support the hypothesis that a Th1-like response is crucial for eradication of Borrelia spirochaetes.
Materials and methods
Mice
Ninety-eight female C3H/HeN mice, susceptible to Borrelia infection, were obtained from Taconic M&B (Ry, Denmark). The mice were housed in groups of seven in steel-wire cages under a 12-h light/12-h dark cycle at the Animal Unit, Faculty of Health Sciences, Linköping University, Linköping with ad libitum access to pellets (Type R 36, Lactamin, Vadstena, Sweden). The mice were 9–10 weeks old at the onset of the experiment. Mice were divided into four groups: 28 normal controls, 14 mercury-treated controls (Hg), 28 infected with Borrelia only (Bb) and 28 treated with mercury and infected with Borrelia (BbHg).
Treatment with mercury
Forty-two mice were exposed to mercury at non-toxic doses (approximately 190 µg Hg/kg body weight/day) by dissolving 10 mg/l HgCl2 (Fluka Chemie, Buchs, Switzerland) in the drinking water, given ad libitum, for 14 days. Fresh solutions were given once a week. Treatment with Hg started simultaneously in the group to be treated with Hg only and in the group also to be treated with B. burgdorferi. Treatment with Hg continued for 3 more days after the day of inoculation of Borrelia, in total 14 days.
Borrelia spirochaetes
B. burgdorferi N40, a proven infectious strain [50], was grown in Barbour Stoenner Kelly (BSK II) medium supplemented with 6% normal rabbit serum (Department of Microbiology, Umeå University, Sweden) at 34°C for 72 h prior to infection.
Borrelia infection
Fifty-six C3H/HeN mice, 28 of which were not exposed to mercury, were anaesthetized with Isofluran (Forene®; Abbott Scandinavia AB, Solna, Sweden) and injected subcutaneously at the tail root with 105B. burgdorferi N40 in 100 µl BSK II. In the 28 BbHg mice the inoculation took place 11 days after onset of Hg treatment.
Experimental plan
Seven animals from each of the Bb, BbHg and normal control groups were killed on days 3, 16, 44 and 65 post-immunization (p.i.) with Borrelia spirochaetes. The mice exposed to mercury only (Hg) were killed on days 3 and 65. On the due day the mice were anaesthetized with Isofluran (Forene®; Abbott Scandinavia AB), a blood sample for Ig analysis was obtained by retroorbital puncture, and the mice were killed by cervical dislocation. Ear and tibiotarsal joints were taken for evaluation of spirochaetal load. Inguinal lymph nodes were taken for cytokine analysis.
Clinical evaluation
From day 0 of mercury exposure, 11 days before inoculation with B. burgdorferi, all mice were weighed and the average thickness of tibiotarsal joints of both hind legs were measured using an electronic digital calliper (Biltema AB, Helsingborg, Sweden) every other day. Measurements were taken at the thickest portion of the joint with the joint extended. Data are reported as the change in swelling. Swelling was defined as increase in joint diameter at the actual day compared with the diameter before onset of treatment.
Histopathology of the tibiotarsal joints
The left rear tibiotarsal joint was taken from each mouse for histology. Samples were fixed in formaldehyde 4% (Histolab Products AB, Göteborg, Sweden), decalcified, embedded in paraffin, and sections were stained with haematoxylin and eosin. Slides were analysed blinded by one of the authors(P. H.) and scored for neutrophil infiltration, mononuclear cell infiltration, synovia thickness and cartilage hyperplasia. The scores for all parameters were: 0, normal; 0·5, minimal; 1, slight; 2, moderate; and 3, severe.
Spirochaetal load
Culture of Borrelia spirochaetes
To determine the extent of spirochaetal dissemination, biopsies from both ears, the left kidney, the right tibiotarsal joint and blood were cultured in BSK II medium supplemented with 100 µg/ml phosphomycin and 50 µg/ml rifampicin (both purchased from Sigma-Aldrich, Steinheim, Germany) at 34°C. The cultures were centrifuged at 900 g for 10 min at room temperature (RT) and counted in a Bürker chamber by phase contrast microscopy after 5 days.
Cytokine analyses
The lymph nodes were ground through a sterile strainer into Click's medium (Sigma, St Lois, MO, USA) supplemented with 0·04 mM b-mercaptoethanol (Sigma), 200 µM l-glutamine (Sigma-Aldrich), 100 U penicillin/ml (Invitrogen, Stockholm, Sweden) and 100 µg streptomycin/ml (Invitrogen). The suspension was centrifuged at 4°C at 400 g for 10 min. The cells were washed once in Click's medium, centrifuged as described above, resuspended in Click's medium with 10% fetal calf serum (FCS) (Sigma-Aldrich, Steinheim, Germany), referred to hereafter as 10% Click's medium, counted under a phase contrast microscopy and diluted to a density of 1 × 106 lymphocytes/ml in 10% Click's medium. To analyse the cytokine production for mouse IL-4, IL-12p70 and IFN-γ, enzyme-linked immunospot assay (ELISpotPLUS) kits (Mabtech AB, Nacka Strand, Sweden) were used according to the manufacturer's instructions. Cell suspension (100 µl containing 1 × 105 cells) with 10% Click's medium (50 µl) or Osp-enriched fraction (OF) of B. burgdorferi (50 µl), prepared as described previously [51], were added to each well and incubated in 5% CO2 at 37°C for 2 days. Cell cultures were run in duplicate. Stimulation with OF was used to evaluate the number of Borrelia-specific cytokine-producing cells. The mitogen concanavalin A (ConA, 40 µg/ml, Sigma) was used as a positive control, while wells with 10% Click's medium only, without cells, worked as negative controls. The spots were counted by dissection light microscopy. ConA stimulation always elicited strong responses for all cytokines. No spots were found in the negative control wells.
Immunoglobulin analyses
Blood samples were taken for assessment of serum IgE and IgG2a concentration to determine the Th1/Th2 status [52]. Blood was centrifuged at 600 g for 10 min at room temperature (RT), the serum removed and frozen in −70°C. IgE analyses were performed by enzyme-linked immunosorbent assay (ELISA) as described previously [53] and IgG2a was analysed utilizing a commercial IgG2a kit (Bethyl Laboratories Inc., Montgomery, TX, USA).
Statistical analysis
All data presented over time are medians. The spread of data is shown as interquartile range. Data were examined by non-parametric Mann–Whitney rank sum test. P-values less than 0·05 were considered significant and P-values > 0·05 < 0·1 were considered a trend.
Ethics
The Ethics Committee for Animal Experimentation in Linköping approved this study.
Results
Clinical signs
Weight
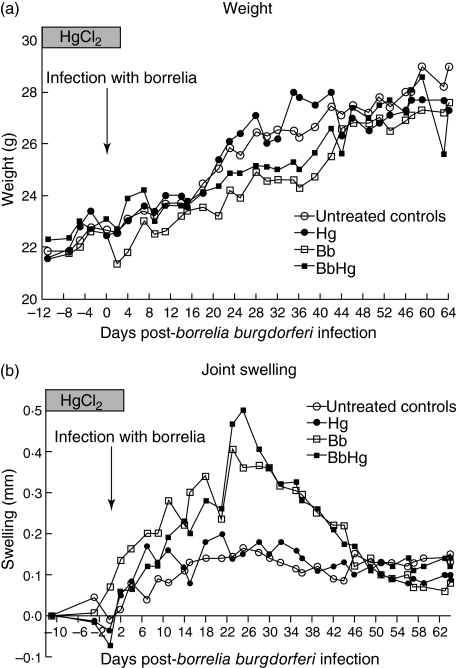
Normal control mice and mice treated with HgCl2 alone had similar weight curves with a steady weight gain during the experiment, whereas Bb and BbHg mice showed a smaller weight gain compared with normal control mice and mice treated with HgCl2 only (Fig. 1a, most pronounced in Bb versus controls on day 11 p.i., P = 0·004; in BbHg versus controls on day 36 p.i., P = 0·021). There were no differences in weight between Bb and BbHg mice at any time during the experiment. After day 44 p.i. the weight curves converged for all four groups of mice.
Fig. 1.
Weight (a) and joint swelling (b) in C3H/HeN mice in the four groups of mice during the experiment. Normal controls; Hg, controls exposed to HgCl2; Bb, infected with Borrelia burgdorferi N40; BbHg, infected with B. burgdorferi N40 and exposed to HgCl2 prior to infection. Joint swelling was calculated as the difference between the joint measure for each day and the first measure. Data represent median values.
Joint diameter
In both Bb and BbHg mice the swelling of the right hind tibiotarsal joints started to increase on day 9 p.i. and reached a maximum on days 23–28 (P < 0·001 for both Bb and BbHg compared to controls, Fig. 1b). The swelling started to decline in both groups after day 28 and was not significantly different from the normal control mice on day 44 p.i. The difference in swelling was not significant (P > 0·05) between the Bb and BbHg groups at any time. The group treated with HgCl2 alone showed no significant difference in joint diameter compared with the normal control group.
Histopathology
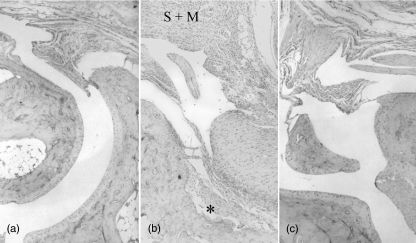
Histological assessment of the left rear tibiotarsal joints in the BbHg group of mice showed no or only minimal infiltration of inflammatory cells, no increased tendon sheath thickness and no cartilage hyperplasia on day 16 p.i. (Fig. 2c, Table 1). In contrast, at this time all mice in the Bb group showed a slight–severe infiltration of mononuclear cells, increased synovial thickness, and in many instances slight or moderate infiltration of neutrophils and cartilage hyperplasia (Fig. 2b, Table 1). The difference was significant between Bb and BbHg mice for all parameters except infiltration of neutrophils (Table 1). On days 3, 44 and 65 p.i. no differences were found between the Bb and BbHg groups (P > 0·05). On day 65 p.i., histological assessment showed normal joints in five of seven mice in both the Bb and BbHg groups, whereas two mice in each group still showed some infiltration of mononuclear cells, increases in tendon sheath thickness and cartilage hyperplasia (data not shown).
Fig. 2.
Histology from the right hindleg joint of C3H mice. (a) Normal control mouse. Ordinary synovia and preserved cartilage–bone interface. (b) Sixteen days after inoculation with Borrelia burgdorferi. Hyperplasia of synovia (S) with massive infiltration of mainly mononuclear inflammatory cells (M). Cartilage destroyed with superficial erosion of the bone structure (*). (c) Sixteen days after inoculation of B. burgdorferi and 27 days after onset of mercury treatment. Minimal synovial hyperplasia with scant inflammatory cells and preserved cartilage–bone interface.
Table 1.
Histopathological assessment of arthritis severity in tibiotarsal joint sections from Borrelia burgdorferi-infected C3H/HeN mice 16 and 44 days after infection.
| 16 days p.i. | 44 days p.i. | |||||
|---|---|---|---|---|---|---|
| Bb n = 7 | BbHg n = 7 | P-value | Bb n = 7 | BbHg n = 7 | P-value | |
| Neutrophils infiltration | 0 (0–2) | 0 (0–0) | n.s. | 0 (0–2) | 0 (0–0) | n.s. |
| Mononuclear cell infiltration | 1 (1–3) | 0 (0–0·5) | P = 0·001 | 0 (0–1) | 1 (0–1) | n.s. |
| Synovia thickness | 2 (1–3) | 0 (0–0·5) | P = 0·001 | 1 (1–2) | 2 (0–3) | n.s. |
| Cartilage hyperplasia | 1 (0–1) | 0 (0–0) | P = 0·026 | 0 (0–1) | 1 (0–1) | n.s. |
Median values (range in parenthesis) of scores are given. The scores for all parameters ranged from 0 (normal) to 3 (severe). p.i., post-immunization, Bb, B. burgdorferi-infected mice, BbHg, B. burgdorferi-infected mice treated with HgCl2. P-values refer to comparisons using the Mann–Whitney U-test; n.s.: non-significant.
Spirochaetal load
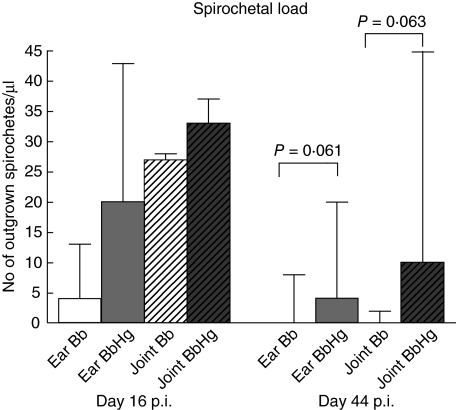
Spirochaetes were detected in cultures from both ear(s) and the right rear hind joint in Bb as well as BbHg mice on days 16 and 44 p.i. (data not shown). On day 44 p.i., BbHg mice showed a trend towards higher spirochaete burden in both ear and joint compared to Bb mice (P = 0·061 and P = 0·063, respectively) (Fig. 3). No spirochaetes were detected in cultures taken on days 3 or 65 p.i. (data not shown). Spirochaetes were not detected in cultures from kidney or blood at any time during the experiment.
Fig. 3.
Effect of mercury exposure on spirochaetal load. Numbers of Borrelia spirochaetes in ear and tibiotarsal joint from C3H/HeN mice 16 and 44 days after experimental infection with Borrelia burgdorferi N40. Bb, mice infected with B. burgdorferi; BbHg, mice infected with B. burgdorferi and treated with HgCl2 prior to infection. Data represent median values from seven mice in each group. Error bars indicate interquartile range. P-values refer to comparisons using the Mann–Whitney U-test.
Immunoglobulin isotypes
Serum IgE
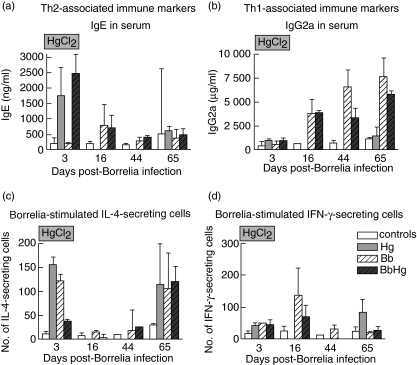
Mice treated with Hg alone had ninefold higher serum IgE levels compared to normal control mice on day 3 p.i. (P = 0·001). The increase in BbHg mice was 12-fold compared to normal controls as well as the Bb mice (P = 0·001, Fig. 4a). On day 16 p.i., the increase of IgE in both Bb and BbHg mice was three- to fourfold compared to normal controls (P = 0·018 and P = 0·003, respectively). On day 44 p.i. the IgE level was twofold higher in BbHg and Bb mice (P = 0·038, Fig. 4a) compared to normal controls, i.e. there was no difference between the BbHg and Bb groups. No further differences in IgE levels were seen.
Fig. 4.
Effect of mercury exposure on T helper 1 (Th1)/Th2 responses in C3HHeN mice infected experimentally with Borrelia burgdorferi. Serum levels of (a) IgE (Th2) and (b) IgG2a (Th1), numbers of Borrelia-stimulated (c) interleukin-4- (Th2), (d) interferon-γ-secreting cells (Th1) in 100 000 lymphocytes from inguinal lymph nodes. Control, normal mice not infected with B. burgdorferi; Hg, mice treated with HgCl2 not infected with B. burgdorferi: Bb, mice infected with B. burgdorferi; BbHg, mice infected with B. burgdorferi and treated with HgCl2 prior to infection. Data represent median values from seven mice in each group. Error bars indicate interquartile range.
Serum IgG2a
Bb, BbHg and Hg mice showed a twofold increase in serum IgG2a level 3 days p.i. compared with normal controls (Fig. 4b). On day 16 p.i. the serum IgG2a level was increased sixfold in both Bb and BbHg mice compared to normal controls (both P = 0·017). On day 44 p.i. the increase was five- and 10-fold in Bb and BbHg mice compared with the normal controls, respectively (P = 0·004 and P = 0·001, respectively, Fig. 4b), i.e. the level in Bb mice was twice as high as in BbHg mice (P = 0·004, Fig. 4b). On day 65 the mice treated with Hg alone showed no increase in serum IgG2a, while Bb and BbHg mice showed a seven- and fivefold increase compared to the normal controls (P = 0·001), respectively.
Cytokines
IL-4
The number of Borrelia-stimulated IL-4-producing lymph node cells was 14-fold higher in mice treated with Hg only for 14 days (3 days p.i.) compared to normal control mice (P = 0·002, Fig. 4c). On days 3 and 16 p.i., Bb mice showed a three- to fourfold higher number of lymph node cells secreting IL-4, both regarding the spontaneous (data not shown) and the Borrelia-stimulated secretion, compared to BbHg mice (Fig. 4c, day 3 p.i. p = 0·002 and P = 0·004 for spontaneous and Borrelia-stimulated secretion, respectively; day 16 p.i., P = 0·032 and P = 0·012, respectively). No further differences between the groups were seen in the number of IL-4-secreting cells.
IFN-γ
On day 3 p.i., the lymph node cells from mice treated with Hg only showed a two- to threefold increase in the number of IFN-γ-producing cells both spontaneously and after Borrelia stimulation compared to normal control mice (P = 0·003). The number of IFN-γ-secreting cells (spontaneous as well as Borrelia-induced) in mice treated with Hg only 3 days p.i. (after 14 days Hg treatment, Fig. 4d) was similar to that in Bb and BbHg mice 3 days p.i. Bb mice showed increased numbers of spontaneously IFN-γ-secreting cells compared with normal controls on day 16 p.i. (P = 0·004, data not shown) and an increased number of Borrelia-induced IFN-γ-secreting cells compared with BbHg mice on day 16 p.i. (P = 0·048, Fig. 4d). No further differences were seen between the groups.
IL-12p70
No difference was observed between the different groups with respect to spontaneous or Borrelia-induced IL-12p70-secreting cells 3 days p.i., or in spontaneous or Borrelia-induced IL-12p70-secreting cells 16 days p.i. (P > 0·05, data not shown). There was, however, a trend towards increased numbers of IL-12p70-secreting cells in Bb compared with BbHg mice on day 16 p.i. (P = 0·073, data not shown). The number of IL-12p70-secreting cells could not be assessed on day 44 p.i. due to low numbers of lymphocytes. There were no differences between the groups on day 65 p.i.
Discussion
The findings of this study indicate that mice exposed to HgCl2 prior to Borrelia infection had increased spirochaetal load but decreased joint inflammation compared with normal, Borrelia-infected mice. Although the increase in spirochaetal load did not reach statistical significance, similar strong trends for increase was seen in both ear-tissue and joints, together supporting a real increase. The increase in spirochaetal load in BbHg mice correlated with reduced levels of IgG2a and increased levels of IgE. Because IgG2a secretion in mice is an effector response generated by Th1 [54] and IgE switching is dependent on the Th2-cytokines IL-4 [55] and IL-13 [56], the Borrelia-induced effector response was Th2-deviated in mercury-treated mice. Thus, our findings indicate that the Th1 response is important for eradication of Borrelia.
When examining the course of Borrelia infection, BbHg mice initially showed a mercury-induced Th2-type effector response (IgE), which decreased gradually after completion of mercury treatment. This was associated with a delayed Th1-response compared with Bb mice, reflected both in the number of IFN-γ-secreting cells and the following Th1-induced isotype switch to IgG2a, which most probably explains the similarly delayed eradication of Borrelia in BbHg mice. The different kinetics of IgE and IgG2a responses seen in the current study, i.e. a rapid increase of IgE following HgCl2-treatment which then declines, and a later increase of IgG2a that gradually increases, has been reported previously [57].
Although significantly less severe histopathology was recorded in BbHg compared to Bb mice on day 16 p.i. (P = 0·001 for both infiltration of mononuclear cells and synovia thickness, respectively), strongly indicating a decreased inflammatory response, the increase in joint swelling (Fig. 1b) was not significantly different between the two groups. The failure to detect differences in joint swelling may possibly be explained by technical difficulties in the measurement of joint diameter using a calliper, handling mice not anaesthetized, leading to a high variability.
So far, no established model of murine chronic borreliosis in mice is available. Thus, all mice recovered at the end of the experiment, making it impossible to draw any conclusions regarding the mechanisms in development of chronic borreliosis. It should, however, be noted that the reparative response in the joints, measured as cartilage hyperplasia, was increased in Bb mice compared to BbHg mice on day 16 p.i., suggesting a more beneficial response for resolving the disease in Bb mice. In the current study the histopathology showed a more severe Borrelia-induced arthritis in mice not exposed to mercury, tallying with a more efficient eradication of Borrelia compared to mercury-treated mice. The delicate balance between eradication of the microbe and protection of the host from immune-mediated tissue damage has been illustrated previously in studies of Borrelia infection. Mice lacking Th1-like responses showed less severe symptoms but a much higher spirochaetal burden than wild-type mice, while mice with decreased Th2-like responses developed more severe arthritis [31,58]. The present study also partly corroborates a previous study where pregnancy, considered to be associated with a Th2 shift [59,60], was shown to markedly reduce arthritis severity in Borrelia-infected mice [61]. The reduced severity was associated with a slight reduction in IFN-γ and markedly increased IL-4.
The decreased inflammatory response recorded in the joints of BbHg mice compared to Bb mice on day 16 p.i. corresponded with reduced numbers of cytokine-secreting cells in BbHg mice on the same occasion. This is in accordance with the observed reduction in clearance of spirochaetes in BbHg mice detected on day 44 p.i. However, the decreased numbers of Borrelia-induced cytokine-secreting cells in BbHg mice included both cells secreting IL-4 (Th2) and IFN-γ (Th1). In addition, there was a trend for decreased numbers of IL-12p70-secreting cells (Th1-inducing). This does not tally with the persistence of Th1 (IgG2a) and Th2 (IgE) type of immunoglobulins on day 16 p.i. The discrepancy between the in vivo observations of serum immunoglobulin isotypes, showing an initial Th2 response which after completion of mercury treatment shifted gradually to Th1, and the in vitro observations of cytokine secretion may be explained by the fact that the number of cytokine-secreting cells does not correlate with the amount of cytokines secreted [62,63], i.e. the cytokine secretion varies between the cells. The reason for using ELISPOT, which detects the number of cytokine-secreting cells instead of measuring cytokine concentration in cell culture supernatants by, for example, ELISA, was that ELISPOT has outstanding sensitivity in the measurement of IL-4, which is extremely difficult to detect [62]. Nevertheless, the serum levels of IgG2a and IgE, which are indicators of cytokine-generated effects, clearly indicated a Th2-deviation also at the cytokine level in BbHg mice. We consider the in vivo results on serum immunoglobulin isotypes, which are linked to in vivo secretion of cytokines [54,55], as the best measurement of the Th1/Th2 balance.
The rebound of IL-4-secreting cells on day 65 in all groups except the untreated controls is surprising. Speculatively, this time-point would reflect a return to homeostasis in the Th1/Th2 balance, which in Borrelia-infected mice was Th1-dominated during the eradication of Borrelia, and thus suppressed Th2-responses. Borrelial lipoproteins are known to be highly immunogenic, inducing unspecific activation of numerous cell types [64], e.g. secretion of cytokines, which would also explain the response in the uninfected HgCl2-treated group. The untreated non-infected group did not, however, show any significant cytokine response to Borrelia antigen on any occasion, which may indicate that the HgCl2 treatment per se also causes hyperreactivity of the Th2-type to highly immunogenic antigens after the initial immunodeviating effects has declined.
In conclusion, this study indicates that mice exposed to HgCl2 prior to infection with B. burgdorferi have decreased arthritis severity but reduced elimination of Borrelia spirochaetes, which was associated with an initial mercury-induced Th2-type response and a delayed Th1-type response compared to unexposed Borrelia-infected mice. Our findings support the hypothesis that Th1-type responses are required for optimal eradication of Borrelia.
Acknowledgments
We would like to thank Klara Martinsson MSc and Christer Bergman, Research Engineer, Division of Molecular and Immunological Pathology, Faculty of Health Sciences at Linköping University, for excellent guidance and help with the analyses of immunoglobulin isotypes and with histological analyses, respectively. This study was supported by grants from the Swedish Research Council branch of Medicine to C. E. (grant no. 14631), to S. B. and to P. H. (grant no. 9453).
References
- 1.Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998;352:557–65. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–47. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- 3.Berglund J, Eitrem R, Ornstein K, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–24. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 4.Ekerfelt C, Masreliez C, Svenvik M, et al. Antibodies and T cell reactivity to Borrelia burgdorferi in asymptomatic population: a study of healthy blood donors in an inland district in the south-east of Sweden. Scand J Infect Dis. 2001;33:806–8. doi: 10.1080/00365540110077376. [DOI] [PubMed] [Google Scholar]
- 5.Ekerfelt C, Forsberg P, Svenvik M, et al. Asymptomatic Borrelia seropositive individuals display the same incidence of Borrelia-specific interferon-gamma (IFN-γ)-secreting cells in blood patients with clinical Borrelia infection. Clin Exp Immunol. 1999;115:498–502. doi: 10.1046/j.1365-2249.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser R. Variable CSF findings in early and late Lyme neuroborreliosis: a follow-up study in 47 patients. J Neurol. 1994;242:26–36. doi: 10.1007/BF00920571. [DOI] [PubMed] [Google Scholar]
- 7.Nocton JJ, Steere AC. Lyme disease. Adv Int Med. 1995;40:69–115. [PubMed] [Google Scholar]
- 8.Oschmann P, Dorndorf W, Hornig C, et al. Stages and syndromes of neuroborreliosis. Neurology. 1998;245:262–72. doi: 10.1007/s004150050216. [DOI] [PubMed] [Google Scholar]
- 9.Vrethem M, Hellblom L, Widlund M, et al. Chronic symptoms are common in patients with neuroborreliosis − a questionnaire follow-up study. Acta Neurol Scand. 2002;106:205–8. doi: 10.1034/j.1600-0404.2002.01358.x. [DOI] [PubMed] [Google Scholar]
- 10.Berglund JL, Stjernberg K, Ornstein K. 5-y follow-up study of patients with neuroborreliosis. Scand J Infect Dis. 2002;34:421–5. doi: 10.1080/00365540110080421. [DOI] [PubMed] [Google Scholar]
- 11.Stricker RB, Lautin A, Burrascano JJ. Lyme disease: point/counterpoint. Exp Rev Anti Infect Ther. 2005;3:155–65. doi: 10.1586/14787210.3.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Frey M, Jaulhac B, Piemont Y, et al. Detection of Borrelia burgdorferi DNA in muscles of patients with chronic myalgia related to Lyme disease. Am J Med. 1998;104:591–4. doi: 10.1016/s0002-9343(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 13.Oksi J, Marjamaki M, Nikoskelainen J, et al. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann Med. 1999;31:225–32. doi: 10.3109/07853899909115982. [DOI] [PubMed] [Google Scholar]
- 14.Preac-Mursic V, Weber K, Pfister HW, et al. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989;17:355–9. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- 15.Hemmer B, Pinilla C, Gran B, et al. VII International conference on Lyme borreliosis and other emerging tick-borne diseases. Munich, Germany: 1999. Cross-recognition of self antigens by Borrelia burgdorferi-specific T cells derived from the cerebrospinal fluid of a patient with CNS Lyme disease. [Google Scholar]
- 16.Sigal LH. Immunologic mechanisms in Lyme neuroborreliosis: the potential role of autoimmunity and molecular mimicry. Semin Neurol. 1997;17:63–8. doi: 10.1055/s-2008-1040915. [DOI] [PubMed] [Google Scholar]
- 17.Wagner EF, Hanna N, Fast LD, et al. Novel diversity in IL-4 mediated responses in resting human naive B-cell versus germinal center/memory B cells. J Immunol. 2000;165:5573–9. doi: 10.4049/jimmunol.165.10.5573. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Ojaimi C, Wu H, et al. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis. 2002;186:782–91. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsberg P, Ernerudh J, Ekerfelt C, et al. The outer surface proteins of Lyme disease Borrelia spirochaetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oksi J, Savolainen J, Pene J, et al. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–3. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Z, Braun J, Neure L, et al. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 22.Ekerfelt C, Ernerudh J, Bunikis J, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-γ predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 23.Ekerfelt C, Ernerudh J, Forsberg P, et al. Augmented intrathecal secretion of interferon-γ in response to Borrelia garinii in neuroborreliosis. J Neuroimmunol. 1998;89:177–81. doi: 10.1016/s0165-5728(98)00136-2. [DOI] [PubMed] [Google Scholar]
- 24.Widhe M, Ekerfelt C, Forsberg P, et al. IgG subclasses in Lyme borreliosis: a study of specific IgG subclass distribution in an IFN-γ predominated disease. Scand J Immunol. 1998;47:575–81. [PubMed] [Google Scholar]
- 25.Grusell M, Widhe M, Ekerfelt C. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J Neuroimmunol. 2002;131:173–8. doi: 10.1016/s0165-5728(02)00255-2. [DOI] [PubMed] [Google Scholar]
- 26.Widhe M, Jarefors S, Ekerfelt C, et al. Borrelia specific IFN-γ and IL-4 secretion in blood and CSF during the course of human Lyme borreliosis: relation to clinical outcome. J Infect Dis. 2004;189:1881–91. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 27.Wang W-Z, Fredriksson S, Sun J-B, et al. Lyme neuroborreliosis: evidence for persistent up-regulation of Borrelia burgdorferi-reactive cells secreting interferon-γ. Scand J Immunol. 1995;42:694–700. doi: 10.1111/j.1365-3083.1995.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 28.Granström M. Borrelios/Lyme disease − serologi/diagnostik. Information Från Läkemedelsverket [Information from the Medical Products Agency] 1998;9:98. [Google Scholar]
- 29.Gross DM, Steere AC, Huber BT. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–8. [PubMed] [Google Scholar]
- 30.Kang I, Barthold SW, Persing DH, et al. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anguita J, Rincón M, Samanta S, et al. Borrelia burgdorferi-infected, interleukin-6 deficient mice have decreased Th2 responses and increased Lyme arthritis. J Infect Dis. 1998;178:1512–5. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 32.Brown CR, Reiner SL. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–33. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christe M, Rutti B, Brossard M. Cytokines (IL-4 and IFN-γ) and antibodies (IgE and IgG2a) produced in mice infected with Borrelia burgdorferi sensu stricto via nymphs of Ixodex ricinus ticks or syringe inoculations. Parasitol Res. 2000;86:491–6. doi: 10.1007/s004360050699. [DOI] [PubMed] [Google Scholar]
- 34.Christopherson JA, Munson EL, England DM, et al. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin Diagn Lab Immunol. 2003;10:44–52. doi: 10.1128/CDLI.10.1.44-52.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoskar AR, Elizondo J, Monteforte GM, et al. Interleukin-4-deficient BALB/c mice develop an enhanced Th1-like response but control cardiac inflammation following Borrelia burgdorferi infection. FEMS Microbiol Lett. 2000;183:319–25. doi: 10.1111/j.1574-6968.2000.tb08978.x. [DOI] [PubMed] [Google Scholar]
- 36.Bagenstose LM, Salgame P, Monestier M. Cytokine regulation of a rodent model of mercuric chloride-induced autoimmunity. Environ Health Perspectives. 1999;107:807–10. doi: 10.1289/ehp.99107s5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 38.Häggqvist B, Hultman P. Murine metal-induced autoimmunity: baseline and stimulated cytokine mRNA expression in genetically susceptible and resistant strains. Clin Exp Immunol. 2001;126:157–64. doi: 10.1046/j.1365-2249.2001.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson U, Sander B, Hultman P. Effects of murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J Autoimmun. 1997;10:347–55. doi: 10.1006/jaut.1997.0149. [DOI] [PubMed] [Google Scholar]
- 40.Mathieson PW. Mercury: god of Th2 cells? Clin Exp Immunol. 1995;102:229–30. doi: 10.1111/j.1365-2249.1995.tb03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochel M, Vohr HW, Pfeiffer C, et al. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991;146:3006–11. [PubMed] [Google Scholar]
- 42.Prigent P, Saoudi A, Pannettier C, et al. Mercuric chloride, a chemical responsible for T helper cell (Th)2-mediated autoimmunity in brown Norway rats, directly triggers T-cells to produce interleukin-4. J Clin Invest. 1995;96:1484–9. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havarinasab S, Hultman P. Organic mercury compounds and mercury. Autoimmun Rev. 2005;4:270–5. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Bagenstose LM, Mentink-Kane MM, Brittingham A, et al. Mercury enhance susceptibility to murine leishmaniasis. Parasite Immunol. 2001;23:633–40. doi: 10.1046/j.1365-3024.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- 45.Morris L, Trout AB, McLeod KS, et al. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993;61:3459–65. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 47.Jarefors S, Janefjord CK, Forsberg P, et al. Decreased up-regulation of the interleukin-12Rb2-chain and interferon-γ secretion and increased number of forkhead box P3-expressing cells in patients with a history of chronic Lyme borreliosis compared with asymptomatic individuals. Clin Exp Immunol. 2006;147:18–27. doi: 10.1111/j.1365-2249.2006.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarefors S, Karlsson M, Forsberg P, et al. Reduced numbers of interleukin-12 secreting cells in patients with Lyme borreliosis previously exposed to Anaplasma phagocytophilum. Clin Exp Immunol. 2005;143:322–8. doi: 10.1111/j.1365-2249.2005.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöwall J, Carlsson A, Vaarala O, et al. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-α and interleukin-12 in asymptomatic individuals in response to live spirochaetes. Clin Exp Immunol. 2005;141:89–98. doi: 10.1111/j.1365-2249.2005.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barthold SW, Beck DS, Hansen GM, et al. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–8. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 51.Bergström S, Sjöstedt A, Dotevall L, et al. Diagnosis of Lyme borreliosis by an enzyme immunoassay detecting immunoglobulin G reactive to purified Borrelia burgdorferi cell components. Eur J Clin Microbiol Infect Dis. 1991;10:422–7. doi: 10.1007/BF01968022. [DOI] [PubMed] [Google Scholar]
- 52.Hovden AO, Cox RJ, Madhun A, et al. Two doses parenterally administered split influenza virus vaccine elicited high serum IgG concentrations which effectively limited viral shedding upon challenge in mice. Scand J Immunol. 2005;62:342–52. doi: 10.1111/j.1365-3083.2005.01666.x. [DOI] [PubMed] [Google Scholar]
- 53.Havarinasab S, Lambertsson L, Qvarnström J, et al. Dose–response study of thimerosal-induced murine systemic immunity. Toxicol Appl Pharmacol. 2004;194:169–79. doi: 10.1016/j.taap.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobuline isotype selection. Ann Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn R, Rajewski K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 56.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 57.Johansson U, Hansson-Georgiadis H, Hultman P. The genotype determines the B cell response in mercury-treated mice. Int Arch Allergy Immunol. 1998;116:295–305. doi: 10.1159/000023959. [DOI] [PubMed] [Google Scholar]
- 58.Anguita J, Persing DH, Rincón M, et al. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J Clin Invest. 1996;97:1028–34. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–82. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 60.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 61.Moro MH, Bjornsson J, Marietta EV, et al. Gestational attenuation of Lyme arthritis is mediated by progesterone and IL-4. J Immunol. 2001;166:7404–9. doi: 10.4049/jimmunol.166.12.7404. [DOI] [PubMed] [Google Scholar]
- 62.Ekerfelt C, Ernerudh J, Jenmalm MC. Detection of spontaneous and antigen-induced human IL-4 responses in vitro; comparison of ELISPOT, a novel ELISA and real time RT–PCR. J Immunol Meth. 2002;260:55–67. doi: 10.1016/s0022-1759(01)00520-8. [DOI] [PubMed] [Google Scholar]
- 63.Rönnelid J. Reactivity to collagen type II and C1q in rheumatic diseases. Stockholm, Sweden: Department of Medicine, Rheumatology Unit, Karolinska Institute; 1997. [Google Scholar]
- 64.Talkington J, Nickell SP. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect Immun. 1999;67:1107–15. doi: 10.1128/iai.67.3.1107-1115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]