Abstract
In this second review on chemokines, we focus on the polymorphisms and alternative splicings and on their consequences in disease. Because chemokines are key mediators in the pathogenesis of inflammatory, autoimmune, vascular and neoplastic disorders, a large number of studies attempting to relate particular polymorphisms of chemokines to given diseases have already been conducted, sometimes with contradictory results. Reviewing the published data, it becomes evident that some chemokine genes that are polymorphic have alleles that are found repeatedly, associated with disease of different aetiologies but sharing some aspects of pathogenesis. Among CXC chemokines, single nucleotide polymorphisms (SNPs) in the CXCL8 and CXCL12 genes stand out, as they have alleles associated with many diseases such as asthma and human immunodeficiency virus (HIV), respectively. Of CC chemokines, the stronger associations occur among alleles from SNPs in CCL2 and CCL5 genes and a number of inflammatory conditions. To understand how chemokines contribute to disease it is also necessary to take into account all the isoforms resulting from differential splicing. The first part of this review deals with polymorphisms and the second with the diversity of molecular species derived from each chemokine gene due to alternative splicing phenomena. The number of molecular species and the level of expression of each of them for every chemokine and for each functionally related group of chemokines reaches a complexity that requires new modelling algorithms akin to those proposed in systems biology approaches.
Keywords: chemokines, human, polymorphisms, splicing, variability
Increasing chemokine variability: polymorphisms and alternative splicing
In a first review [1] we examined data which indicated that, during evolution, the variability of the chemokine superfamily grew in complexity, and we took advantage of the conservation of physiological functions among chemokines located in the different genetic clusters and miniclusters to improve our perspective of their functions. As for many gene families, the main mechanism that has generated this diversity of chemokines is gene duplication, which is particularly evident in the chemokine clusters. However, another important mechanism by which variation has been increasing at the genomic level is the existence of single nucleotide polymorphisms (SNPs), which are the most common form of DNA sequence variation. SNPs are highly abundant, stable and distributed throughout the genome. SNPs are an increasingly important tool for the study of the structure and history of human genome and they are also useful polymorphic markers to investigate genetic susceptibility to disease or to pharmacological sensitivity [2]. Other types of polymorphisms such as deletion/insertion polymorphisms (DIPs), copy number polymorphisms (CNPs) or those due to repeated elements (as minisatellites and microsatellites) also contribute importantly to the genomic variation but their distribution is more restricted. In addition to DNA sequence variation, alternative mRNA splicing is becoming recognized increasingly as an important mechanism for the generation of structural and functional variability in proteins. Several studies indicate that alternative splicing in humans is more the rule than the exception: primary transcripts from more than 50% of all human genes undergo alternative splicing, with a bias towards genes that are expressed in the nervous and immune systems [3,4].
In this second review, we focused upon the polymorphisms and disease associations of chemokine genes as well as variations in splicing which should be taken into account in order to understand these disease associations more clearly. We proceeded by collecting all information available in public databases, organized it by families following the systematic nomenclature [5], and finally we highlighted the cases in which disease association is stronger. In the course of this process we also analysed data available on isoforms generated by differential splicing. Even though we can expect that new data will still be produced on the polymorphisms and isoforms of chemokines, we now have a picture of their complexity and we can begin to discern patterns of disease association; this is also the subject of this review.
Polymorphisms and disease in the human chemokine superfamily
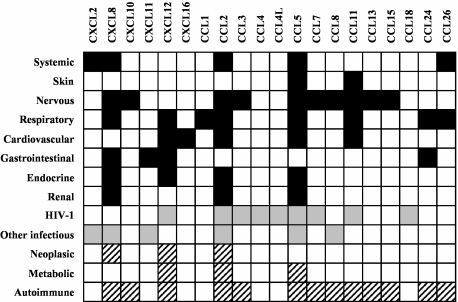
Polymorphisms in the genes of the immune system can influence the immune response markedly, human leucocyte antigen (HLA) genes being the paradigm. After the HLA genes, chemokine genes are probably one of the most polymorphic sets of genes in the immune system and it is becoming increasingly clear that chemokine polymorphisms influence the immune response to a remarkable extent. As the genome project progressed and the abundance of SNPs became evident, databases began to record SNPs and now millions of them are registered. However, the quality of the initial data contained in these databases had been questioned, because a considerable proportion of the initial SNPs may simply represent sequencing errors. Fortunately, the validation status of SNPs is improving and in this review we have included only well-documented and functionally relevant SNPs. The number of reports on disease-associated SNPs including members of the chemokine superfamily is increasing and will probably continue to rise during the next few years, as the importance of chemokines in the immune response gains recognition. As has already been documented for cytokines, the majority of SNPs found in the chemokines genes or their receptors are not located in the coding sequence but either in the promoter, the introns or the 3′ untranslated regions, and they can affect all aspects of gene expression and mRNA levels. Interestingly, most polymorphisms associated with disease in the chemokine superfamily affect their inflammatory members, thus confirming that they are the genes under stronger evolutionary pressure (Fig. 1).
Fig. 1.
Overview of chemokine polymorphisms and disease. Columns represent chemokines with disease-involved polymorphisms. Rows represent different disease categories: upper lines refer to diseases grouped by systems (black squares show polymorphism involvement), middle lines refer to infectious diseases (grey squares) and lower lines refer to other interesting physiopathological groups of diseases (dashed squares).
Human CXC chemokines
Several interesting polymorphisms affecting both inflammatory and homeostatic CXC ligands have been described (Table 1), CXCL8 [interleukin (IL)-8] and CXCL12 stromal cell-derived factor (SDF-1) being the chemokines that accumulate most of them.
Table 1.
Polymorphisms and disease in the human CXC subfamily.
| Ligand | Polymorphism | Location | Symbol | Disease involved | References |
|---|---|---|---|---|---|
| CXCL2 | Tandem repeat -665(AC)n | Promoter | Severe sepsis | [28] | |
| CXCL8 | Microsatellite | D4S2641 | Diffuse parabronchiolitis | [64] | |
| SNP -845 (C/T) | Promoter | rs2227532 | SLE nephritis | [16] | |
| SNP -251 (A/T) | Promoter | rs4073 | Asthma | [13] | |
| RSV infection | [8, 9, 14] | ||||
| Smoking behaviour | [7] | ||||
| Oral squamous cell carcinoma | [65] | ||||
| EAEC diarrhoea | [10] | ||||
| Clostridium difficile diarrhoea | [66] | ||||
| Helicobacter pylori-induced gastric diseases | [11, 67–70] | ||||
| Acute pancreatitis severity | [71] | ||||
| Gastric cancer | [12, 72, 73] | ||||
| Colorectal cancer | [74] | ||||
| Prostate cancer | [75] | ||||
| Parkinson's disease | [76] | ||||
| Multiple sclerosis | [77] | ||||
| Multiple system atrophy | [78] | ||||
| AIDS-related Kaposi's sarcoma | [79] | ||||
| Acute pyelonephritis | [17] | ||||
| SNP +781 (C/T) | Intron 1 | rs2227306 | Asthma | [13, 15] | |
| SNP +1633 (C/T) | Intron 3 | rs2227543 | Asthma | [13] | |
| SNP +2767 (A/T) | 3′UTR | rs1126647 | Asthma | [13] | |
| Nephritis in cutaneous vasculitis | [18] | ||||
| Acute pyelonephritis | [17] | ||||
| Haplotypes | Behcet's disease | [80] | |||
| CXCL10 | Haplotypes | Multiple sclerosis | [81] | ||
| CXCL11 | DIP -599del5 | Promoter | Hepatitis C virus (HCV) infection | [29] | |
| CXCL12 | SNP +801 (G/A) | 3′UTR | rs1801157 | HIV-1 infection | [20, 22–24] |
| Atherosclerosis in HIV patients | [82] | ||||
| Breast and lung cancer | [83–85] | ||||
| Acute myeloid leukemia | [86] | ||||
| Lymphoma | [87] | ||||
| Chronic myeloproliferative disease | [88] | ||||
| Liver transplantation | [89] | ||||
| Type 1 diabetes | [90] | ||||
| CXCL16 | SNP +599 (C/T) | Exon 4 | rs2277680 | Coronary artery stenosis | [30] |
CXCL8, a proinflammatory chemokine, is a potent chemoattractant for neutrophils, basophils and T lymphocytes. High levels of CXCL8 have been detected in biofluids from various acute inflammatory diseases, which is in keeping with neutrophilic infiltration into inflammatory sites as one of the hallmarks of acute inflammation. Among all CXCL8 described SNPs, the presence of −251A/T in the transcription start site is known to exert a strong influence on protein synthesis. The distribution of this SNP shows a remarkable heterogeneity among world populations [6] and has been associated with a spectrum of diseases (for references see Table 1): (a) airway diseases such as asthma, respiratory syncytial virus (RSV) infection and oral squamous cell carcinoma. Anecdotally, the inflammatory prone allele A may influence the initiation or characteristics of the smoking habit [7]; (b) gastrointestinal diseases such as Clostridium difficile and enteroaggregative Escherichia coli diarrhoea, Helicobacter pylori-induced gastric ulcer, atrophic gastritis, severe acute pancreatitis and gastrointestinal tract cancer; (c) central nervous system (CNS) diseases such as Parkinson's, multiple sclerosis (MS) and multiple system atrophy; and (d) a miscellany of diseases such as AIDS-related Kaposi's sarcoma and acute pyelonephritis has also shown to be influenced by the −251A/T CXCL8 polymorphism. However, in spite of the numerous studies on this polymorphism, data are still far from clear. Since the publication of the original association of the CXCL8 −251A/T polymorphism [8,9], there have been reports showing higher CXCL8 production by the A allele [8,10,11] while others showed higher production by allele T [12]. These contradictory data have their counterpart in association studies: the −251 T allele frequency has been increased significantly in asthma [13] and reduced significantly in RSV bronchiolitis [8]. In fact, it seems that the differences in CXCL8 expression are not linked directly to the −251A/T polymorphism. In one haplotype-based association study, Hacking et al. [14] have shown that there are two main CXCL8 haplotypes including six SNPs (−251A/T, +396G/T, +781T/C, +1238delA/insA, +1633T/C, +2767T/A), constituting the so-called haplotype 2 (A/G/T/delA/T/T), the haplotype associated with significantly higher CXCL8 transcription levels relative to the mirror haplotype 1. Strikingly, the −251A allele present on the high producer haplotype had no significant effect on the allele-specific level of transcription when analysed in reporter gene experiments. This indicates that the functional allele might be in linkage disequilibrium (LD) with haplotype 2 and that the −251A/T is not the functional SNP. Four SNPs of the previously described haplotypes (−251A/T, +781T/C, +1633T/C and +2767T/A) have been found to be associated with asthma in different studies [13,15]. These multiple SNPs associations are due probably to the existence of a very tight LD among them. The −845C/T SNP in the CXCL8 promoter region has been associated with severe systemic lupus erythematosus (SLE) nephritis [16]. Another distant SNP in 3′UTR (+2767A/T) has been associated with acute pyelonephritis [17] and nephritis in cutaneous vasculitis [18].
CXCL12 is a homeostatic CXC chemokine widely expressed which possesses a broad range of actions (from attraction of mature T and B cells to migration of haematopoietic progenitor cells from the bone marrow). CXCL12 plays an especially important role in two non-related diseases such as human immunodeficiency virus (HIV) and cancer, because its receptor (CXCR4) is also the co-receptor used by HIV T-tropic strains and because it is the most widely expressed chemokine receptor in many different types of cancers [19]. The +801G/A SNP, located in 3′UTR, is the best-studied polymorphism in CXCL12 gene. It has been associated extensively with clinical features of HIV infection but, as in CXCL8–251A/T polymorphism, there are some contradictory reports. The published effect of the mutated allele (−801A) ranges from strong protection of HIV infection progression to AIDS [20,21] to enhanced progression to AIDS and shorter survival [22,23]. Even though it was proposed originally that the −801A allele was associated with higher CXCL12 production [20], later studies indicated the opposite [24], and other reports claimed that there were no differences in the CXCL12 production by the A or G alleles [25,26]. A recent haplotype-based study [27] demonstrated that other polymorphisms in LD with the CXCL12 + 801G/A SNP, rather than CXCL12 + 801G/A itself, are responsible for the different transcription levels. Therefore, the discrepancy among the previous epidemiological studies may be attributed to the haplotype structures and frequencies in the studied populations. CXCL12 + 801G/A has also been associated with many different types of cancers such as breast and lung cancer, acute myeloid leukaemia, lymphoma and chronic myeloproliferative disease.
Relatively few reports deal with the effect of polymorphisms of other CXC chemokines on disease. A short tandem repeat (STR) in CXCL2 may contribute to the development of severe sepsis [28], one haplotype in CXCL10 possibly contributes to reduce the rate of progression in MS patients, a 5-base pairs (bp) deletion in the promoter of CXCL11 may favour hepatitis C virus (HCV) infection to evolve towards chronicity [29] and, finally, a SNP in exon 4 of CXCL16, leading to an amino acid change (V200A), seemed to influence the severity of coronary artery stenosis [30].
Human CC chemokines
As many as 14 of the 26 members of CC chemokine subfamily have polymorphisms associated with disease, and most affect the inflammatory chemokines (Table 2). CCL2 [monocyte chemoattractant protein (MCP)-1] attracts specifically monocytes and memory T cells and tissue expression is found in a large variety of diseases characterized by mononuclear cell infiltration, with an essential role in atherosclerosis and multiple sclerosis. CCL2 has a SNP located in the 5′ distal regulatory region (−2518G/A) and it seems clear that the −2518G allele is associated with an increased CCL2 production (at both mRNA and protein levels) [31–34]. This −2518G/A polymorphism has been associated with a large variety of diseases: (a) systemic inflammatory diseases such as systemic lupus erythematosus (SLE), juvenile rheumatoid arthritis, systemic sclerosis and HLA-B27-associated acute uveitis; (b) conditions affecting the kidney such as renal transplantation, long-term haemodialysis and IgA nephropathy; (c) heart diseases such as myocardial infarction, coronary artery disease and the cardiomyopathy of Chagas' disease; (d) CNS diseases such as Alzheimer's and major depression; (e) endocrine diseases such as type 1 and type 2 diabetes: (f) infectious diseases such as those caused by HIV-1, HCV, HBV and Mycobacterium tuberculosis; and (g) other diseases such as breast cancer and asthma. It is important to note that there are also many negative reports showing a lack of association of this SNP with various diseases (including some of those cited previously as associated diseases).
Table 2.
Polymorphisms and disease in the human CC subfamily.
| Ligand | Polymorphism | Location | Symbol | Disease involved | References |
|---|---|---|---|---|---|
| CCL1 | SNP (A/T) | Intron 2 | rs2282691 | Chronic obstructive pulmonary disease | [91] |
| CCL2 | SNP -2518 (G/A) | Promoter | rs1024611 | Systemic sclerosis | [92] |
| Asthma | [93, 94] | ||||
| Systemic lupus erythematosus | [95–97] | ||||
| Juvenile rheumatoid arthritis | [98] | ||||
| Renal transplantation | [99] | ||||
| Breast cancer | [100] | ||||
| Long-term haemodialysis | [101] | ||||
| Long-term haemodialysis | [101] | ||||
| IgA nephropathy | [102] | ||||
| HLA-B27 associated disease | [103] | ||||
| Coronary artery disease | [104, 105] | ||||
| Myocardial infarction | [106] | ||||
| Alzheimer's disease association | [107] | ||||
| Major depressive disorder | [108] | ||||
| Type 1 diabetes | [109] | ||||
| Type 2 diabetes | [110] | ||||
| HIV-1 infection | [33] | ||||
| Pulmonary tuberculosis | [111] | ||||
| Cardiomyopathy in human Chagas' disease | [112] | ||||
| Hepatitis B virus (HBV) clearance | [113] | ||||
| Hepatitis C virus (HCV) severity | [34] | ||||
| Haplotypes | Multiple sclerosis | [114, 115] | |||
| Haplotypes | HIV-1 infection | [116] | |||
| CCL3 | Haplotypes | Multiple sclerosis | [114, 115, 117] | ||
| Haplotypes | HIV-1 infection | [35, 36] | |||
| CCL4 | Haplotypes | HIV-1 infection | [35] | ||
| CCL4L | SNP +59 (C/T) | Exon 2 | rs3744595 | HIV-1 infection | [39] |
| SNP +590 (A/G) | Intron 2 | rs4796195 | HIV-1 infection | [38] | |
| CCL5 | SNP -403 (G/A) | Promoter | rs2107538 | Allergic rhinitis | [118] |
| Atopy and asthma | [119–121] | ||||
| Atopic dermatits | [40–42] | ||||
| Renal damage in SLE | [122] | ||||
| Rheumatoid arthritis | [123, 124] | ||||
| Multiple sclerosis | [125] | ||||
| HIV-1 infection | [36, 126, 127] | ||||
| HCV infection | [128, 129] | ||||
| Sarcoidosis | [130] | ||||
| Coronary arteriosclerosis | [131] | ||||
| Hypercholesterolaemia | [132] | ||||
| Cardiac mortality in type 2 diabetes | [133] | ||||
| SNP -28 (C/G) | Promoter | rs2280788 | Allergic rhinitis | [118] | |
| Asthma | [134, 135] | ||||
| Nephropathy in type 2 diabetes | [136] | ||||
| HIV-1 infection | [36, 43, 127] | ||||
| Multiple sclerosis | [125] | ||||
| Systemic lupus erythematosus | [137] | ||||
| Atopic dermatitis | [42] | ||||
| SNP In1.1 (T/C) | Intron 1 | rs2280789 | Cardiac mortality in type 2 diabetes | [133] | |
| HIV-1 infection | [37, 127, 138] | ||||
| Haplotypes | HIV-1 infection | [36] | |||
| Haplotypes | Type 1 diabetes | [139] | |||
| CCL7 | Microsatellite | Promoter | Multiple sclerosis | [140, 141] | |
| Haplotypes | HIV-1 infection | [116] | |||
| CCL8 | SNP +11 (A/C) | Exon 3 | rs1133763 | HCV infection | [128] |
| Haplotypes | Multiple sclerosis | [114] | |||
| CCL11 | SNP -576 (C/T) | Promoter | rs4795896 | Asthma | [44, 45] |
| SNP -426 (C/T) | Promoter | rs16969415 | Atopic dermatitis | [45, 142] | |
| Asthma | [45] | ||||
| SNP -384 (A/G) | Promoter | rs17809012 | Atopic dermatitis | [44, 142] | |
| Asthma | [45] | ||||
| SNP +67 (G/A) | Exon 1 | rs3744508 | Asthma | [46, 47] | |
| Myocardial infarction | [143] | ||||
| Haplotypes | Multiple sclerosis | [114] | |||
| Haplotypes | HIV-1 infection | [116] | |||
| CCL13 | Haplotypes | Multiple sclerosis | [114] | ||
| CCL15 | Haplotypes | Multiple sclerosis | [114, 115] | ||
| CCL18 | Haplotypes | HIV-1 infection | [35] | ||
| CCL24 | SNP +179 (T/C) | Intron 1 | rs2302004 | Asthma | [144] |
| Ulcerative colitis | [145] | ||||
| SNP +275 (C/T) | Intron 1 | rs2302005 | Asthma | [144] | |
| Ulcerative colitis | [145] | ||||
| SNP +1265 (A/G) | Intron 2 | rs11465310 | Asthma | [47, [48] | |
| CCL26 | SNP +77 (C/T) | Intron 2 | rs2240478 | Asthma | [144] |
| SNP +1577 (G/A) | Intron 3 | rs6965556 | Rheumatoid arthritis | [146] | |
| SNP +2497 (T/G) | 3′UTR | rs2302009 | Rheumatoid arthritis | [146] | |
| Asthma | [144] | ||||
| Allergic rhinitis | [147] |
CCL3 [macrophage inflammatory protein (MIP)-1α], CCL4 (MIP-1β), CCL4L [lymphocyte activation gene (LAG)-1] and CCL5 [regulated upon activation normal T cell expressed and secreted (RANTES)] have a diversity of polymorphisms that have an important impact on susceptibility to HIV-1 infection. This is not surprising, as they are ligands of the CCR5 receptor, which is the co-receptor used by HIV M-tropic strains to enter into the cells. Haplotypes defined on the region containing the genes CCL18, CCL3 and CCL4 (chromosome 17 q11–q21) have been found to be associated with HIV infection susceptibility and progression [35]. Although CCL18 has not yet been implicated in HIV-1/AIDS pathogenesis and its receptor is not known, this genetic analysis points to this gene as a candidate for modulating HIV-1 pathogenesis. CCL5 haplotypes have also been shown to influence the clinical progression of HIV infection [36,37]. Two interesting SNPs in the CCL4L gene have been associated with different aspects of HIV-1 infection: (a) the +590A/G is located at the intron 2 acceptor splice site. The G allele disrupts the original acceptor splice site and provokes a new complex transcription pattern. This allele modifies susceptibility to HIV-1 infection [38]. (b) The +59C/T is located in exon 2, leading to an amino acid change (R22H). The H variant has been associated with a lower overall survival of HIV-1 infected individuals [39]. Three CCL5 individual SNPs have also been associated with HIV-1 infection, two of them located in the promoter region (−403G/A and −28C/G) and the other in intron 1 (In1.1T/C). It has been demonstrated clearly that the −403A and −28G alleles enhance CCL5 production [40–43] and, conversely, the In1.1C allele reduces CCL5 gene transcription [37]. The two CCL5 promoter polymorphisms, −403G/A and −28C/G, have also been associated with a variety of other diseases such as allergic diseases (i.e. asthma, atopy, allergic rhinitis and atopic dermatitis), inflammatory diseases [i.e. SLE, MS, rheumatoid arthritis (RA), sarcoidosis and polymyalgia rheumatica] and infectious diseases (i.e. HIV-1 and HCV). Additionally, the −403G/A polymorphism has been found to be associated with metabolic risk-related conditions such as hypercholesterolaemia, coronary arteriosclerosis and cardiac mortality in type 2 diabetes.
CCL11 (eotaxin-1), CCL24 (eotaxin-2) and CCL26 (eotaxin-3) are CCR3 ligands and potent eosinophil chemoattractants, playing a fundamental role in asthma and other allergic diseases and in eosinophil-associatedgastrointestinal diseases. Not unexpectedly, SNPs in these three chemokines have been found to be associated with allergic diseases such as asthma, allergic rhinitis and atopic dermatitis. Four SNPs of the CCL11 gene have been found to be associated independently with asthma in several studies [44–47]. Three of them are located in the promoter region (−576C/T, −426C/T and −384 A/G) and the other (+67G/A) in the signal peptide (exon 1) leading to an amino acid change (T23A). Interestingly, both the −384G and +67A alleles are associated with lower CCL11 production [44,46]. The three polymorphisms of the CCL24 gene associated with asthma are intronic SNPs: +179TC and +275C/T in intron 1 and +1265A/G in intron 2. There are data indicating that the +1265A allele is associated with lower CCL24 levels than the G allele [48]). Finally, CCL26 has two SNPs affecting asthma differently: the +2497T/G (in the 3′UTR region) have been associated with susceptibility and the +77C/T (in intron 2) seem to play a critical role in attracting eosinophils and maintaining high IgE levels.
Regarding the C and CX3C subfamilies, no relevant polymorphisms in their members have so far been described.
Transcriptional variability: alternative splicing in the chemokine superfamily
The mRNA of several chemokines is known to undergo alternative splicing (Table 3), some of them with repercussions in the molecular activity and/or in the tissue distribution of the differentially spliced variants. However, to date, there are no reports on their implication in disease pathogenesis.
Table 3.
Alternative splicing in the human chemokine superfamily.
| Ligand | Splicing phenomena | Isoforms | Length | Isoforms identity | Refs |
|---|---|---|---|---|---|
| CXCL12 | Exon 4 alternative splicing | SDF-1α | 68 aa | All variants share the same first three exons but contain different fourth exons | [49, 50, 148] |
| SDF-1β | 72 aa | ||||
| SDF-1γ | 98 aa | ||||
| SDF-1δ | 119 aa | ||||
| SDF-1ε | 69 aa | ||||
| SDF-1φ | 79 aa | ||||
| CCL4 | Exon 2 skipping | CCL4 | 69 aa | The Δ2 isoform keeps only the two first amino acids due to a frameshift | [38] |
| CCL4Δ2 | |||||
| CCL4L1 | Exon 2 skipping | CCL4L1 | 69 aa | The Δ2 isoform keeps only the two first amino acids due to a frameshift | [38] |
| CCL4L1Δ2 | 29 aa | ||||
| CCL4L2 | Alternative acceptor splice sites in exon 3 | CCL4L2 | 64 aa | The Δ2 isoforms keep only the two first amino acids due to a frameshift | [38] |
| Exon 2 skipping | CCL4L2Δ2 | 24 aa | The rest of the isoforms share the same first two exons but contain different third exons | ||
| CCL4L2b | 41 aa | ||||
| CCL4L2bΔ2 | 45 aa | ||||
| CCL4L2c | 80 aa | ||||
| CCL4L2d | 73 aa | ||||
| CCL4L2e | 63 aa | ||||
| CCL4L2f | 80 aa | ||||
| CCL20 | Alternative acceptor splice site in exon 2 | CCL20 Ala | 70 aa | 100% (CCL20 Ala has 1 additional aa in N-terminus) | [51, 52] |
| CCL20 Ser | 69 aa | ||||
| CCL23 | Alternative acceptor splice site in exon 3 | CKβ8-1 | 116 aa | 99% (CK8 lacks 17 aa before the two first cysteines) | [53, 54] |
| CKβ8 | 99 aa | ||||
| CCL27 | Alternative first exon usage | CCL27 | 95 aa | PESKY and canonical CCL27 differ only in the first of three exons | [56, 57, 149] |
| Intron retention | PESKY | 127 aa | The partially spliced and unspliced variants of CCL27 retains the intron 1 and intron 1 and 2, respectively | ||
| CCL27 unspliced | 32 aa | ||||
| CCL27 partially spliced | 32 aa |
CXCL12 is the only CXC chemokine known to generate isoforms by alternative splicing. The two main splice forms of CXCL12 (SDF-1α and SDF-1β) have similar amino acid sequences except for the presence of four additional amino acids at the carboxy terminus of SDF-1β. Both isoforms display a similar tissue expression pattern, but SDF-1α mRNA can be detected in the adult human brain, whereas SDF-1β cannot. The two isoforms are subjected to different proteolytic processing, and this fact could explain functional differences [49]. Recently, four additional human SDF-1 isoforms derived from alternative splicing events have been identified (SDF-1γ, SDF-1δ, SDF-1ε and SDF-1φ), showing some differential distribution of tissue expression [50].
CCL4 and CCL4L, two closely related chemokines, have different isoforms due to alternative splicing. Both chemokines have exon 2 skipped variants that keep only the two first amino acids from the original protein due to a frameshift in the new junction between exon 1 and exon 3. Additionally, CCL4L2 (an allelic variant of CCL4L) has a nucleotide change in the acceptor splice site of intron 2 leading to a complex transcription pattern due to multiple usage of new alternative acceptor splice sites surrounding the original mutated one [38].
Two alternative splice isoforms of CCL20 have been identified, resulting from the alternative usage of two potential acceptor splice sites separated by three nucleotides in the junction of intron 1 and exon 2. The longer form (CCL20Ala) has an alanine (Ala27) as its predicted N-terminal amino acid, whereas the deletion of Ala27 leads to Ser27 as the predicted N-terminal amino acid in the short form (CCL20Ser) [51,52]. The biological activity of CCL20Ala and CCL20Ser and the tissue-specific preference of different acceptor splice-sites usages are not yet known.
CCL23 has two variants originated by alternative splicing in exon 3: the originally described CKβ8 and the splicing variant CKβ8-1, which is 17 amino acids longer. The mature proteins CKβ8 and CKβ8-1 consist of 99 and 116 amino acids, respectively. It has been shown that CKβ8 differed from CKβ8-1 in the monocyte chemoattraction and in the binding to human formyl peptide-receptor-like-1 (FPRL-1), suggesting that these two CCL23 isoforms could possibly have different a kinetic and specificity of chemotactic function in vivo [53,54].
Finally, CCL27 is produced as two splice variants. One of these variants encodes a classical chemokine with an associated signal peptide (CCL27), while the other variant (PESKY) maintains the sequence of the mature chemokine, but the signal peptide has been replaced by an alternative stretch of amino acids that directs this isoform to the nucleus where it modulates transcription. Surprisingly, secreted CCL27 can also reach the nucleus after CCR10-mediated internalization, and in this way directly modulates transcription and influences several cellular processes [55]. Expression studies have revealed differential tissue expression of CCL27 and PESKY. Interestingly, while CCL27 is highly expressed in the placenta, PESKY is expressed mainly in the testes and brain and weakly in the developing embryo [56]. Recently, several novel CCL27 variants have been identified in mouse but their presence in humans has not yet been demonstrated [57].
Concluding remarks
The high variability of the chemokine superfamily includes mechanisms of genomic and transcriptional variation. There is already a good number of well-described polymorphisms of chemokines with functional relevance and we made a detailed review of those involved significantly in disease. In spite of the many reports on the association of these polymorphisms to diseases, there are still confusing and contradictory data. Many factors in the epidemiological investigation could explain this phenomenon (covered widely in several reviews [58–60]), but it is clear that further studies are necessary to define more clearly the role of genetic variants of chemokines in disease. The recently developed high-throughput methods for SNP genotyping should make it easy to carry out larger association studies using a high number of SNPs, covering from one or a few genes (candidate gene approach) to the whole genome (genome-wide approach). In fact, the single SNP association studies are currently being replaced by the haplotype-based studies using tagSNPs, as this approach ensures the capture of most of the genetic variation in a relatively transferable manner among global populations [61]. With regard to the alternative splicing phenomena in the chemokine superfamily, several members with different splice variants have been identified but there are still few available data about its functional role. Molecular analyses during the last decade demonstrate that alternative splicing determines the binding properties, intracellular localization, enzymatic activity, protein stability and post-translational modifications of a large number of proteins [62,63]. Efforts are now being directed at establishing the full repertoire of functionally relevant transcript variants generated by alternative splicing, the specific roles of such variants in normal and disease physiology, and how alternative splicing is co-ordinated on a global level to achieve cell- and tissue-specific functions. Although the interaction between all these factors will probably provide us with the true key to understanding their real effect on pathology, future studies will be necessary to achieve all these goals in the chemokine superfamily.
Acknowledgments
This work was supported by grants from the FIPSE (Fundación para la Investigación y la Prevención del Sida en España) (project 36487/05), FIS (Fondo de Investigaciones Sanitarias) (project PI 02/0104) and PEI (Pla Estratègic d'Investigació) from BST (Banc de Sang i Teixits).
References
- 1.Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Exp Immunol. 2007;148:208–17. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookes AJ. The essence of SNPs. Gene. 1999;234:177–86. doi: 10.1016/s0378-1119(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 3.Modrek B, Resch A, Grasso C, Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucl Acids Res. 2001;29:2850–9. doi: 10.1093/nar/29.13.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JM, Castle J, Garrett-Engele P, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 6.Fujihara J, Shiwaku K, Yasuda T, et al. Variation of interleukin 8 −251 A>T polymorphism in worldwide populations and intra-ethnic differences in Japanese populations. Clin Chim Acta. 2007;377:79–82. doi: 10.1016/j.cca.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Matsuo K, Hamajima N, Okuma K, Saito T, Tajima K. Significant association of interleukin 8 −251T/A polymorphism with smoking behavior in a Japanese population. J Hum Genet. 2005;50:567–73. doi: 10.1007/s10038-005-0296-y. [DOI] [PubMed] [Google Scholar]
- 8.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–7. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull J, Ackerman H, Isles K, et al. Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virus. Am J Hum Genet. 2001;69:413–9. doi: 10.1086/321291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang ZD, Okhuysen PC, Guo DC, et al. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J Infect Dis. 2003;188:506–11. doi: 10.1086/377102. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi A, Ohmiya N, Shirai K, et al. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487–93. doi: 10.1158/1055-9965.EPI-05-0326. [DOI] [PubMed] [Google Scholar]
- 12.Lee WP, Tai DI, Lan KH, et al. The -251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res. 2005;11:6431–41. doi: 10.1158/1078-0432.CCR-05-0942. [DOI] [PubMed] [Google Scholar]
- 13.Heinzmann A, Ahlert I, Kurz T, Berner R, Deichmann KA. Association study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2004;114:671–6. doi: 10.1016/j.jaci.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Hacking D, Knight JC, Rockett K, et al. Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibility. Genes Immun. 2004;5:274–82. doi: 10.1038/sj.gene.6364067. [DOI] [PubMed] [Google Scholar]
- 15.Puthothu B, Krueger M, Heinze J, Forster J, Heinzmann A. Impact of IL8 and IL8-Receptor alpha polymorphisms on the genetics of bronchial asthma and severe RSV infections. Clin Mol Allergy. 2006;4:2. doi: 10.1186/1476-7961-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovin BH, Lu L, Zhang X. A novel interleukin-8 polymorphism is associated with severe systemic lupus erythematosus nephritis. Kidney Int. 2002;62:261–5. doi: 10.1046/j.1523-1755.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 17.Artifoni L, Negrisolo S, Montini G, et al. Interleukin-8 and CXCR1 receptor functional polymorphisms and susceptibility to acute pyelonephritis. J Urol. 2007;177:1102–6. doi: 10.1016/j.juro.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Amoli MM, Thomson W, Hajeer AH, et al. Interleukin 8 gene polymorphism is associated with increased risk of nephritis in cutaneous vasculitis. J Rheumatol. 2002;29:2367–70. [PubMed] [Google Scholar]
- 19.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 20.Winkler C, Modi W, Smith MW, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998;279:389–93. doi: 10.1126/science.279.5349.389. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) [DOI] [PubMed] [Google Scholar]
- 21.Vissoci Reiche EM, Ehara Watanabe MA, Bonametti AM, et al. The effect of stromal cell-derived factor 1 (SDF1/CXCL12) genetic polymorphism on HIV-1 disease progression. Int J Mol Med. 2006;18:785–93. [PubMed] [Google Scholar]
- 22.van Rij RP, Broersen S, Goudsmit J, Coutinho RA, Schuitemaker H. The role of a stromal cell-derived factor-1 chemokine gene variant in the clinical course of HIV-1 infection. Aids. 1998;12:F85–90. [PubMed] [Google Scholar]
- 23.Brambilla A, Villa C, Rizzardi G, et al. Shorter survival of SDF1–3′A/3′A homozygotes linked to CD4+ T cell decrease in advanced human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:311–5. doi: 10.1086/315650. [DOI] [PubMed] [Google Scholar]
- 24.Soriano A, Martinez C, Garcia F, et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3′A genotype, and expression of CXCR4 on T lymphocytes: their impact on resistance to human immunodeficiency virus type 1 infection and its progression. J Infect Dis. 2002;186:922–31. doi: 10.1086/343741. [DOI] [PubMed] [Google Scholar]
- 25.Arya SK, Ginsberg CC, Davis-Warren A, D'Costa J. In vitro phenotype of SDF1 gene mutant that delays the onset of human immunodeficiency virus disease in vivo. J Hum Virol. 1999;2:133–8. [PubMed] [Google Scholar]
- 26.Kimura R, Nishioka T, Ishida T. The SDF1-G801A polymorphism is not associated with SDF1 gene expression in Epstein–Barr virus-transformed lymphoblastoid cells. Genes Immun. 2003;4:356–61. doi: 10.1038/sj.gene.6363978. [DOI] [PubMed] [Google Scholar]
- 27.Kimura R, Nishioka T, Soemantri A, Ishida T. Allele-specific transcript quantification detects haplotypic variation in the levels of the SDF-1 transcripts. Hum Mol Genet. 2005;14:1579–85. doi: 10.1093/hmg/ddi166. [DOI] [PubMed] [Google Scholar]
- 28.Flores C, Maca-Meyer N, Perez-Mendez L, et al. A CXCL2 tandem repeat promoter polymorphism is associated with susceptibility to severe sepsis in the Spanish population. Genes Immun. 2006;7:141–9. doi: 10.1038/sj.gene.6364280. [DOI] [PubMed] [Google Scholar]
- 29.Helbig KJ, George J, Beard MR. A novel I-TAC promoter polymorphic variant is functional in the presence of replicating HCV in vitro. J Clin Virol. 2005;32:137–43. doi: 10.1016/j.jcv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Lundberg GA, Kellin A, Samnegard A, et al. Severity of coronary artery stenosis is associated with a polymorphism in the CXCL16/SR-PSOX gene. J Intern Med. 2005;257:415–22. doi: 10.1111/j.1365-2796.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- 31.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–8. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 32.Jibiki T, Terai M, Shima M, et al. Monocyte chemoattractant protein 1 gene regulatory region polymorphism and serum levels of monocyte chemoattractant protein 1 in Japanese patients with Kawasaki disease. Arthritis Rheum. 2001;44:2211–2. doi: 10.1002/1529-0131(200109)44:9<2211::aid-art375>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez E, Rovin BH, Sen L, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muhlbauer M, Bosserhoff AK, Hartmann A, et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085–93. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 35.Modi WS, Lautenberger J, An P, et al. Genetic variation in the CCL18–CCL3–CCL4 chemokine gene cluster influences HIV Type 1 transmission and AIDS disease progression. Am J Hum Genet. 2006;79:120–8. doi: 10.1086/505331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez E, Dhanda R, Bamshad M, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1alpha: impact on the epidemiology of the HIV-1 pandemic. Proc Natl Acad Sci USA. 2001;98:5199–204. doi: 10.1073/pnas.091056898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An P, Nelson GW, Wang L, et al. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc Natl Acad Sci USA. 2002;99:10002–7. doi: 10.1073/pnas.142313799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colobran R, Adreani P, Ashhab Y, et al. Multiple products derived from two CCL4 loci: high incidence of a new polymorphism in HIV+ patients. J Immunol. 2005;174:5655–64. doi: 10.4049/jimmunol.174.9.5655. [DOI] [PubMed] [Google Scholar]
- 39.Capoulade-Metay C, Meyer L, Tran T, et al. Influence of the R22H variant of macrophage inflammatory protein 1beta/Lag-1 in HIV-1 survival. Aids. 2005;19:831–3. doi: 10.1097/01.aids.0000168979.97584.18. [DOI] [PubMed] [Google Scholar]
- 40.Nickel RG, Casolaro V, Wahn U, et al. Atopic dermatitis is associated with a functional mutation in the promoter of the C-C chemokine RANTES. J Immunol. 2000;164:1612–6. doi: 10.4049/jimmunol.164.3.1612. [DOI] [PubMed] [Google Scholar]
- 41.Bai B, Tanaka K, Tazawa T, Yamamoto N, Sugiura H. Association between RANTES promoter polymorphism -401A and enhanced RANTES production in atopic dermatitis patients. J Dermatol Sci. 2005;39:189–91. doi: 10.1016/j.jdermsci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K, Roberts MH, Yamamoto N, Sugiura H, Uehara M, Hopkin JM. Upregulating promoter polymorphisms of RANTES relate to atopic dermatitis. Int J Immunogenet. 2006;33:423–8. doi: 10.1111/j.1744-313X.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Chao D, Nakayama EE, et al. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci U S A. 1999;96:4581–5. doi: 10.1073/pnas.96.8.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang HS, Kim JS, Lee JH, et al. A single nucleotide polymorphism on the promoter of eotaxin 1 associates with its mRNA expression and asthma phenotypes. J Immunol. 2005;174:1525–31. doi: 10.4049/jimmunol.174.3.1525. [DOI] [PubMed] [Google Scholar]
- 45.Raby BA, Van Steen K, Lazarus R, Celedon JC, Silverman EK, Weiss ST. Eotaxin polymorphisms and serum total IgE levels in children with asthma. J Allergy Clin Immunol. 2006;117:298–305. doi: 10.1016/j.jaci.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura H, Luster AD, Nakamura T, et al. Variant eotaxin: its effects on the asthma phenotype. J Allergy Clin Immunol. 2001;108:946–53. doi: 10.1067/mai.2001.120135. [DOI] [PubMed] [Google Scholar]
- 47.Shin HD, Kim LH, Park BL, et al. Association of eotaxin gene family with asthma and serum total IgE. Hum Mol Genet. 2003;12:1279–85. doi: 10.1093/hmg/ddg142. [DOI] [PubMed] [Google Scholar]
- 48.Min JW, Lee JH, Park CS, et al. Association of eotaxin-2 gene polymorphisms with plasma eotaxin-2 concentration. J Hum Genet. 2005;50:118–23. doi: 10.1007/s10038-005-0230-3. [DOI] [PubMed] [Google Scholar]
- 49.De La Luz Sierra M, Yang F, Narazaki M, et al. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–9. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 50.Yu L, Cecil J, Peng SB, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–9. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Nelson RT, Boyd J, Gladue RP, et al. Genomic organization of the CC chemokine mip-3alpha/CCL20/larc/exodus/SCYA20, showing gene structure, splice variants, and chromosome localization. Genomics. 2001;73:28–37. doi: 10.1006/geno.2001.6482. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka Y, Imai T, Baba M, et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–42. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 53.Youn BS, Zhang SM, Broxmeyer HE, et al. Characterization of CKbeta8 and CKbeta8-1: two alternatively spliced forms of human beta-chemokine, chemoattractants for neutrophils, monocytes, and lymphocytes, and potent agonists at CC chemokine receptor 1. Blood. 1998;91:3118–26. [PubMed] [Google Scholar]
- 54.Elagoz A, Henderson D, Babu PS, et al. A truncated form of CKbeta8-1 is a potent agonist for human formyl peptide-receptor-like 1 receptor. Br J Pharmacol. 2004;141:37–46. doi: 10.1038/sj.bjp.0705592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nibbs RJ, Graham GJ. CCL27/PESKY: a novel paradigm for chemokine function. Expert Opin Biol Ther. 2003;3:15–22. doi: 10.1517/14712598.3.1.15. [DOI] [PubMed] [Google Scholar]
- 56.Baird JW, Nibbs RJ, Komai-Koma M, et al. ESkine, a novel beta-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J Biol Chem. 1999;274:33496–503. doi: 10.1074/jbc.274.47.33496. [DOI] [PubMed] [Google Scholar]
- 57.Ledee DR, Chen J, Tonelli LH, Takase H, Gery I, Zelenka PS. Differential expression of splice variants of chemokine CCL27 mRNA in lens, cornea, and retina of the normal mouse eye. Mol Vis. 2004;10:663–7. [PubMed] [Google Scholar]
- 58.Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet. 2002;3:391–7. doi: 10.1038/nrg796. [DOI] [PubMed] [Google Scholar]
- 59.Hoh J, Ott J. Genetic dissection of diseases: design and methods. Curr Opin Genet Dev. 2004;14:229–32. doi: 10.1016/j.gde.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 61.Gu S, Pakstis AJ, Li H, Speed WC, Kidd JR, Kidd KK. Significant variation in haplotype block structure but conservation in tagSNP patterns among global populations. Eur J Hum Genet. 2007;15:302–12. doi: 10.1038/sj.ejhg.5201751. [DOI] [PubMed] [Google Scholar]
- 62.Blencowe BJ. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Stamm S, Ben-Ari S, Rafalska I, et al. Function of alternative splicing. Gene. 2005;344:1–20. doi: 10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Emi M, Keicho N, Tokunaga K, et al. Association of diffuse panbronchiolitis with microsatellite polymorphism of the human interleukin 8 (IL-8) gene. J Hum Genet. 1999;44:169–72. doi: 10.1007/s100380050135. [DOI] [PubMed] [Google Scholar]
- 65.Vairaktaris E, Yapijakis C, Serefoglou Z, et al. The interleukin-8 (-251A/T) polymorphism is associated with increased risk for oral squamous cell carcinoma. Eur J Surg Oncol. 2006;33:504–7. doi: 10.1016/j.ejso.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Jiang ZD, DuPont HL, Garey K, et al. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am J Gastroenterol. 2006;101:1112–6. doi: 10.1111/j.1572-0241.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 67.Gyulai Z, Klausz G, Tiszai A, et al. Genetic polymorphism of interleukin-8 (IL-8) is associated with Helicobacter pylori-induced duodenal ulcer. Eur Cytokine Netw. 2004;15:353–8. [PubMed] [Google Scholar]
- 68.Ohyauchi M, Imatani A, Yonechi M, et al. The polymorphism interleukin 8–251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330–5. doi: 10.1136/gut.2003.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato I, van Doorn LJ, Canzian F, et al. Host–bacterial interaction in the development of gastric precancerous lesions in a high risk population for gastric cancer in Venezuela. Int J Cancer. 2006;119:1666–71. doi: 10.1002/ijc.21979. [DOI] [PubMed] [Google Scholar]
- 70.Hofner P, Gyulai Z, Kiss ZF, et al. Genetic polymorphisms of NOD1 and IL-8, but not polymorphisms of TLR4 genes, are associated with Helicobacter pylori-induced duodenal ulcer and gastritis. Helicobacter. 2007;12:124–31. doi: 10.1111/j.1523-5378.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 71.Hofner P, Balog A, Gyulai Z, et al. Polymorphism in the IL-8 gene, but not in the TLR4 gene, increases the severity of acute pancreatitis. Pancreatology. 2006;6:542–8. doi: 10.1159/000097363. [DOI] [PubMed] [Google Scholar]
- 72.Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–6. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 73.Shirai K, Ohmiya N, Taguchi A, et al. Interleukin-8 gene polymorphism associated with susceptibility to non-cardiac gastric carcinoma with microsatellite instability. J Gastroenterol Hepatol. 2006;21:1129–35. doi: 10.1111/j.1440-1746.2006.04443.x. [DOI] [PubMed] [Google Scholar]
- 74.Landi S, Moreno V, Gioia-Patricola L, et al. Association of common polymorphisms in inflammatory genes interleukin (IL) 6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560–6. [PubMed] [Google Scholar]
- 75.McCarron SL, Edwards S, Evans PR, et al. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369–72. [PubMed] [Google Scholar]
- 76.Ross OA, O'Neill C, Rea IM, et al. Functional promoter region polymorphism of the proinflammatory chemokine IL-8 gene associates with Parkinson's disease in the Irish. Hum Immunol. 2004;65:340–6. doi: 10.1016/j.humimm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 77.Kamali-Sarvestani E, Nikseresht AR, Aliparasti MR, Vessal M. IL-8 (-251 A/T) and CXCR2 (+1208 C/T) gene polymorphisms and risk of multiple sclerosis in Iranian patients. Neurosci Lett. 2006;404:159–62. doi: 10.1016/j.neulet.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 78.Infante J, Llorca J, Berciano J, Combarros O. Interleukin-8, intercellular adhesion molecule-1 and tumour necrosis factor-alpha gene polymorphisms and the risk for multiple system atrophy. J Neurol Sci. 2005;228:11–13. doi: 10.1016/j.jns.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 79.van der Kuyl AC, Polstra AM, Weverling GJ, Zorgdrager F, van den Burg R, Cornelissen M. An IL-8 gene promoter polymorphism is associated with the risk of the development of AIDS-related Kaposi's sarcoma: a case–control study. Aids. 2004;18:1206–8. doi: 10.1097/00002030-200405210-00016. [DOI] [PubMed] [Google Scholar]
- 80.Lee EB, Kim JY, Zhao J, Park MH, Song YW. Haplotype association of IL-8 gene with Behcet's disease. Tissue Antigens. 2007;69:128–32. doi: 10.1111/j.1399-0039.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 81.Galimberti D, Scalabrini D, Fenoglio C, et al. CXCL10 haplotypes and multiple sclerosis: association and correlation with clinical course. Eur J Neurol. 2007;14:162–7. doi: 10.1111/j.1468-1331.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 82.Coll B, Alonso-Villaverde C, Parra S, et al. The stromal derived factor-1 mutated allele (SDF1-3′A) is associated with a lower incidence of atherosclerosis in HIV-infected patients. Aids. 2005;19:1877–83. doi: 10.1097/01.aids.0000183516.22266.dd. [DOI] [PubMed] [Google Scholar]
- 83.Razmkhah M, Talei AR, Doroudchi M, Khalili-Azad T, Ghaderi A. Stromal cell-derived factor-1 (SDF-1) alleles and susceptibility to breast carcinoma. Cancer Lett. 2005;225:261–6. doi: 10.1016/j.canlet.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 84.Razmkhah M, Doroudchi M, Ghayumi SM, Erfani N, Ghaderi A. Stromal cell-derived factor-1 (SDF-1) gene and susceptibility of Iranian patients with lung cancer. Lung Cancer. 2005;49:311–5. doi: 10.1016/j.lungcan.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 85.Zafiropoulos A, Crikas N, Passam AM, Spandidos DA. Significant involvement of CCR2-64I and CXCL12-3a in the development of sporadic breast cancer. J Med Genet. 2004;41:e59. doi: 10.1136/jmg.2003.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dommange F, Cartron G, Espanel C, et al. CXCL12 polymorphism and malignant cell dissemination/tissue infiltration in acute myeloid leukemia. FASEB J. 2006;20:1913–5. doi: 10.1096/fj.05-5667fje. [DOI] [PubMed] [Google Scholar]
- 87.de Oliveira Cavassin GG, De Lucca FL, Delgado Andre N, et al. Molecular investigation of the stromal cell-derived factor-1 chemokine in lymphoid leukemia and lymphoma patients from Brazil. Blood Cells Mol Dis. 2004;33:90–3. doi: 10.1016/j.bcmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Gerli G, Vanelli C, Turri O, et al. SDF1–3′A gene polymorphism is associated with chronic myeloproliferative disease and thrombotic events. Clin Chem. 2005;51:2411–4. doi: 10.1373/clinchem.2005.057802. [DOI] [PubMed] [Google Scholar]
- 89.Schroppel B, Fischereder M, Ashkar R, et al. The impact of polymorphisms in chemokine and chemokine receptors on outcomes in liver transplantation. Am J Transplant. 2002;2:640–5. doi: 10.1034/j.1600-6143.2002.20709.x. [DOI] [PubMed] [Google Scholar]
- 90.Ide A, Kawasaki E, Abiru N, et al. Stromal-cell derived factor-1 chemokine gene variant is associated with type 1 diabetes age at onset in Japanese population. Hum Immunol. 2003;64:973–8. doi: 10.1016/s0198-8859(03)00176-9. [DOI] [PubMed] [Google Scholar]
- 91.Takabatake N, Shibata Y, Abe S, et al. A single nucleotide polymorphism in the CCL1 gene predicts acute exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:875–85. doi: 10.1164/rccm.200603-443OC. [DOI] [PubMed] [Google Scholar]
- 92.Karrer S, Bosserhoff AK, Weiderer P, et al. The -2518 promotor polymorphism in the MCP-1 gene is associated with systemic sclerosis. J Invest Dermatol. 2005;124:92–8. doi: 10.1111/j.0022-202X.2004.23512.x. [DOI] [PubMed] [Google Scholar]
- 93.Szalai C, Kozma GT, Nagy A, et al. Polymorphism in the gene regulatory region of MCP-1 is associated with asthma susceptibility and severity. J Allergy Clin Immunol. 2001;108:375–81. doi: 10.1067/mai.2001.117930. [DOI] [PubMed] [Google Scholar]
- 94.Keszei M, Nagy A, Kozma GT, et al. Pediatric asthmatic patients have low serum levels of monocyte chemoattractant protein-1. J Asthma. 2006;43:399–404. doi: 10.1080/02770900600710433. [DOI] [PubMed] [Google Scholar]
- 95.Tucci M, Barnes EV, Sobel ES, et al. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004;50:1842–9. doi: 10.1002/art.20266. [DOI] [PubMed] [Google Scholar]
- 96.Ye DQ, Hu YS, Li XP, et al. The correlation between monocyte chemoattractant protein-1 and the arthritis of systemic lupus erythematosus among Chinese. Arch Dermatol Res. 2005;296:366–71. doi: 10.1007/s00403-004-0531-y. [DOI] [PubMed] [Google Scholar]
- 97.Kim HL, Lee DS, Yang SH, et al. The polymorphism of monocyte chemoattractant protein-1 is associated with the renal disease of SLE. Am J Kidney Dis. 2002;40:1146–52. doi: 10.1053/ajkd.2002.36858. [DOI] [PubMed] [Google Scholar]
- 98.Ozyurek AR, Gurses D, Ulger Z, Levent E, Bakiler AR, Berdeli A. Allelic frequency of the MCP-1 promoter -2518 polymorphism in the Turkish population and in Turkish patients with juvenile rheumatoid arthritis. Clin Rheumatol. 2006;26:546–50. doi: 10.1007/s10067-006-0347-6. [DOI] [PubMed] [Google Scholar]
- 99.Kruger B, Schroppel B, Ashkan R, et al. A monocyte chemoattractant protein-1 (MCP-1) polymorphism and outcome after renal transplantation. J Am Soc Nephrol. 2002;13:2585–9. doi: 10.1097/01.asn.0000031701.53792.54. [DOI] [PubMed] [Google Scholar]
- 100.Ghilardi G, Biondi ML, La Torre A, Battaglioli L, Scorza R. Breast cancer progression and host polymorphisms in the chemokine system: role of the macrophage chemoattractant protein-1 (MCP-1) -2518 G allele. Clin Chem. 2005;51:452–5. doi: 10.1373/clinchem.2004.041657. [DOI] [PubMed] [Google Scholar]
- 101.Omori K, Kazama JJ, Song J, et al. Association of the MCP-1 gene polymorphism A-2518G with carpal-tunnel syndrome in hemodialysis patients. Amyloid. 2002;9:175–82. doi: 10.3109/13506120209114819. [DOI] [PubMed] [Google Scholar]
- 102.Mori H, Kaneko Y, Narita I, et al. Monocyte chemoattractant protein-1 A-2518G gene polymorphism and renal survival of Japanese patients with immunoglobulin A nephropathy. Clin Exp Nephrol. 2005;9:297–303. doi: 10.1007/s10157-005-0375-6. [DOI] [PubMed] [Google Scholar]
- 103.Wegscheider BJ, Weger M, Renner W, et al. Role of the CCL2/MCP-1-2518A>G gene polymorphism in HLA-B27 associated uveitis. Mol Vis. 2005;11:896–900. [PubMed] [Google Scholar]
- 104.Szalai C, Duba J, Prohaszka Z, et al. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1-2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233–9. doi: 10.1016/s0021-9150(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 105.Kim MP, Wahl LM, Yanek LR, Becker DM, Becker LC. A monocyte chemoattractant protein-1 gene polymorphism is associated with occult ischemia in a high-risk asymptomatic population. Atherosclerosis. 2006;193:366–72. doi: 10.1016/j.atherosclerosis.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 106.McDermott DH, Yang Q, Kathiresan S, et al. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112:1113–20. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- 107.Pola R, Flex A, Gaetani E, et al. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism and risk of Alzheimer's disease in Italians. Exp Gerontol. 2004;39:1249–52. doi: 10.1016/j.exger.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Pae CU, Yu HS, Kim TS, et al. Monocyte chemoattractant protein-1 (MCP1) promoter -2518 polymorphism may confer a susceptibility to major depressive disorder in the Korean population. Psychiatry Res. 2004;127:279–81. doi: 10.1016/j.psychres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Yang B, Houlberg K, Millward A, Demaine A. Polymorphisms of chemokine and chemokine receptor genes in Type 1 diabetes mellitus and its complications. Cytokine. 2004;26:114–21. doi: 10.1016/j.cyto.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Simeoni E, Hoffmann MM, Winkelmann BR, et al. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia. 2004;47:1574–80. doi: 10.1007/s00125-004-1494-4. [DOI] [PubMed] [Google Scholar]
- 111.Flores-Villanueva PO, Ruiz-Morales JA, Song CH, et al. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med. 2005;202:1649–58. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramasawmy R, Cunha-Neto E, Fae KC, et al. The monocyte chemoattractant protein-1 gene polymorphism is associated with cardiomyopathy in human chagas disease. Clin Infect Dis. 2006;43:305–11. doi: 10.1086/505395. [DOI] [PubMed] [Google Scholar]
- 113.Park BL, Kim YJ, Cheong HS, et al. Association of common promoter polymorphisms of MCP1 with hepatitis B virus clearance. Exp Mol Med. 2006;38:694–702. doi: 10.1038/emm.2006.82. [DOI] [PubMed] [Google Scholar]
- 114.Vyshkina T, Shugart YY, Birnbaum G, Leist TP, Kalman B. Association of haplotypes in the beta-chemokine locus with multiple sclerosis. Eur J Hum Genet. 2005;13:240–7. doi: 10.1038/sj.ejhg.5201295. [DOI] [PubMed] [Google Scholar]
- 115.Vyshkina T, Kalman B. Haplotypes within genes of beta-chemokines in 17q11 are associated with multiple sclerosis: a second phase study. Hum Genet. 2005;118:67–75. doi: 10.1007/s00439-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 116.Modi WS, Goedert JJ, Strathdee S, et al. MCP-1–MCP-3–Eotaxin gene cluster influences HIV-1 transmission. Aids. 2003;17:2357–65. doi: 10.1097/00002030-200311070-00011. [DOI] [PubMed] [Google Scholar]
- 117.Vyshkina T, Kalman B. Analyses of a MS-associated haplotype encompassing the CCL3 gene. J Neuroimmunol. 2006;176:216–8. doi: 10.1016/j.jneuroim.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 118.Kim JJ, Lee JH, Jang CH, et al. Chemokine RANTES promoter polymorphisms in allergic rhinitis. Laryngoscope. 2004;114:666–9. doi: 10.1097/00005537-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 119.Leung TF, Tang NL, Lam CW, et al. RANTES G-401A polymorphism is associated with allergen sensitization and FEV1 in Chinese children. Respir Med. 2005;99:216–9. doi: 10.1016/j.rmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 120.Fryer AA, Spiteri MA, Bianco A, et al. The -403 G–>A promoter polymorphism in the RANTES gene is associated with atopy and asthma. Genes Immun. 2000;1:509–14. doi: 10.1038/sj.gene.6363717. [DOI] [PubMed] [Google Scholar]
- 121.Al-Abdulhadi SA, Helms PJ, Main M, Smith O, Christie G. Preferential transmission and association of the -403 G–>A promoter RANTES polymorphism with atopic asthma. Genes Immun. 2005;6:24–30. doi: 10.1038/sj.gene.6364151. [DOI] [PubMed] [Google Scholar]
- 122.Ye DQ, Yang SG, Li XP, et al. Polymorphisms in the promoter region of RANTES in Han Chinese and their relationship with systemic lupus erythematosus. Arch Dermatol Res. 2005;297:108–13. doi: 10.1007/s00403-005-0581-9. [DOI] [PubMed] [Google Scholar]
- 123.Makki RF, al Sharif F, Gonzalez-Gay MA, Garcia-Porrua C, Ollier WE, Hajeer AH. RANTES gene polymorphism in polymyalgia rheumatica, giant cell arteritis and rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:391–3. [PubMed] [Google Scholar]
- 124.Wang CR, Guo HR, Liu MF. RANTES promoter polymorphism as a genetic risk factor for rheumatoid arthritis in the Chinese. Clin Exp Rheumatol. 2005;23:379–84. [PubMed] [Google Scholar]
- 125.Gade-Andavolu R, Comings DE, MacMurray J, et al. RANTES: a genetic risk marker for multiple sclerosis. Mult Scler. 2004;10:536–9. doi: 10.1191/1352458504ms1080oa. [DOI] [PubMed] [Google Scholar]
- 126.McDermott DH, Beecroft MJ, Kleeberger CA, et al. Chemokine RANTES promoter polymorphism affects risk of both HIV infection and disease progression in the Multicenter AIDS Cohort Study. Aids. 2000;14:2671–8. doi: 10.1097/00002030-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 127.Ahlenstiel G, Iwan A, Nattermann J, et al. Distribution and effects of polymorphic RANTES gene alleles in HIV/HCV coinfection − a prospective cross-sectional study. World J Gastroenterol. 2005;11:7631–8. doi: 10.3748/wjg.v11.i48.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hellier S, Frodsham AJ, Hennig BJ, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–76. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 129.Promrat K, McDermott DH, Gonzalez CM, et al. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352–60. doi: 10.1053/gast.2003.50061. [DOI] [PubMed] [Google Scholar]
- 130.Takada T, Suzuki E, Ishida T, et al. Polymorphism in RANTES chemokine promoter affects extent of sarcoidosis in a Japanese population. Tissue Antigens. 2001;58:293–8. doi: 10.1034/j.1399-0039.2001.580502.x. [DOI] [PubMed] [Google Scholar]
- 131.Simeoni E, Winkelmann BR, Hoffmann MM, et al. Association of RANTES G-403A gene polymorphism with increased risk of coronary arteriosclerosis. Eur Heart J. 2004;25:1438–46. doi: 10.1016/j.ehj.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Yamaguchi S, Yamada Y, Metoki N, et al. Genetic risk for atherothrombotic cerebral infarction in individuals stratified by sex or conventional risk factors for atherosclerosis. Int J Mol Med. 2006;18:871–83. [PubMed] [Google Scholar]
- 133.Boger CA, Fischereder M, Deinzer M, et al. RANTES gene polymorphisms predict all-cause and cardiac mortality in type 2 diabetes mellitus hemodialysis patients. Atherosclerosis. 2005;183:121–9. doi: 10.1016/j.atherosclerosis.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 134.Hizawa N, Yamaguchi E, Konno S, Tanino Y, Jinushi E, Nishimura M. A functional polymorphism in the RANTES gene promoter is associated with the development of late-onset asthma. Am J Respir Crit Care Med. 2002;166:686–90. doi: 10.1164/rccm.200202-090OC. [DOI] [PubMed] [Google Scholar]
- 135.Yao TC, Kuo ML, See LC, et al. The RANTES promoter polymorphism: a genetic risk factor for near-fatal asthma in Chinese children. J Allergy Clin Immunol. 2003;111:1285–92. doi: 10.1067/mai.2003.1506. [DOI] [PubMed] [Google Scholar]
- 136.Nakajima K, Tanaka Y, Nomiyama T, et al. RANTES promoter genotype is associated with diabetic nephropathy in type 2 diabetic subjects. Diabetes Care. 2003;26:892–8. doi: 10.2337/diacare.26.3.892. [DOI] [PubMed] [Google Scholar]
- 137.Liao CH, Yao TC, Chung HT, See LC, Kuo ML, Huang JL. Polymorphisms in the promoter region of RANTES and the regulatory region of monocyte chemoattractant protein-1 among Chinese children with systemic lupus erythematosus. J Rheumatol. 2004;31:2062–7. [PubMed] [Google Scholar]
- 138.Cooke GS, Tosh K, Ramaley PA, et al. A polymorphism that reduces RANTES expression is associated with protection from death in HIV-seropositive Ugandans with advanced disease. J Infect Dis. 2006;194:666–9. doi: 10.1086/505875. [DOI] [PubMed] [Google Scholar]
- 139.Zhernakova A, Alizadeh BZ, Eerligh P, et al. Genetic variants of RANTES are associated with serum RANTES level and protection for type 1 diabetes. Genes Immun. 2006;7:544–9. doi: 10.1038/sj.gene.6364326. [DOI] [PubMed] [Google Scholar]
- 140.Fiten P, Vandenbroeck K, Dubois B, et al. Microsatellite polymorphisms in the gene promoter of monocyte chemotactic protein-3 and analysis of the association between monocyte chemotactic protein-3 alleles and multiple sclerosis development. J Neuroimmunol. 1999;95:195–201. doi: 10.1016/s0165-5728(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 141.Nelissen I, Dubois B, Goris A, Ronsse I, Carton H, Opdenakker G. Gelatinase B, PECAM-1 and MCP-3 gene polymorphisms in Belgian multiple sclerosis. J Neurol Sci. 2002;200:43–8. doi: 10.1016/s0022-510x(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 142.Tsunemi Y, Saeki H, Nakamura K, et al. Eotaxin gene single nucleotide polymorphisms in the promoter and exon regions are not associated with susceptibility to atopic dermatitis, but two of them in the promoter region are associated with serum IgE levels in patients with atopic dermatitis. J Dermatol Sci. 2002;29:222–8. doi: 10.1016/s0923-1811(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 143.Zee RY, Cook NR, Cheng S, et al. Threonine for alanine substitution in the eotaxin (CCL11) gene and the risk of incident myocardial infarction. Atherosclerosis. 2004;175:91–4. doi: 10.1016/j.atherosclerosis.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 144.Chae SC, Lee YC, Park YR, et al. Analysis of the polymorphisms in eotaxin gene family and their association with asthma, IgE, and eosinophil. Biochem Biophys Res Commun. 2004;320:131–7. doi: 10.1016/j.bbrc.2004.05.136. [DOI] [PubMed] [Google Scholar]
- 145.Park YR, Choi SC, Lee ST, Kim KS, Chae SC, Chung HT. The association of eotaxin-2 and eotaxin-3 gene polymorphisms in a Korean population with ulcerative colitis. Exp Mol Med. 2005;37:553–8. doi: 10.1038/emm.2005.68. [DOI] [PubMed] [Google Scholar]
- 146.Chae SC, Park YR, Shim SC, Lee IK, Chung HT. Eotaxin-3 gene polymorphisms are associated with rheumatoid arthritis in a Korean population. Hum Immunol. 2005;66:314–20. doi: 10.1016/j.humimm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 147.Chae SC, Park YR, Oh GJ, Lee JH, Chung HT. The suggestive association of eotaxin-2 and eotaxin-3 gene polymorphisms in Korean population with allergic rhinitis. Immunogenetics. 2005;56:760–4. doi: 10.1007/s00251-004-0746-2. [DOI] [PubMed] [Google Scholar]
- 148.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 149.Gortz A, Nibbs RJ, McLean P, et al. The chemokine ESkine/CCL27 displays novel modes of intracrine and paracrine function. J Immunol. 2002;169:1387–94. doi: 10.4049/jimmunol.169.3.1387. [DOI] [PubMed] [Google Scholar]