Abstract
Human type II deiodinase is a master regulator of thyroid hormone activation in several tissues. In placenta, type II deiodinase mRNA levels and enzymatic activity are elevated only during the first trimester of pregnancy and then progressively decline. During this early stage, mitogens such as epidermal growth factor (EGF) have been shown to promote the proliferation of the trophoblast by acting through multiple mechanisms. Here we show that EGF modulates transcription of human type II deiodinase gene (Dio2) through distinct signaling pathways, leading to the assembly of a heterogeneous transcription factor complex. Gene expression and deiodination assays have shown that EGF promptly induces a short-lived Dio2 mRNA and enzymatic activity. The induction is mediated by ERK and p38 kinases, as demonstrated by selective inhibition or overexpression of different mitogen-activated kinases. Reporter assays of mutant constructs indicate that EGF-induced transcriptional activity on Dio2 promoter is mediated by the cAMP response element (CRE) and does not involve the activating protein 1 site. With functional and biochemical approaches, we have demonstrated that the EGF stimulation culminates with the assembly and recruitment over the Dio2 CRE of a composite complex, which consists of c-Jun, c-Fos, and CRE-binding protein. These results further support the hypothesis that placental iodothyronine metabolism is critical during early pregnancy.
HUMAN TYPE 2 DEIODINASE (hD2) catalyzes outer ring deiodination of T4 and regulates the intracellular concentrations of T3 in several organs, such as brain, pituitary, heart, skeletal muscle, and placenta. Tissues where D2 is highly expressed are usually very sensitive to low thyroid hormone levels, implying that in these districts, the production of T3 needs to be promptly and finely regulated, according to the cellular needs. This is achieved by an orchestrated control of D2 at multiple levels and in response to several cues. A critical and well characterized mechanism used to maintain proper levels of D2 within the cells is ubiquitination and subsequent protein degradation (1,2). In addition, pretranslational mechanisms control the amount of intracellular D2 (3). Transcription of the Dio2 gene is regulated by multiple stimuli and transcription factors, such as TSH, norepinephrine, phorbol-12-myristate-13-acetate, lipopolysaccharide, thyroid transcription factor-1, NKX-2.5, GATA-4, cAMP response element-binding protein (CREB), Fos-related antigen 2, and nuclear factor-κB (4,5,6,7,8,9,10). Furthermore, posttranscriptional events also contribute to regulate the amount of D2 in the cells. Indeed, 5′ and 3′ untranslated regions have been shown to affect translation efficiency and mRNA stability of the Dio2 gene, respectively (11).
Several studies have shown the importance of D2 regulation in different tissues. In brown adipose tissue, catecholamines and cold exposure promptly increase D2 activity via cAMP, with consequent generation of T3 and heat production (12). Also, it has been demonstrated that the tissue-specific expression of D2 in thyroid and heart is related to the activity of thyroid transcription factor-1 and NKX-2.5/GATA-4 transcription factors, respectively (7,8).
As far as the role of deiodinases in placenta is concerned, very little is known about the function and regulation of D2 in this tissue. Some data indicate that thyroid hormones are critical during the growth and development of early trophoblast and that D2 might function as a biological amplifier of trophoblast endocrine function, by providing T3 (13). Supporting this notion is the observation that D2 activity and mRNA levels in the trophoblast are elevated only during the first trimester of pregnancy and then progressively decline (14). In our previous studies, we have found that Dio2 mRNA is strongly induced in placenta by cAMP via CREB and that this regulation occurs via a conserved CRE and a single TATA box/transcriptional start site unit located in the promoter region (15).
In a previous report, it has been observed that epidermal growth factor (EGF) induces Dio2 transcription in mammary epithelium (16), but the molecular mechanism underlying this regulation has not been addressed. Because trophoblast produces EGF and expresses EGF receptors only during the early stage of development (17), we hypothesized that the ability to respond to this mitogen could be a mechanism used by early trophoblast to enhance the expression of genes important at this stage of development, in collaboration with cAMP.
Using as a model choriocarcinoma JEG3 cells, a cell line very similar to early trophoblast that expresses EGF receptors, here we show that EGF promotes the expression of Dio2 gene in synergy with cAMP and that this regulation occurs through a composite transcription factor module, which includes CREB, c-Jun, and c-Fos.
Materials and Methods
Plasmids, cell culture, transient transfections, and luciferase assays
Plasmids 1.3-kb Dio2 Luc, ΔCRE, ΔAP1, Zeo A-CREB, and Zeo A-Fos have been previously described (9,18).
Plasmids encoding MAPK kinase kinase 1 (MEKK1) and MAPK kinase 6 (MKK6) were a generous gift of P. L. Puri.
To generate the plasmid Dio2CRE-TK, we used the following oligonuleotides: forward, 5′-AGCTTTAAAGCCCTCTTTCTCAATGACGTCAAGATCTTTACCAAGATTAG-3′, and reverse, 5′-GATCCTAATCTTGGTAAAGATCTTGACGTCATTGAGAAAGAGGGCTTTAA-3′.
Oligos were annealed and cloned into the HindIII/BamHI sites of the pRLTK luc plasmid.
The plasmids encoding Flag CREB, HA c-Jun, and HA c-Fos were constructed by PCR amplification of the human coding regions of CREB, c-Jun and c-Fos. The PCR products were digested and cloned into the EcoRI/XhoI sites of Flag- or HA-tagged pcDNA3 vectors (Invitrogen, Carlsbad, CA).
JEG3 cells were cultured in MEM supplemented with 10% fetal bovine serum and 1% l-glutamine. The day before transfection 200,000 cells per well were seeded in six-well plates and grown overnight. For transfection, cells were incubated overnight in OPTIMEM (Invitrogen) with 0.5 μg luciferase reporter plasmid, 0.5 μg of the indicated expression vectors, and 4 μl lipofectin/well (Invitrogen). Plasmid RSV β-gal (100 ng/well), which expresses β-galactosidase under the control of the RSV promoter, was used in all transfections to normalize the luciferase activity. The empty expression vector pCDNA3 (Invitrogen) has been used to maintain a total of 1 μg plasmid DNA. The day after transfection, cells were incubated for 4 h in MEM containing 0.1% BSA and 10 μm forskolin (Sigma Chemical Co., St. Louis, MO) or control dimethylsulfoxide vehicle or 12 h in MEM containing 0.1% BSA and 100 ng/ml EGF (Invitrogen). When MAPK inhibitors were used, cells were preincubated with them for 60 min before stimulus. The inhibitors used were PD98059 (ERK kinase inhibitor; Sigma), SB203580 (p38 inhibitor; Sigma), and SP600125 [c-Jun N-terminal kinase (JNK) inhibitor; Sigma] at the concentration of 50, 20, and 10 μm, respectively.
Knockdown of c-Jun and c-Fos was performed as described (19) with small interfering RNAs (siRNAs) from Dharmacon (Lafayette, CO) (c-Fos: M-003265-01, c-Jun: M-003268-02). Fifty nanograms of siRNA were transfected in JEG3 cells with lipofectine. After transfection, cells were grown 48 h and treated as indicated. The actual knock down of the proteins was verified by Western blot analysis.
After transfections, cells were lysed in 0.5% Triton X-100 and 0.25 m Tris (pH 8), and luciferase activity was measured. Results are expressed as fold change compared with control and represent the average ± sd of three independent experiments, each performed in triplicate.
D2 activity assay
JEG3 cells were seeded in six-well plates and incubated in serum-free medium containing 0.1% BSA overnight. They were then exposed to 100 ng/ml EGF and/or 10 μm forskolin or vehicle for 6 h, harvested, and sonicated. Assays for D2 activity were performed as described (20), using 1 nm outer ring labeled [125I]T4 as substrate with approximately 200 μg sonicated protein for 16 h. Blank counts were obtained by incubating sonicates with 100 nm [125I]T4, and the 125I released (about 3%) subtracted from that in the experimental samples. Results are expressed as the femtomoles of T4 converted to T3 per milligram per 16 h.
RNA isolation and RT-PCR
JEG3 cells were grown in 100-mm dishes until they reached 70–80% confluence, and 10 μm forskolin or 100 ng/ml EGF was added for the indicated times. To determine the stability of the mRNAs, EGF-treated cells were incubated with 5 μg/ml actinomycin D (Sigma) for the indicated times.
Total RNA was isolated with RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. RT has been performed with 1 μg total RNA using Superscript II (Invitrogen) and random hexamers. Quantitative and semiquantitative PCR was performed as described (21) using primers specific to Dio2 and β-actin or GAPDH (15).
Densitometric analysis was performed using NIH ImageJ software.
Chromatin immunoprecipitation (ChIP) and Re-ChIP
ChIP has been performed as previously described (22). Briefly, cells were incubated with 1% formaldehyde for 20 min at room temperature to cross-link proteins to DNA. After cross-linking, cells were lysed (3% sarkosyl, 5 mm EDTA, 50 mm Tris-Hcl, pH 8.1, plus protease inhibitors) and sonicated to reduce the genomic DNA into small fragments (400–600 bp). The lysates were precleared with protein A-agarose and normal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4 C. After preclearing, the lysates were incubated with a rabbit polyclonal CREB (provided by Marc Montminy), c-Jun, c-Fos antisera (Santa Cruz), or, as a negative control, with normal rabbit IgG overnight at 4 C.
For Re-ChIP experiments, after the first IP and washings, the immunocomplexes were eluted in 10 mm dithiothreitol, diluted with Re-ChIP buffer (1% Triton X-100, 2 mm EDTA, 150 mm NaCl, 20 mm Tris-HCl, pH 8) and immunoprecipitated again overnight at 4 C with the specified antibodies.
At the end of the first (ChIP) or second (Re-ChIP) IP, the immunocomplexes were washed extensively and eluted, and the cross-linking was reversed by overnight incubation at 65 C. Samples were digested with proteinase K and the DNA purified, precipitated, and resuspended in Tris-EDTA.
Real-time quantitative PCR was performed with the SYBR-Green technique, using primers encompassing the Dio2 CRE or the unrelated GAPDH gene (15) and analyzed with the comparative cycle threshold (ΔΔCT) method as described in detail elsewhere (23).
Results are expressed as fold difference values compared with the control samples.
The fold difference value in each case compares the specific antibody sample (CREB, c-Jun, and c-Fos) to the corresponding IgG control sample. The results represent the average ± sd of at least three different experiments, each performed in triplicate.
Co-IP and Western blot
Co-IP were performed as described (22). Briefly, cells were transfected for 36 h with the indicated expression plasmids.
After transfection, cell lysates were incubated with anti-Flag M2 agarose-conjugated beads (Sigma) for 2 h. After incubation, the beads were washed extensively, the immunocomplexes were eluted, and Western blot was performed.
To perform Western blot experiments on EGF-treated JEG3, cells were lysed in SDS/urea buffer (22) and sonicated. Forty micrograms of cell lysates were loaded into a gel, run, transferred in a nitrocellulose membrane, and incubated with the different antibodies.
Statistical analysis
The results presented in figures and tables are representative for at least three experiments with comparable results. Statistical differences were tested using the Mann-Whitney U test when treatment and control samples were analyzed or with nonparametric ANOVA (Kruskal-Wallis test) when more than two treatments were analyzed. Where significant differences were observed (P < 0.05) using ANOVA, pairwise comparisons were carried out using Dunn’s multiple comparisons test. All statistical tests are indicated in the figure legends. Differences were considered significant at P < 0.05. Statistical analyses were performed using Instat GraphPad 3.06 statistical software (Graphpad Inc., San Diego, CA).
Results
EGF and cAMP induce hD2 mRNA and activity in choriocarcinoma cells
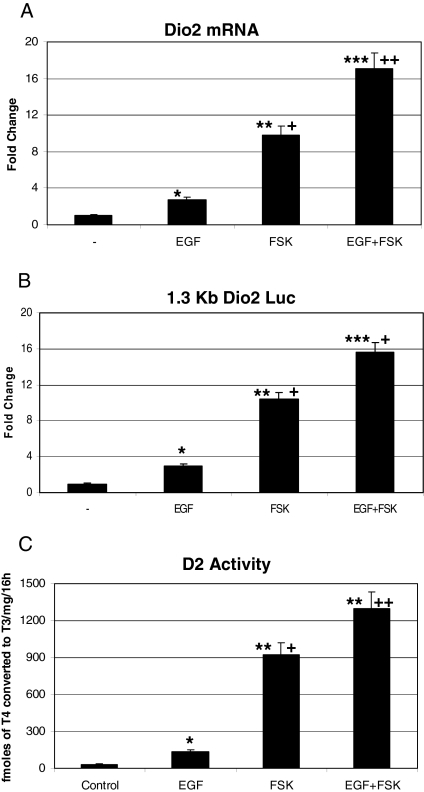
The ability of EGF to induce the expression and activity of D2 in mammary epithelium (16) prompted us to investigate whether this effect occurs also in JEG3 cells, a cell line similar to early trophoblast, where Dio2 gene is highly induced by cAMP. Incubation with 100 ng/ml EGF resulted in a 3-fold increase of Dio2 mRNA after 2 h that disappeared after 8 h (Fig. 1, A and B). Consistently, D2 enzymatic activity was increased 3.5-fold after 6 h of EGF treatment (Fig. 1C).
Figure 1.
EGF stimulates Dio2 mRNA and activity in JEG3 cells. A, Time course of EGF-treated JEG3 cells. Cells were incubated overnight with serum-free medium and then treated with 100 ng/ml EGF for the indicated times. RT-PCR was performed using primers specific for Dio2 and β-actin. B, Quantitative real-time PCR. Cells were treated as described above for 2 h with EGF. SYBR-Green quantitative PCR was performed using primers specific for human Dio2 and GAPDH. *, P < 0.05, EGF vs. control, Mann-Whitney U test. C, Effect of EGF treatment on D2 enzymatic activity. D2 assays were performed on sonicates of JEG3 cells stimulated for 6 h with EGF. *, P < 0.05, EGF vs. control, Mann-Whitney U test. D, Luciferase assay of 1.3-kb Dio2 Luc promoter construct. Cells were seeded in six multiwell plates and transfected with the 1.3-kb Dio2 Luc and RSV β-gal reporter plasmids. The day after transfection, cells were incubated for the indicated times with EGF. At the end of the incubation, luciferase and β-galactosidase assays were performed. *, P < 0.05, EGF vs. control, Mann-Whitney U test. Results (B–D) represent the average value ± sd of at least three different experiments, each performed in triplicate.
To determine whether the stimulatory effect of EGF occurs at the promoter level, we performed luciferase assays with a 1.3-kb Dio2-Luc reporter construct. As shown in Fig. 1D, EGF increased the activity of Dio2 promoter at 6, 12, and 24 h with the best inducibility at 12 h.
Because we have previously observed that cAMP is a potent inducer of Dio2 transcription in this cell line (15), we next tested whether EGF and the cAMP agonist forskolin could work synergistically. As expected, forskolin caused a robust increase of Dio2 transcription, detectable by real-time quantitative RT-PCR, luciferase, and deiodination assays. Cotreatment with both drugs resulted in a stimulation of the gene that was more than additive, representing a synergistic activation. This effect was detectable in all three assays and was statistically significant (see Fig. 2, A–C).
Figure 2.
Synergistic effect of EGF and cAMP. A, Effect of cAMP and EGF alone or in combination on Dio2 mRNA. JEG3 cells were serum deprived overnight and incubated for 2 h with 100 ng/ml EGF and/or 10 μm FSK. Real-time quantitative PCR was performed as described above, using primers specific for Dio2 and the housekeeping gene GAPDH. ANOVA test: *, EGF vs. control P < 0.05; **, FSK vs. control P < 0.01; ***, EGF+FSK vs. control P < 0.001; +, FSK vs. EGF P < 0.05; ++, EGF+FSK vs. FSK P < 0.01. B, Luciferase assay on JEG3 cells, transfected with 1.3-Kb Dio2 Luc reporter and treated with 100 ng/ml EGF, 10 μm FSK, alone or in combination. ANOVA test: *, EGF vs. control P < 0.05; **, FSK vs. control P < 0.01; ***, EGF+FSK vs. control P < 0.001; +, FSK vs. EGF and EGF+FSK vs. FSK P < 0.05. C, D2 assay, showing effect of FSK, alone or in combination with EGF, on D2 activity. ANOVA test: *, EGF vs. control P < 0.05; **, FSK vs. control and EGF+FSK vs. control P < 0.001; +, FSK vs. EGF P < 0.01; ++, EGF+FSK vs. FSK P < 0.05.
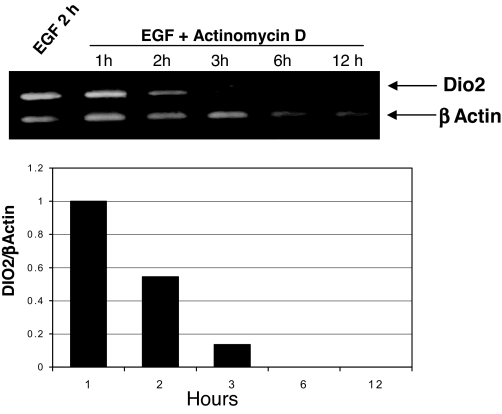
To measure the stability of the EGF-induced Dio2, we incubated EGF-treated JEG3 cells with actinomycin D for different times. As shown in Fig. 3, and consistent with previous studies (24), the EGF-induced transcript showed a short, 1-h half-life.
Figure 3.
Stability of EGF-induced transcript. Cells were treated for 2 h with EGF and then incubated with 5 μg/ml actinomycin D for the indicated times. At the end of the incubation, total RNA was extracted and reverse-transcribed, and the cDNA was amplified with primers complementary to Dio2 and β-actin genes. Top, Ethidium bromide staining; bottom, densitometric analysis. Bars represent the Dio2/β-actin ratio.
Signal transduction pathway and promoter sequences involved in the response to EGF
Upon stimulation of JEG3 cells with EGF, three MAPKs are mainly activated: ERK, p38, and JNK (25). To study the signal transduction pathway involved in this effect, we started using the inhibitors PD98059 and SB203580 to suppress ERK and p38 kinase activity, respectively. Both inhibitors were able to block transcription of Dio2 gene as shown by RT-PCR and luciferase assays (Fig. 4A, top and bottom, respectively). The presence of both drugs completely suppressed the effect of EGF. This latter observation suggested that JNK does not play a major role in this context. Indeed, the selective JNK inhibitor SP 600125 failed to inhibit EGF inducibility of Dio2 mRNA (Fig. 4B), confirming that this kinase is not involved in this effect. Consistent with these data, overexpression of the ERK activating kinase MEKK1 and the p38 activating kinase MKK6 resulted in a strong enhancement of Dio2 transcription (Fig. 4C).
Figure 4.
Effect of MAPK inhibition and overexpression on Dio2 transcription. A, Top, RT-PCR. Cells were pretreated for 1 h with the MAPK inhibitors PD98059 (PD) (ERK kinase inhibitor), SB203580 (SB) (p38 inhibitor; Sigma), or vehicle control and then treated with 100 ng/ml EGF for 2 h. At the end of the incubation, total RNA was extracted and reverse-transcribed, and the cDNA was amplified with primers complementary to Dio2 and β-actin genes. Bottom, Luciferase assay. Cells were transfected with the 1.3-kb Dio2 Luc and RSV β-gal constructs. The day after transfection, cells were pretreated for 1 h with the indicated MAPK inhibitors and then incubated 12 h with 100 ng/ml EGF. After treatments, the luciferase and β-galactosidase assays were performed. ANOVA test: *, EGF vs. NT P < 0.05; +, EGF/PD vs. EGF and EGF/SB vs. EGF P < 0.05; ++, EGF/PD+SB vs. EGF P < 0.01. NT, Nontreated cells. B, RT-PCR. Cells were pretreated for 1 h with JNK inhibitor SP600125 (SP) or vehicle control (NT) and then treated with 100 ng/ml EGF for 2 h. At the end of the incubation, total RNA was extracted and reverse-transcribed, and the cDNA was amplified with primers complementary to Dio2 and β-actin genes. C, Luciferase assay. Cells were transfected with 1.3-kb Dio2 Luc, RSV β-gal, MEKK1, and MKK6 expression constructs. At the end of the incubation, luciferase and β-galactosidase assays were performed. ANOVA test: *, MEKK1 vs. control P < 0.001; **, MKK6 vs. control P < 0.01; +, MKK6 vs. MEKK1 P < 0.05. Results represent the average value ± sd of at least three different experiments, each performed in triplicate.
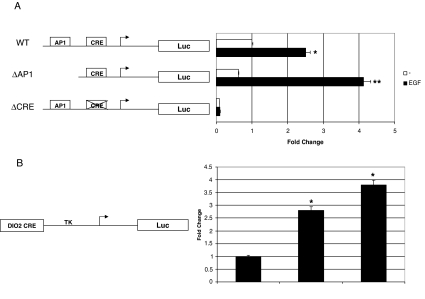
To map the promoter sequences involved in the EGF response, we performed luciferase assays. Because Dio2 promoter contains consensus activating protein 1 (AP1) and CRE sites (9), and the ability of EGF to activate transcription has been linked to the presence of both these promoter elements, we used CRE- and AP1-mutated constructs, as depicted in Fig. 5A. Remarkably, deletion of the region containing the AP1 site did not affect the response to EGF, which was even more robust in the absence of this site. In contrast, mutation of the CRE disrupted both basal and EGF-induced luciferase activity. To determine whether the CRE of Dio2 promoter was sufficient to impart EGF inducibility, we cloned an oligonucleotide sequence containing the Dio2 CRE in front of the thymidine kinase minimal promoter (Dio2CRE TK). As shown in Fig. 5B, this construct was readily induced by EGF and forskolin (FSK), thus demonstrating that the Dio2 CRE is sufficient to impart EGF inducibility to the gene.
Figure 5.
Contribution of AP1 and CRE elements on EGF inducibility. A, JEG3 cells were transfected with wild-type (WT), ΔAP1, and ΔCRE-Dio2 Luc, RSV β-gal plasmids and treated 12 h with 100 ng/ml EGF. *, P < 0.05, WT EGF vs. control; **, P < 0.01, ΔAP1 EGF vs. control, Mann-Whitney test U test. NT, Nontreated cells. B, JEG3 cells were transfected with Dio2CRE TK Luc, RSV β-gal plasmids and treated for 12 h with 100 ng/ml EGF or 5 h with 10 μm FSK. After treatments, cells were lysed and luciferase and β-galactosidase assays were performed. *, P < 0.05, EGF vs. control and FSK vs. control, ANOVA test. Results represent the average value ± sd of at least three different experiments, each performed in triplicate.
Analysis of the transcription factors involved
Having found that the CRE is necessary and sufficient to confer EGF inducibility to Dio2 promoter, we next sought to identify the transcription factors involved in this process and their promoter occupancy.
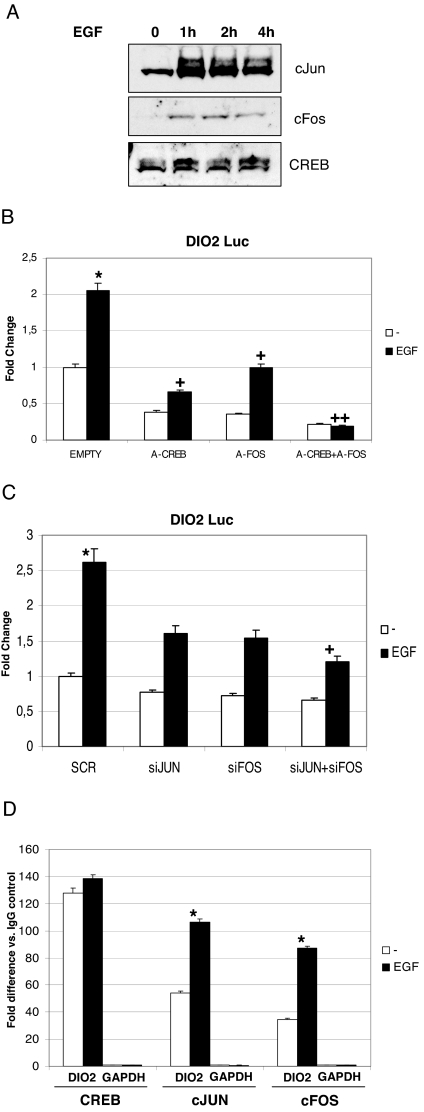
As previously reported, EGF stimulates the CREB-target gene α-chorionic gonadotropin (α-CG) in placenta, by increasing c-Jun and c-Fos levels and activity (25). To determine whether a similar mechanism could bring about the mitogen-mediated Dio2 transcription, we first performed Western blot experiments on EGF-treated JEG3 cells. As shown in Fig. 6A, both c-Jun and c-Fos were strongly induced by EGF, whereas CREB levels did not change. To study the effect of the functional ablation of these transcription factors, we used the acidic dominant-negative polypeptides A-Fos and A-CREB (26). Both A-CREB and A-Fos significantly inhibited basal and EGF-induced promoter activity. The presence of both inhibitors completely abolished the response to EGF (Fig. 6B), indicating that all these transcription factors are engaged to impart the response to the mitogen. To confirm the role of AP1 in this regulation, we performed knockdown experiments by transfecting siRNAs of c-Jun and c-Fos (19) along with the reporter plasmids. The actual knockdown was verified by Western blot analysis and was more than 80% of the total protein (not shown). Consistent with our model, the combined ablation of c-Jun and c-Fos significantly decreased the response to EGF (Fig. 6C).
Figure 6.
The effect of EGF on hD2 transcription is mediated by c-Jun, c-Fos, and CREB. A, Effect of EGF on total c-Jun, c-Fos, and CREB levels. JEG3 cells were serum deprived and then treated with 100 ng/ml EGF for the indicated times. At the end of the incubation, cells were lysed with denaturing SDS/urea lysis buffer. Equal amounts of protein extracts were loaded and run onto a SDS-PAGE gel. Western blot was performed using CREB, c-Jun, and c-Fos antisera. B, Luciferase assay. Cells were transfected with 1.3-kb Dio2 Luc, Zeo A-CREB, Zeo A-Fos, and RSV β-gal plasmids. The day after transfection, cells were treated with 100 ng/ml EGF for 12 h and lysed, and luciferase and β-galactosidase assays were performed. Results represent the average value ± sd of three different experiments, each performed in triplicate. ANOVA test: *, EGF vs. control P < 0.05; +, EGF/ACREB vs. EGF and EGF/AFOS vs. EGF P < 0.05; ++, EGF/ACREB+AFOS vs. EGF P < 0.01. NT, Nontreated cells. C, Luciferase assay. Cells were transfected with the indicated siRNAs, 1.3-kb Dio2 Luc and RSV β-gal plasmids. After 48 h, cells were treated for 12 h with 100 ng/ml EGF. After treatments, cells were lysed, and luciferase and β-galactosidase assays were performed. SCR, Scrambled. ANOVA test: *, SCR/EGF vs. SCR/control P < 0.05; +, siJUN+siFOS/EGF vs. SCR/EGF P < 0.05. SCR, Scrambled. D, ChIP. JEG3 cells were treated with 100 ng/ml EGF or vehicle for 2 h and then cross-linked with formaldehyde. Cells were lysed, and the lysates were sonicated and immunoprecipitated with the indicated antisera. After extensive washings, the DNA/protein complexes were eluted, the cross-linking was reversed, and the DNA was purified with phenol/chloroform/isoamyl alcohol (25:24:1). Real-time quantitative PCR was performed using primers encompassing the Dio2 CRE or the unrelated GAPDH gene and analyzed with the comparative cycle threshold (ΔΔCT) method as described in text. The fold difference value in each case compares the specific antibody sample (CREB, c-Jun, and c-Fos) to the corresponding IgG control sample. The results represent the average ± sd of three different experiments, each performed in triplicate. *, P < 0.05, c-Jun/EGF and c-Fos/EGF vs. nontreated controls, ANOVA test.
To analyze the promoter occupancy of these proteins upon stimulation with EGF, we performed ChIP assays. Stimulation with EGF significantly increased the recruitment of both c-Jun and c-Fos over Dio2 promoter (Fig. 6D). In contrast, CREB recruitment was unaffected by the treatment.
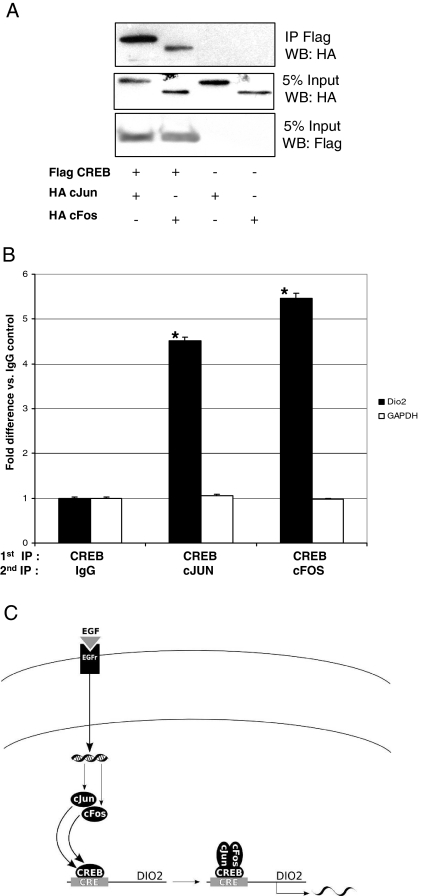
Because these results suggested that CREB, c-Jun, and c-Fos are recruited together over the Dio2 CRE upon EGF treatment, we next performed binding assays to test whether they can associate in vivo. Toward this end, we coimmunoprecipitated epitope-tagged proteins (Fig. 7A) and found that CREB can actually interact with both c-Jun and c-Fos. To confirm that the AP1 complex interacts with the Dio2 CRE through CREB, we performed Re-ChIP experiments. To this end, we performed a first IP with CREB antisera followed by a second IP with c-Jun, c-Fos, or normal rabbit IgG in EGF-treated JEG3 cells. As shown in Fig. 7B, both c-Jun and c-Fos were found associated with Dio2 CRE after CREB IP, thus demonstrating that AP1 and CREB are able to interact and form a composite complex that is assembled over the Dio2 CRE.
Figure 7.
A, Co-IP. JEG3 cells were transfected with Flag CREB, HA c-Jun, and HA c-Fos expression plasmids, as indicated, for 36 h. After transfection, cells were lysed and immunoprecipitated for 2 h with anti-Flag agarose-conjugated beads. At the end of the incubation, the beads were washed, and the immunocomplexes were eluted and loaded onto a SDS-PAGE gel. Western blot was performed with anti-HA and anti-Flag antibodies. B, Re-Chip. EGF-treated JEG3 cells were cross-linked with formaldehyde, lysed, sonicated, and immunoprecipitated with CREB antisera (first IP). After extensive washings, the DNA/protein complexes were eluted with 10 mm dithiothreitol. After elution, a second IP was performed with the indicated antibodies. The DNA/protein complexes were washed again, eluted, and the cross-linking reversed. The DNA was then purified with phenol/chloroform/isoamyl alcohol (25:24:1), precipitated, and resuspended. Real-time quantitative PCR was performed using primers encompassing the Dio2 CRE or the unrelated GAPDH gene and analyzed with the comparative cycle threshold (ΔΔCT) method as described in text. The fold difference value in each case compares the specific antibody sample (CREB, c-Jun, and c-Fos) with the corresponding IgG control sample. The results represent the average ± sd of three different experiments, each performed in triplicate. *, P < 0.01 CREB-c-Jun vs. CREB-IgG and CREB-c-Fos vs. CREB-IgG, ANOVA. C, Model of EGF-mediated activation of Dio2 gene. After EGF interacts with its receptor, distinct signaling cascades are activated leading to increased levels of c-Jun and c-Fos. These two transcription factors are then recruited over the Dio2 CRE together with CREB, which occupies constitutively the same site.
Discussion
The development and the endocrine functions of early trophoblast are dependent on the appropriate levels of several hormones, including T3. The maintenance of the peripheral euthyroid state is achieved by regulating the local activity of D2 at multiple levels and with different mechanisms. In this report, we have demonstrated that the expression of Dio2 gene in placenta is regulated by EGF, through an articulated transcriptional mechanism.
The EGF regulation of Dio2 is reminiscent of that of the α-subunit of the gonadotropin gene (αCG). Indeed, α-CG is highly expressed and secreted at the beginning of the pregnancy in response to cAMP and EGF, as is D2 activity in human placenta.
The early trophoblast expresses EGF receptors and produces EGF to promote the rapid expansion of this tissue in the first trimester of pregnancy (17). Therefore, the ability of some genes to respond to EGF in synergy with cAMP might represent a mechanism used by this tissue to maintain elevated levels of proteins that are critical at this stage, like CG and 5′-deiodinase.
We have demonstrated that EGF stimulates transcription of a short-lived Dio2 mRNA. In contrast, we previously found that FSK-induced transcript is stable (15), suggesting that, in addition to the transcriptional control, cAMP might also regulate stability of Dio2 mRNA. This hypothesis is intriguing, because it would imply a signal-dependent regulation of mRNA stability, which is likely to be related to the 3′-untranslated region of Dio2 mRNA. Indeed the 3′-untranslated region of Dio2 mRNA has been shown to have potent effects on the stability of the transcript (11).
As shown by RT-PCR and luciferase assays, the signaling pathways triggered by EGF are mediated by ERK and p38 kinases. In addition, the effect of cAMP and EGF is synergistic, suggesting that a combination of different signaling cascades might be used to integrate and potentiate the transcriptional response, as observed for the αCG gene (25).
With reporter assays, we have demonstrated that the response to EGF occurs at the transcriptional level. Deletion of the AP1 site did not suppress the response to EGF, whereas mutation of the CRE completely abolished the transcriptional activity.
This observation further confirms the nonfunctionality of the AP1 site of the Dio2 promoter (4) and implies that the CRE functions as a platform of convergence for multiple stimuli, working through different pathways.
It has been shown that the response of αCG gene to EGF and cAMP is dependent on the combined action of CREB, c-Jun, and c-Fos (25). These three transcription factors are recruited over αCG promoter thanks to a peculiar promoter structure, which consists of a tandem repeat of two CREs, located close to the TATA/TSS unit. In contrast, in the present study, we have demonstrated that in this context a single CRE is sufficient to impart the response to EGF and cAMP. Also, we have shown that these three transcription factors can occupy the same element when cells are exposed to EGF. Indeed, ChIP experiments have revealed that EGF does not affect CREB occupancy, which is constitutively bound to the CRE, whereas c-Jun and c-Fos occupancy is increased by EGF treatment. Notably, as demonstrated by Re-ChIP and coimmunoprecipitation experiments, AP1 interacts with CREB forming a composite and previously uncharacterized complex, indicating a novel mechanism of transcriptional regulation in response to hormonal and mitogenic signals in placenta cells.
In conclusion, we have found that EGF regulates D2 activity through distinct signaling cascades in JEG3 cells, leading to increased levels of intracellular c-Jun and c-Fos. The AP1 transcriptional complex is then recruited over the Dio2 CRE and, together with CREB, activates transcription (Fig. 7C). These findings provide new insights to understand the molecular basis of Dio2 gene regulation and further support the hypothesis that thyroid hormone activation is critical during trophoblast development.
Acknowledgments
We thank Charles Vinson for the gift of Zeo-ACREB and Zeo A-Fos plasmids, Lorenzo Puri for MEKK1 and MKK6 expression vectors, and Marc Montminy for CREB antisera.
Footnotes
This work was supported by a Ministero dell’Università e della Ricerca Grant C8969-G105826. P.R.L. and J.W.H. were supported by National Institutes of Health Grant DK 36256.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 8, 2007
Abbreviations: AP1, Activating protein 1; α-CG, α-chorionic gonadotropin; ChIP, chromatin immunoprecipitation; CREB, cAMP response element-binding protein; EGF, epidermal growth factor; FSK, forskolin; hD2, human type 2 deiodinase; JNK, c-Jun N-terminal kinase; MEKK1, MAPK kinase kinase 1; MKK6, MAPK kinase 6; siRNA, small interfering RNA.
References
- Bianco AC, Larsen PR 2005 Cellular and structural biology of the deiodinases. Thyroid 15:777–786 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Kim BW 2006 Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116:2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Salvatore D 2005 Pretranslational regulation of type 2 deiodinase. Thyroid 15:855–864 [DOI] [PubMed] [Google Scholar]
- Bartha T, Kim SW, Salvatore D, Gereben B, Tu HM, Harney JW, Rudas P, Larsen PR 2000 Characterization of the 5′-flanking and 5′-untranslated regions of the cyclic adenosine 3′,5′-monophosphate-responsive human type 2 iodothyronine deiodinase gene. Endocrinology 141:229–237 [DOI] [PubMed] [Google Scholar]
- Imai Y, Toyoda N, Maeda A, Kadobayashi T, Fangzheng G, Nishikawa M, Iwasaka T 2001 Type 2 iodothyronine deiodinase expression is upregulated by the protein kinase A-dependent pathway and is downregulated by the protein kinase C-dependent pathway in cultured human thyroid cells. Thyroid 11:899–907 [DOI] [PubMed] [Google Scholar]
- Zeold A, Doleschall M, Haffner MC, Capelo LP, Menyhert J, Liposits Z, da Silva WS, Bianco AC, Kacskovics I, Fekete C, Gereben B 2006 Characterization of the nuclear factor-κB responsiveness of the human dio2 gene. Endocrinology 147:4419–4429 [DOI] [PubMed] [Google Scholar]
- Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR 2001 The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol Endocrinol 15:112–124 [DOI] [PubMed] [Google Scholar]
- Dentice M, Morisco C, Vitale M, Rossi G, Fenzi G, Salvatore D 2003 The different cardiac expression of the type 2 iodothyronine deiodinase gene between human and rat is related to the differential response of the dio2 genes to Nkx-2.5 and GATA-4 transcription factors. Mol Endocrinol 17:1508–1521 [DOI] [PubMed] [Google Scholar]
- Canettieri G, Celi FS, Baccheschi G, Salvatori L, Andreoli M, Centanni M 2000 Isolation of human type 2 deiodinase gene promoter and characterization of a functional cyclic adenosine monophosphate response element. Endocrinology 141:1804–1813 [DOI] [PubMed] [Google Scholar]
- Smith M, Burke Z, Humphries A, Wells T, Klein D, Carter D, Baler R 2001 Tissue-specific transgenic knockdown of Fos-related antigen 2 (Fra-2) expression mediated by dominant negative Fra-2. Mol Cell Biol 21:3704–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Kollar A, Harney JW, Larsen PR 2002 The mRNA structure has potent regulatory effects on type 2 iodothyronine deiodinase expression. Mol Endocrinol 16:1667–1679 [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR 1983 Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712–713 [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Mochizuki M 1991 Thyroid hormone as a biological amplifier of differentiated trophoblast function in early pregnancy. Acta Endocrinol (Copenh) 125:58–66 [DOI] [PubMed] [Google Scholar]
- Chan S, Kachilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, Driver PM, Bradwell AR, Kester M, Visser TJ, Franklyn JA, Kilby MD 2003 Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab 88:4488–4495 [DOI] [PubMed] [Google Scholar]
- Canettieri G, Franchi A, Sibilla R, Guzman E, Centanni M 2004 Functional characterisation of the CRE/TATA box unit of type 2 deiodinase gene promoter in a human choriocarcinoma cell line. J Mol Endocrinol 33:51–58 [DOI] [PubMed] [Google Scholar]
- Song S, Oka T 2003 Regulation of type II deiodinase expression by EGF and glucocorticoid in HC11 mouse mammary epithelium. Am J Physiol Endocrinol Metab 284:E1119–E1124 [DOI] [PubMed] [Google Scholar]
- Amemiya K, Kurachi H, Adachi H, Morishige KI, Adachi K, Imai T, Miyake A 1994 Involvement of epidermal growth factor (EGF)/EGF receptor autocrine and paracrine mechanism in human trophoblast cells: functional differentiation in vitro. J Endocrinol 143:291–301 [DOI] [PubMed] [Google Scholar]
- Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C 1997 A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem 272:18586–18594 [DOI] [PubMed] [Google Scholar]
- Baron S, Escande A, Alberola G, Bystricky K, Balaguer P, Richard-Foy H 2007 Estrogen receptor α and the activating protein-1 complex cooperate during insulin-like growth factor-I-induced transcriptional activation of the pS2/TFF1 gene. J Biol Chem 282:11732–11741 [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR 2005 Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr BM, Canettieri G, Montminy MR 2001 Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc Natl Acad Sci USA 98:10936–10941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canettieri G, Morantte I, Guzman E, Asahara H, Herzig S, Anderson SD, Yates JR, Montminy M 2003 Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol 10:175–181 [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG 2002 Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 277:13286–13293 [DOI] [PubMed] [Google Scholar]
- Kim SW, Harney JW, Larsen PR 1998 Studies of the hormonal regulation of type 2 5′-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology 139:4895–4905 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Ban M, Zhang T, Mulvaney JM 2000 Role of the cyclic AMP response element binding complex and activation of mitogen-activated protein kinases in synergistic activation of the glycoprotein hormone α-subunit gene by epidermal growth factor and forskolin. Mol Cell Biol 20:3331–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M 2002 Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol 22:6321–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]