Abstract
Two highly homologous oocyte-secreted growth factors, bone morphogenetic protein (BMP)-15 and growth and differentiation factor (GDF)-9, are known to control folliculogenesis and ovulation through direct effects on granulosa cells in the developing follicles. Although much is known about the expression and biology of these proteins, the impact of posttranslational modifications of BMP-15 and GDF-9 is unknown. Here, we report that: 1) recombinant human (rh) BMP-15 and rhGDF-9 are phosphorylated; 2) the phosphorylation is essential for bioactivity; and 3) the dephosphorylated forms of rhBMP-15 and rhGDF-9 can abolish the bioactivity of rhBMP-15, rhGDF-9, and rhBMP-7, but not rh activin A. These results indicate that the phosphorylation state of rhBMP-15 and rhGDF-9 is a determinant of their agonistic and antagonistic activities.
ELUCIDATION OF THE role of the oocyte in regulating folliculogenesis and ovulation has been a major focus of interest in the field of female reproduction for nearly a decade (1,2). Two oocyte-secreted factors, bone morphogenetic protein (BMP)-15 and growth and differentiation factor (GDF)-9, are of particular interest in the regulation of folliculogenesis and ovulation because genetic studies of mutations in these genes have recently uncovered the critical role of these factors in regulating ovulation rates and litter size in mammals (1).
The first naturally occurring bmp15 point mutations, FecXI and FecXH, were discovered by Galloway et al. (3), in which the homozygous carriers are infertile with folliculogenesis arrested at the primary stage, whereas the heterozygous carriers have an increased ovulation rate. The importance of BMP-15 in regulating sheep fertility was further confirmed by subsequent studies that identified three additional naturally occurring bmp15 point mutations, FecXB, FecXG, and FecXL (4,5) that resulted in the same phenotype as the ewes carrying FecXI and FecXH (3). A point mutation in the gdf9 gene (FecGH) in sheep was also discovered, which had a similar phenotype to the bmp15 mutations, further emphasizing the importance of these growth factors in ovarian function (4).
In humans, a point mutation in the bmp15 gene has been discovered in women with infertility due to hypergonadotropic ovarian failure (6). Interestingly, the recombinant protein with this mutation lacks biological activity, and, importantly, the mutant protein had an antagonistic effect toward the wild-type BMP-15 protein. More recent studies have also found several point mutations in the bmp15 and gdf9 genes that are associated with premature ovarian failure (7,8,9,10,11). Although the phenotypes of these mutations are reported, the mechanisms by which the mutations affect the protein structure and function of BMP-15 and GDF-9 are still unknown.
Like other members of the TGF-β superfamily, BMP-15 and GDF-9 are synthesized as precursors composed of a signal peptide, a pro-region, and a biologically active mature protein (12,13,14). The bioactive mature form of recombinant human (rh) BMP-15 migrates as two distinct bands corresponding to 16 and 17 kDa (15). The structural difference between the two forms is most likely due to posttranslational modification. However, treatment of rhBMP-15 with N- and O-glycosidases did not change the migration pattern of the two bands (15). Another well-studied and commonly occurring posttranslational modification regulating various cellular functions is phosphorylation. Therefore, to investigate the structural differences between the two forms of the rhBMP-15 mature protein, we examined its phosphorylation status. In this study we have also included other ovarian TGF-β superfamily members, namely, GDF-9, BMP-7, and activin A.
The fact that BMP-15 and GDF-9 play critical roles in the regulation of female fertility, and that the point mutations in these proteins can have an enormous effect on the bioactivity and function of these factors, motivated us to characterize the structure of rhBMP-15 and rhGDF-9. Accordingly, in the present study, we have focused on determining the status and effect of phosphorylation on the function of rhBMP-15 and rhGDF-9. Until now, none of the TGF-β superfamily members was known to be phosphorylated. Therefore, the findings in the present study may open new avenues for understanding novel regulatory mechanisms underpinning the biological functions of TGF-β superfamily members.
Materials and Methods
Reagents and supplies
rhBMP-15 and rhGDF-9, both tagged with a Flag epitope at the C terminus, and rh activin were prepared in our laboratory as described earlier (15,16,17). rhBMP-7 was generously provided by Dr. Kuber Sampath (Creative BioMolecules Inc., Boston, MA). Calf intestine alkaline phosphatase (AP), Pro-Q diamond, and SYPRO ruby stain were from Invitrogen (Carlsbad, CA), and anti-FLAG antibody agarose was from Sigma-Aldrich (St. Louis, MO). Female Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). A human granulosa cell line (COV-434) and a mouse embryo teratocarcinoma epithelial cell line (P19) were generously provided by Drs. Peter Schrier (Leiden University Medical Center, The Netherlands) and Sylvia Evans (University of California, San Diego, CA), respectively. Phospho Smad1/5/8, Smad2/3, and Smad5 antibodies were from Cell Signaling Technology (Beverly, MA). Phospho Smad2 antibody was generously provided by Dr. Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Sweden). Recombinant extracellular domain (ECD) of human BMP receptor (BMPR)-II, ALK-6 (BMPR-IB), and ALK-5 (TβR-I) that are fused to the Fc region of human IgG (receptor-ECD/Fc chimera) were purchased from R & D Systems (Minneapolis, MN). Horseradish peroxidase-conjugated goat antihuman IgG antiserum was from Santa Cruz Biotechnology (Santa Cruz, CA).
SDS-PAGE and phosphoprotein analysis of recombinant proteins
rhBMP-15, rhGDF-9, rhBMP-7, and rh activin A (500 ng) were subjected to SDS-PAGE analysis using a 15% gel (18). This was followed by phosphorylation analysis using Pro-Q diamond stain according to the manufacturer’s instructions. After capturing the phosphostained image using a Typhoon 9400 variable mode imager (Amersham Biosciences, Piscataway, NJ), the same gel was stained for total protein using SYPRO ruby per the manufacturer’s instructions.
Dephosphorylation protocol
Recombinant proteins (500 ng) were incubated with 1 U AP at 37 C for 2 h. After incubation, the proteins were analyzed by SDS-PAGE and used for subsequent biological studies.
Primary rat granulosa cell culture and thymidine incorporation assay
Rat granulosa cell culture and the thymidine incorporation assay were performed as reported previously (15). All animal protocols were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Coimmunoprecipitation using receptor ECD/Fc chimeras
As described previously (19), phosphorylated and dephosphorylated rhBMP-15 or rhGDF-9 (300 ng/ml) were incubated with the indicated receptor-ECD/Fc chimeric proteins (2 μg/ml) in PBS at 4 C for 4 h. Anti-FLAG monoclonal antibodies conjugated to agarose beads (10 μl) were then added for an additional 2 h and incubated at 4 C. After incubation, the agarose beads were collected by centrifugation and washed five times with 1 ml PBS. Bound proteins were eluted from the agarose beads in SDS-PAGE sample buffer. Proteins eluted from the agarose beads were subjected to Western immunoblotting analysis using a horseradish peroxidase-conjugated goat-antihuman IgG to detect the receptor/Fc chimeric proteins.
Analysis of Smad phosphorylation
COV-434 and P19 cells were cultured in 24-well plate in DMEM/high glucose in the presence of 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. When the cells reached 80% confluency, the medium was replaced with serum-free medium. The cells were then precultured for 3 h and then treated with ligands at the indicated concentrations for 60 min. Cells were washed once in chilled PBS and solubilized in radioimmune precipitation lysis buffer containing Protease-Arrest (Geno Technology, Inc., St. Louis, MO), 2% sodium dodecyl sulfate, and 4% β-mercaptoethanol. Lysates were then subjected to Western immunoblotting analysis using anti-Phospho Smad1/5/8 or anti-Phospho Smad2 antibodies. For total protein analysis, the same lysates were subjected to Western immunoblotting analysis using antitotal Smad5 or antitotal Smad2/3 antibodies.
Statistical analysis
All thymidine incorporation and SDS-PAGE data presented here are representative examples of at least three separate experiments. Differences between groups are shown as mean ± sem of at least three separate experiments and analyzed for statistical significance using ANOVA (JMP IN 5.1; SAS Institute Inc., Cary, NC). A P value less than or equal to 0.05 was considered statistically significant.
Results
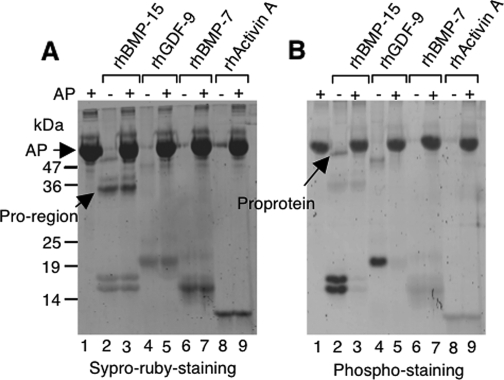
We produced rhBMP-15 tagged with a Flag epitope at the C terminus in human embryonic kidney 293 cells and purified by affinity chromatography using Flag antibody-conjugated agarose (15). The purified rhBMP-15 preparation showed two distinct mature protein bands migrating at 16 and 17 kDa detected by the SYPRO ruby protein staining (Fig. 1A, lane 2), which is consistent with our previous data obtained by immunostaining using Flag antibody. Interestingly, these mature protein bands in the same gel were also detected by phosphostaining using Pro-Q diamond (Fig. 1B, lane 2), indicating that both the rhBMP-15 mature proteins migrating at 16 and 17 kDa are phosphorylated.
Figure 1.
Phosphorylation status of rhBMP-15, rhGDF-9, rhBMP-7, and rh activin A. Five hundred nanograms of each protein were treated for 2 h at 37 C with or without 1 U calf intestine AP. Proteins were resolved by SDS-PAGE under reducing conditions. The gel was first stained with Pro-Q diamond to evaluate phosphorylation status (B), and then the same gel was stained with SYPRO ruby to examine total protein (A). The positions of the pro-region, proprotein and AP are indicated by arrows.
As described previously (15,16,20,21), the rhBMP-15 proprotein migrating at 50 kDa is occasionally detected, albeit faintly, and it is also positive for phosphostaining (Fig. 1B, lane 2). It is known that the pro-regions of TGF-β superfamily members generally dissociate after cleavage from the mature proteins; however, in cases such as TGF-β-1, -2, and -3, BMP-7, and GDF-8, the pro-region remains associated with the mature protein, and thereby regulates the targeting and binding of the ligand to its receptor (22,23,24,25). Because the pro-region (33 kDa) of rhBMP-15 was also copurified by Flag antibody-conjugated agarose (Fig. 1A, lane 2), it can be concluded that it is associated with the mature protein. However, the finding that the pro-region of rhBMP-15 is negative for phosphostaining (Fig. 1B, lane 2) argues that only the mature region of rhBMP-15 is phosphorylated.
To evaluate further this finding, the purified rhBMP-15 was treated with calf intestine AP to catalyze the removal of phosphate groups. As shown in Fig. 1B (lane 3, compare with lane 2), AP-treated rhBMP-15 mature proteins as well as proprotein were not detected by phosphostaining. This was despite the fact that the same amount of protein was loaded as evidenced by SYPRO ruby staining of the same gel (Fig. 1A, lanes 2 and 3).
We wondered whether other key ovarian TGF-β superfamily members, namely, GDF-9, BMP-7, and activin A, might also be phosphorylated. Examination of rhGDF-9 mature protein revealed positive phosphostaining that was eliminated by AP treatment (Fig. 1B, lanes 4 and 5). In contrast, neither rhBMP-7 nor rh activin A could be detected by phosphostaining and altered by the AP treatment (Fig. 1B, lanes 6–9), indicating that these proteins are not phosphorylated.
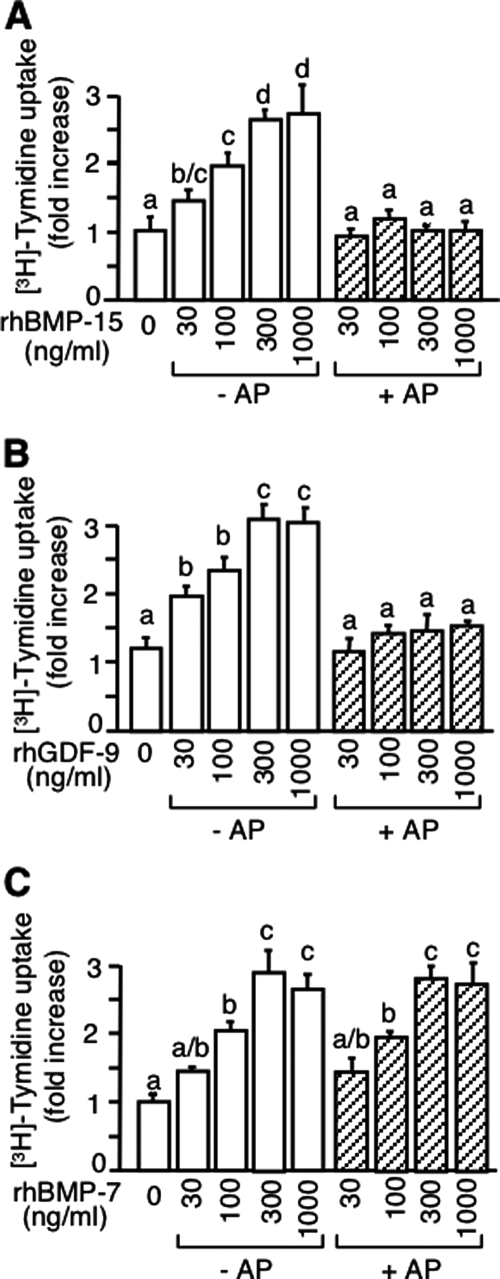
To determine the potential role of the phosphorylation on bioactivity, we used a granulosa cell thymidine incorporation assay because it is known as a common assay for all these TGF-β superfamily members (15,16,17,26). As shown in Fig. 2 (open bars), rhBMP-15, rhGDF-9, and rhBMP-7 all caused a dose-dependent increase in DNA synthesis. In striking contrast, the dephosphorylated rhBMP-15 and rhGDF-9 (shaded bars in Fig. 2, A and B) had no effect on DNA synthesis. Furthermore, the AP treatment of rhBMP-7, which is not phosphorylated, had no effect on its bioactivity (shaded bars in Fig. 2C), indicating that the loss of the activity of rhBMP-15 and rhGDF-9 upon AP treatment is neither due to the AP treatment itself nor to AP remaining in the sample but, indeed, due to the removal of phosphate group(s) from the molecule. Collectively, these results support the conclusion that phosphorylation is an essential posttranslational modification for the bioactivity of rhBMP-15 and rhGDF-9, but not rhBMP-7.
Figure 2.
Effect of AP treatment of rhBMP-15, rhGDF-9, and rhBMP-7 on rat granulosa cell mitosis. Granulosa cells were treated for 24 h with [methyl-3H]thymidine plus the indicated concentrations of rhBMP-15 (A), rhGDF-9 (B), and rhBMP-7 (C) with or without AP pretreatment. The labeled thymidine incorporated into the cells was counted.
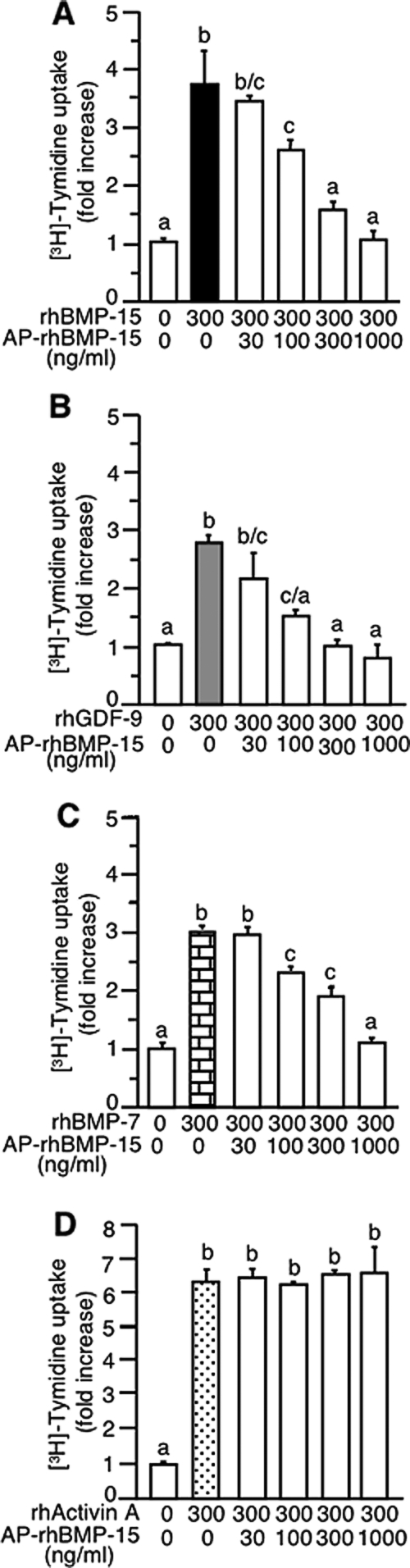
It has previously been reported that dephosphorylation may change some ligands into functional antagonists (27,28). Therefore, we investigated whether dephosphorylated BMP-15 or GDF-9 might have antagonistic effects on their phosphorylated counterparts. As shown in Fig. 3A, cotreatment with increasing concentrations of dephosphorylated rhBMP-15 caused a dose-dependent decrease in the stimulatory effects of the phosphorylated rhBMP-15 on granulosa cell DNA synthesis.
Figure 3.
Effect of AP-treated rhBMP-15 on the granulosa cell mitotic activity of rhBMP-15, rhGDF-9, rhBMP-7, and rh activin A. Cells were treated for 24 h with [methyl-3H]thymidine and 300 ng/ml rhBMP-15 (A), rhGDF-9 (B), rhBMP-7 (C), or rh activin A (D) plus the indicated concentrations of AP-treated rhBMP-15 (AP-rhBMP-15).
We next examined whether dephosphorylated rhBMP-15 could also antagonize the activity of rhGDF-9, rhBMP-7, and rh activin A. Interestingly, the dephosphorylated form of rhBMP-15 was also able to antagonize the bioactivity of rhGDF-9 (Fig. 3B) and rhBMP-7 (Fig. 3C). However, dephosphorylated rhBMP-15 did not impair rh activin A-stimulated DNA synthesis (Fig. 3D). The negative effect of rhBMP-15 treated with AP on the bioactivity of rh activin A again indicates that AP remaining in the sample has no effect.
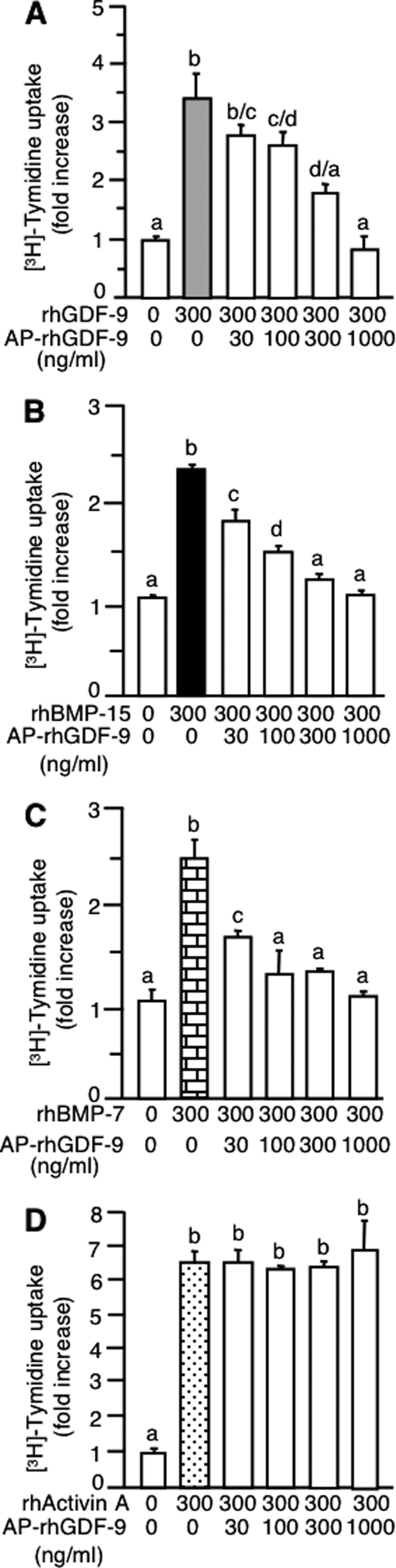
A similar strategy was used to determine whether dephosphorylated rhGDF-9 is a functional antagonist. Interestingly, the effects of dephosphorylated rhGDF-9 were similar to those observed with dephosphorylated rhBMP-15, i.e. dephosphorylated rhGDF-9 profoundly inhibited DNA synthesis stimulated by rhGDF-9 (Fig. 4A), rhBMP-15 (Fig. 4B), and rhBMP-7 (Fig. 4C) but had no effect on the stimulatory activity of rh activin A (Fig. 4D).
Figure 4.
Effect of AP-treated rhGDF-9 on the granulosa cell mitotic activity of rhGDF-9, rhBMP-15, rhBMP-7, and rh activin A. Cells were treated for 24 h with [methyl-3H]thymidine and 300 ng/ml rhGDF-9 (A), rhBMP-15 (B), rhBMP-7 (C), or rh activin A (D) plus the indicated concentrations of AP-treated rhGDF-9 (AP-rhGDF-9).
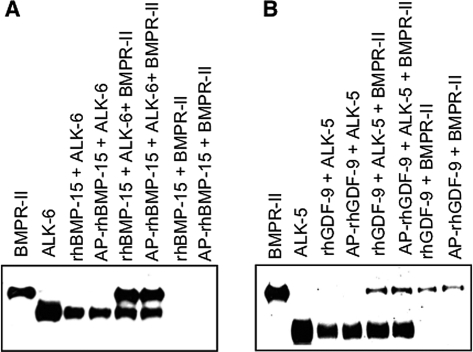
To investigate the mechanism responsible for the antagonistic activity of dephosphorylated rhBMP-15 and rhGDF-9, we compared the biochemical binding ability of the phosphorylated and dephosphorylated proteins. It has been previously reported that the type I and II receptors for BMP-15 are ALK-6 and BMPR-II (19), whereas those for GDF-9 are ALK-5 and BMPR-II, respectively (29,30). In this study we used receptor ECD/Fc chimeric proteins of BMPR-II, ALK-6, or ALK-5, as described previously (19,29). Specifically, the type I (ALK-5 or ALK-6) and type II (BMPR-II) receptors were incubated, separately or in combination, with rhBMP-15, dephosphorylated rhBMP-15, rhGDF-9, or dephosphorylated rhGDF-9, and they were coimmunoprecipitated with anti-FLAG antibody, followed by Western immunoblotting analysis using antihuman IgG to detect the receptor/Fc chimeric proteins.
As shown in Fig. 5A, ALK-6 was coimmunoprecipitated with either rhBMP-15 or dephosphorylated rhBMP-15 with a comparable amount, indicating that they have similar affinity for ALK-6. However, BMPR-II was not coimmunoprecipitated at all with rhBMP-15 or dephosphorylated rhBMP-15. Interestingly, when both ALK-6 and BMPR-II were added, they were coimmunoprecipitated together with either rhBMP-15 or dephosphorylated rhBMP-15. Slightly different results were obtained for rhGDF-9 and dephosphorylated rhGDF-9, in which either ALK-5, BMPR-II, or both receptors together were coimmunoprecipitated with rhGDF-9 or dephosphorylated rhGDF-9 (Fig. 5 B). Thus, no clear difference between rhBMP-15 and dephosphorylated rhBMP-15, and between rhGDF-9 and dephosphorylated rhGDF-9 was observed in this receptor binding assay.
Figure 5.
Coimmunoprecipitation of type I and II receptor ECD/Fc chimeric proteins with rhBMP-15 (A) or rhGDF-9 (B). The indicated receptor-ECDs were coimmunoprecipitated with specified ligands using an anti-FLAG monoclonal antibody conjugated to agarose beads. Proteins eluted from agarose beads were subjected to Western immunoblotting analysis using antihuman IgG antibody. The indicated receptor-ECD alone was loaded in the first two lanes of both panels to show the location of each ECD/Fc chimeric protein.
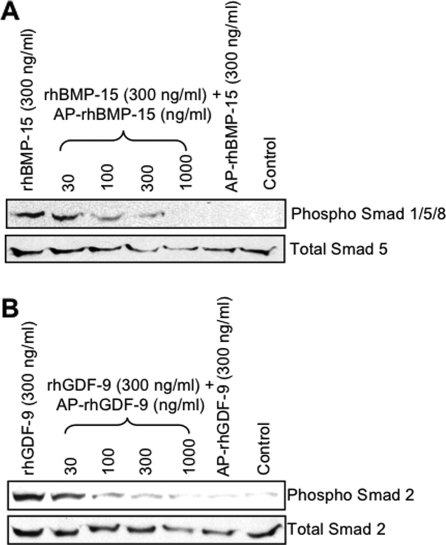
Because dephosphorylation of rhBMP-15 and rhGDF-9 exhibited no effect on the ability of these proteins to bind their respective receptors, we next investigated whether the dephosphorylated proteins are able to activate their respective Smad pathways. BMP-15 is known to signal through Smad1/5/8 (19), whereas GDF-9 signals through the Smad2/3 pathway (30). As expected from the previous reports (19), rhBMP-15 caused a pronounced increase in phospho-Smad1/5/8, whereas dephosphorylated rhBMP-15 had no effect (Fig. 6A). Moreover, the effect of rhBMP-15 was suppressed by increasing doses of dephosphorylated rhBMP-15. Similar results were observed for the activation of Smad2 by rhGDF-9, dephosphorylated rhGDF-9, or combination thereof (Fig. 6B). These findings suggest that the antagonistic activity of dephosphorylated rhBMP-15 and dephosphorylated rhGDF-9 occurs at the level of Smad activation.
Figure 6.
Activation of the Smad signaling pathways. COV-434 (A) and P19 (B) cells were cultured with the indicated concentrations of ligands for 1 h. Cell lysates were then subjected to Western immunoblotting analysis using indicated Smad antibodies.
Discussion
In this study we have provided compelling evidence that rhBMP-15 and rhGDF-9 are phosphorylated. It is remarkable that these two oocyte-secreted growth factors are the first members of the TGF-β superfamily to be reported as phosphoproteins. We have also demonstrated that phosphorylation is essential for the bioactivity of rhBMP-15 and rhGDF-9. Moreover, dephosphorylated rhBMP-15 and rhGDF-9 can abolish the bioactivity of phosphorylated rhBMP-15 and rhGDF-9, respectively. Interestingly, dephosphorylated rhBMP-15 was also able to antagonize the bioactivity of rhGDF-9 and rhBMP-7, which are known to bind the same BMPR-II as rhBMP-15. However, dephosphorylated rhBMP-15 did not impair the DNA synthesis induced by rh activin A, which shares neither type I nor II receptors with rhBMP-15 or rhGDF-9 (1). Similar results were obtained for dephosphorylated rhGDF-9, which antagonized the bioactivity of rhBMP-15 and rhBMP-7 but had no effect on the bioactivity of rh activin A. These findings suggest that the dephosphorylated forms of rhBMP-15 and rhGDF-9 antagonize the biological activity of rhBMP-15, rhGDF-9, and rhBMP-7 at the level of receptor binding.
To understand the mechanism of this antagonistic activity, we examined the ability of the dephosphorylated ligands to bind to their type I and II receptors. Interestingly, both dephosphorylated rhBMP-15 and rhGDF-9 could bind their respective type I and II receptors with similar affinity to the wild-type ligands, suggesting a possible competition in the receptor binding between the phosphorylated and dephosphorylated ligands. Intriguingly, both rhBMP-15 and dephosphorylated rhBMP-15 did not bind BMPR-II alone; however, they could bind BMPR-II in the presence of ALK-6. This finding supports our previous proposition for the mechanism that rhBMP-15 binds to ALK-6 on the surface of rat granulosa cells, and after a conformational change in the ALK-6 receptor, the rhBMP-15/ALK-6 complex recruits BMPR-II, followed by the Smad1/5/8 activation.
The next possible site of the antagonistic effect after the receptor binding is at the level of Smad activation. Either rhBMP-15 or rhGDF-9 alone stimulated phosphorylation of Smad1/5/8 or Smad2, respectively. However, the dephosphorylated forms of these proteins did not increase phosphorylation of their respective Smads above the control level. Moreover, when increasing doses of dephosphorylated rhBMP-15 were added to rhBMP-15, the level of Smad1/5/8 phosphorylation was reduced to the control levels. A similar effect was found when increasing amounts of dephosphorylated rhGDF-9 were added to rhGDF-9. These findings suggest that the antagonistic effects of the dephosphorylated forms of rhBMP-15 and rhGDF-9 are attributed to the inability of these proteins to activate Smad phosphorylation, while they are capable of binding to their type I and II receptors. Together, these results suggest that dephosphorylated rhBMP-15 and rhGDF-9 antagonize their wild-type counterparts by binding to their receptors and failing to activate Smad phosphorylation, thus rendering the receptors unavailable for use by the wild-type ligands.
Phosphorylation is one of the most common and important regulatory modifications (31,32). This posttranslational modification has major influences on various cellular functions, such as signal transduction, metabolic maintenance, and cell division (33). Given that BMP-15 and GDF-9 are secreted proteins, phosphorylation most likely takes place in the secretory pathways within the cells. A number of phosphorylated secretory proteins, such as the milk caseins, IL-6 (34), IGF binding protein-1 (35), ACTH (36,37), matrix Gla protein (38), progastrin (39,40,41) and osteopontin (42,43), have a consensus sequence of (Ser)-(Xaa)-(Glu or previously phosphorylated Ser) that is a target of a casein kinase termed Golgi apparatus casein kinase (44,45,46). Interestingly, this sequence is also present in the mature proteins of human BMP-15 (Ser6-Ala7-Glu8) and GDF-9 (Ser6-Ser7-Glu8), suggesting that BMP-15 and GDF-9 may be targets of Golgi apparatus casein kinase.
Collectively, our data indicate that phosphorylation is a critical posttranslational event for the bioactivity of rhBMP-15 and rhGDF-9. Moreover, dephosphorylated rhBMP-15 and rhGDF-9 exhibit antagonistic activity toward not only their phosphorylated counterparts but also toward each other, as well as rhBMP-7, suggesting that the nonphosphorylated forms of these oocyte-secreted factors may act more broadly as functional antagonists for other members of the TGF-β superfamily. Although these effects have not yet been shown to be directly involved in any of the in vivo actions of either BMP-15 or GDF-9, our findings may initiate a new area of research into phosphorylation as a method of regulation of the bioactivity of TGF-β superfamily members.
Acknowledgments
We thank Dr. Niwa for allowing us access to the phosphoimager, Dr. Erickson for helpful discussions, and Ms. Hartgrove for editorial assistance.
Footnotes
=Address all correspondence and requests for reprints to: Shunichi Shimasaki, Ph.D., Department of Reproductive Medicine, University of California San Diego, 9500 Gilman Drive, La Jolla, California 92093-0633. E-mail: sshimasaki@ucsd.edu.
This work was supported by the National Institutes of Health Grant RO1 HD41494 and the University of California San Diego Academic Senate Grants RG008B and RH040H (to S.Shi.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 15, 2007
Abbreviations: AP, Alkaline phosphatase; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; ECD, extracellular domain; GDF, growth and differentiation factor; rh, recombinant human.
References
- Shimasaki S, Moore RK, Otsuka F, Erickson GF 2004 The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101 [DOI] [PubMed] [Google Scholar]
- Erickson GF, Shimasaki S 2000 The role of the oocyte in folliculogenesis. Trends Endocrinol Metab 11:193–198 [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O 2000 Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM 2004 Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 70:900–909 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL 2005 Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol 234:57–66 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L 2004 Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 75:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L 2005 Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause 12:749–754 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M, Wasniewska M, Cole T, Beck-Peccoz P, Nelson LM, Persani L 2006 Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 91:1976–1979 [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L 2006 Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet 119:408–415 [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss A-C, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA 2006 Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol 154:739–744 [DOI] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA 2007 Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 87:143–146 [DOI] [PubMed] [Google Scholar]
- Massague J 1990 The transforming growth factor-β family. Ann Rev Cell Biol 6:597–641 [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM 2002 Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
- Juengel JL, McNatty KP 2005 The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 11:143–160 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S 2000 Bone morphogenetic protein-15: identification of target cells and biological functions. J Biol Chem 275:39523–39528 [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Shimasaki S 2004 Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors bone morphogenetic protein-15 and growth and differentiation factor-9. J Biol Chem 279:17391–17396 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S 2002 A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA 99:8060–8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S 2003 Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 278:304–310 [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Otsuka F, Shimasaki S 2003 Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9. Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J Biol Chem 278:3713–3719 [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Moore RK, Shimasaki S 2005 Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci USA 102:5426–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC 2001 Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies RS, Chen TJ, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, Wolfman NM 2000 GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors 18:251–259 [DOI] [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bachinger HP, Sakai LY 2005 The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix J Biol Chem 280:27970–27980 [DOI] [PubMed] [Google Scholar]
- Jiang MS, Liang LF, Wang S, Ratovitski T, Holmstrom J, Barker C, Stotish R 2004 Characterization and identification of the inhibitory domain of GDF-8 propeptide. Biochem Biophys Res Commun 315:525–531 [DOI] [PubMed] [Google Scholar]
- Lee W, Otsuka F, Moore RK, Shimasaki S 2001 The effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 65:994–999 [DOI] [PubMed] [Google Scholar]
- Wang LG, Liu XM, Kreis W, Budman DR 1999 Phosphorylation/dephosphorylation of androgen receptor as a determinant of androgen agonistic or antagonistic activity. Biochem Biophys Res Commun 259:21–28 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tomida A, Tsuruo T 2001 Dephosphorylated hypoxia-inducible factor 1 as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 20:5779–5788 [DOI] [PubMed] [Google Scholar]
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJ 2002 Bone morphogenetic protein receptor type ii is a receptor for growth differentiation factor-9. Biol Reprod 67:473–480 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJ 2004 Growth differentiation factor-9 signaling is mediated by the type i receptor, activin receptor-like kinase 5. Mol Endocrinol 18:653–665 [DOI] [PubMed] [Google Scholar]
- Cohen P 2002 The origins of protein phosphorylation. Nat Cell Biol 4:E127–E130 [DOI] [PubMed] [Google Scholar]
- Reinders J, Sickmann A 2005 State-of-the-art in phosphoproteomics. Proteomics 5:4052–4061 [DOI] [PubMed] [Google Scholar]
- Hunter T 2000 Signaling–2000 and beyond. Cell 100:113–127 [DOI] [PubMed] [Google Scholar]
- May LT, Sehgal PB 1992 Phosphorylation of interleukin-6 at serine54: an early event in the secretory pathway in human fibroblasts. Biochem Biophys Res Commun 185:524–530 [DOI] [PubMed] [Google Scholar]
- Jones JI, Busby Jr WH, Wright G, Smith CE, Kimack NM, Clemmons DR 1993 Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J Biol Chem 268:1125–1131 [PubMed] [Google Scholar]
- Eipper BA, Mains RE 1982 Phosphorylation of pro-adrenocorticotropin/endorphin-derived peptides. J Biol Chem 257:4907–4915 [PubMed] [Google Scholar]
- Bennett HP, Brubaker PL, Seger MA, Solomon S 1983 Human phosphoserine 31 corticotropin1–39. Isolation and characterization. J Biol Chem 258:8108–8112 [PubMed] [Google Scholar]
- Price PA, Rice JS, Williamson MK 1994 Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci 3:822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ, Varro A, Desmond H, Young J, Gregory H, Gregory RA 1987 Post-translational processing of the porcine gastrin precursor by phosphorylation of the COOH-terminal fragment. J Biol Chem 262:8643–8647 [PubMed] [Google Scholar]
- Varro A, Desmond H, Pauwels S, Gregory H, Young J, Dockray GJ 1988 The human gastrin precursor. Characterization of phosphorylated forms and fragments. Biochem J 256:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond H, Varro A, Young J, Gregory H, Nemeth J, Dockray GJ 1989 The constitution and properties of phosphorylated and unphosphorylated c-terminal fragments of progastrin from dog and ferret antrum. Regul Pept 25:223–2233 [DOI] [PubMed] [Google Scholar]
- Sorensen ES, Hojrup P, Petersen TE 1995 Posttranslational modifications of bovine osteopontin: identification of twenty-eight phosphorylation and three o-glycosylation sites. Protein Sci 4:2040–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M, Chang PL, Prince CW, Pinna LA 1997 Phosphorylation of osteopontin by Golgi apparatus casein kinase. Biochem Biophys Res Commun 240:602–605 [DOI] [PubMed] [Google Scholar]
- Meggio F, Boulton AP, Marchiori F, Borin G, Lennon DP, Calderan A, Pinna LA 1988 Substrate-specificity determinants for a membrane-bound casein kinase of lactating mammary gland. A study with synthetic peptides. Eur J Biochem 177:281–284 [DOI] [PubMed] [Google Scholar]
- Meggio F, Perich JW, Meyer HE, Hoffmann-Posorske E, Lennon DP, Johns RB, Pinna LA 1989 Synthetic fragments of β-casein as model substrates for liver and mammary gland casein kinases. Eur J Biochem 186:459–464 [DOI] [PubMed] [Google Scholar]
- Lasa-Benito M, Marin O, Meggio F, Pinna LA 1996 Golgi apparatus mammary gland casein kinase: monitoring by a specific peptide substrate and definition of specificity determinants. FEBS Lett 382:149–152 [DOI] [PubMed] [Google Scholar]